High-purity ranolazine and preparation method thereof
A ranolazine and high-purity technology, applied in the field of high-purity ranolazine and its preparation, can solve the problems of complicated operation, high operation cost, long steps and the like, and achieve the effects of good appearance and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
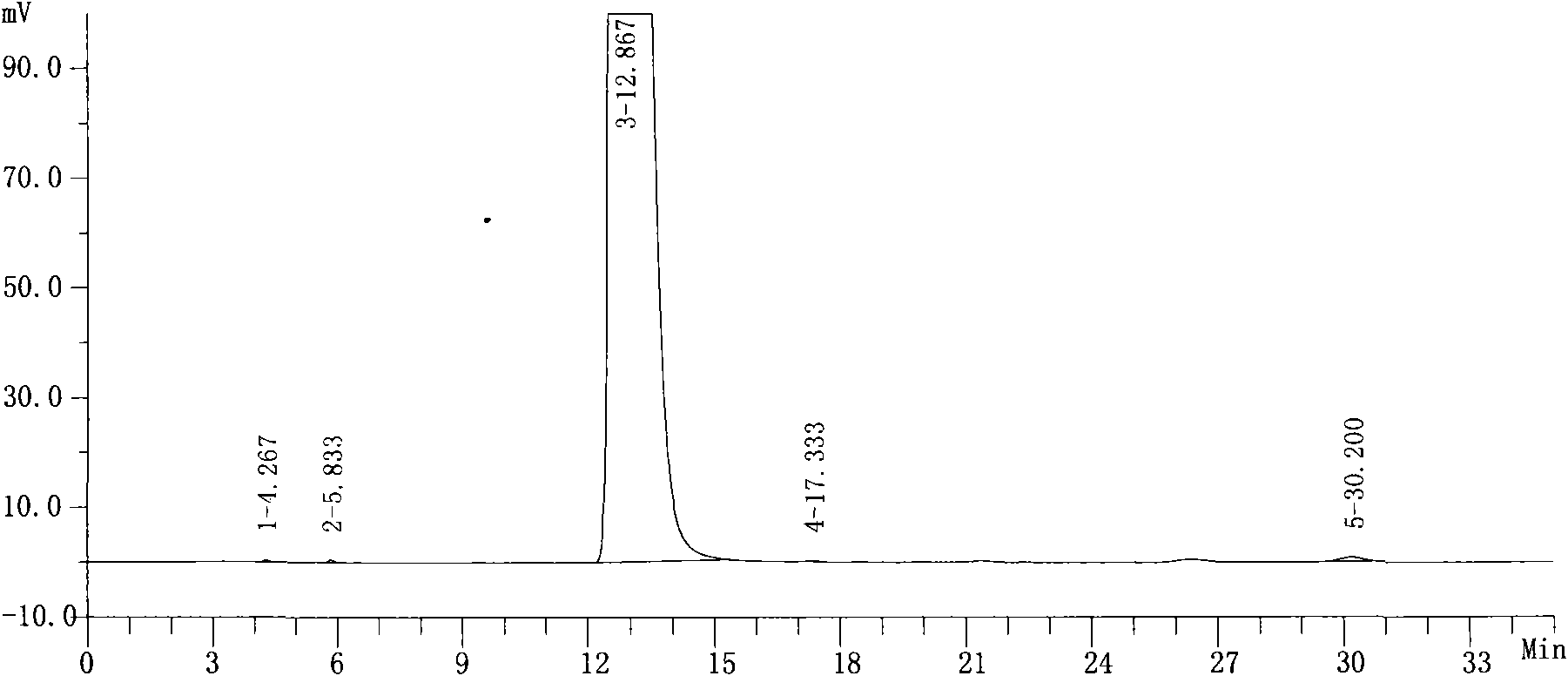
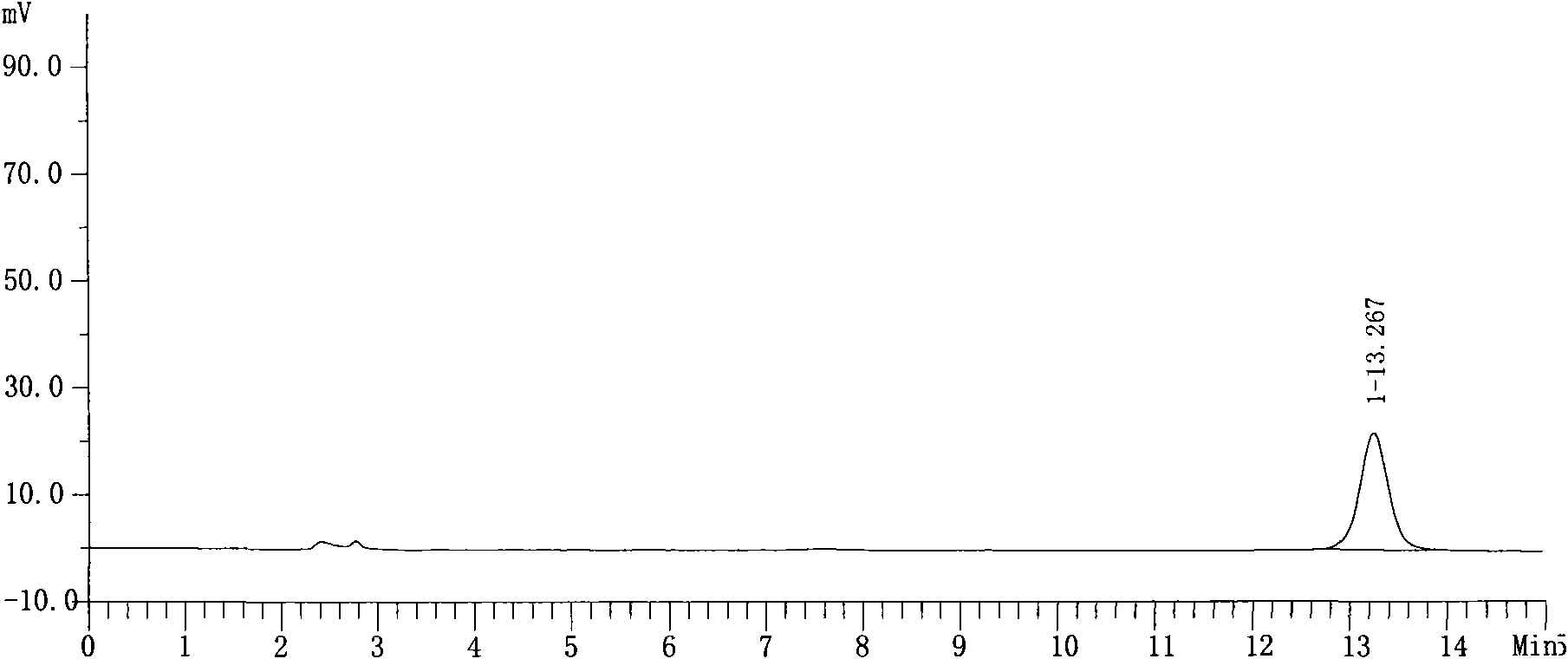
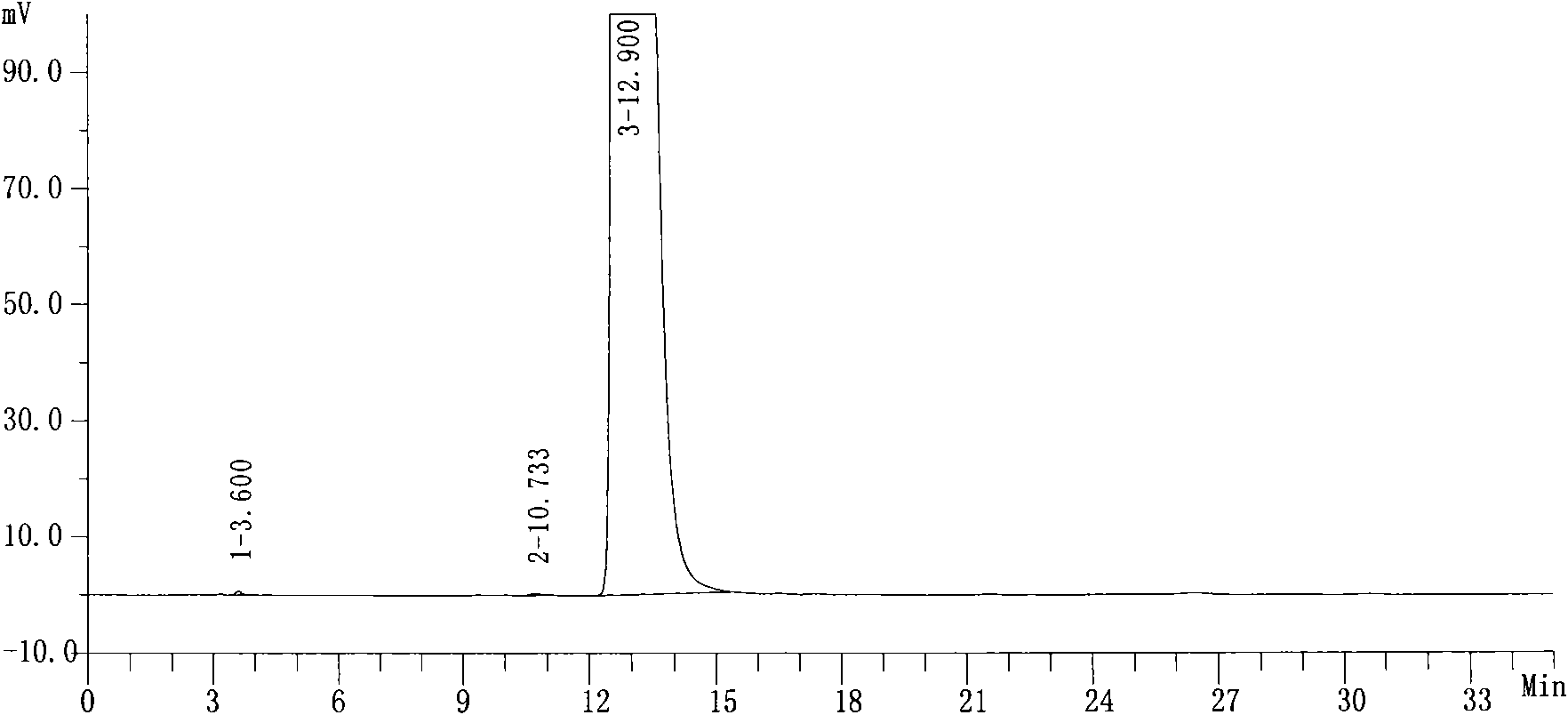
Image
Examples
Embodiment 1
[0032] In a 20L three-neck flask, add 7L of ethanol and 1.4kg (11.5mol) of 2,6-dimethylaniline respectively, and add 1.4kg (13.8mol) of triethylamine at temperature control T=10°C-20°C. Temperature control T=10°C-20°C, 1.9 kg (17.3 mol) of chloroacetyl chloride was added dropwise. After dropping, the temperature was controlled at T=10°C to 20°C to continue the reaction for 2 hours. Add distilled water, stir, and filter with suction. The filter cake was air-dried at 60° C. for 12 hours to obtain 2.0 kg of N-(2,6-dimethylphenyl)chloroacetamide off-white solid, yield: 90%.
Embodiment 2
[0034] In a 20L three-neck flask, add 7L of isopropanol and 1.4kg (11.5mol) of 2,6-dimethylaniline respectively, and add 1.4kg (13.8mol) of triethylamine at temperature control T=10°C-20°C. Temperature control T=10°C-20°C, 1.9 kg (17.3 mol) of chloroacetyl chloride was added dropwise. After dropping, the reaction was continued for 2 hours under temperature control T=0°C-10°C. Add distilled water, stir, and filter with suction. The filter cake was air-dried at 60° C. for 12 hours to obtain 2.06 kg of N-(2,6-dimethylphenyl)chloroacetamide off-white solid, yield: 94%.
Embodiment 3
[0036] Into a 20L three-necked flask, add 12L of toluene and 2.4kg (12.1mol) of N-(2,6-dimethylphenyl)chloroacetamide, respectively. Add 9.4 kg (48.5 mol) of piperazine hexahydrate under stirring, and heat to reflux for 4 hours. Stop the reaction, spin out the solvent under reduced pressure, and add ethyl acetate to dissolve. Suction filtration, the filtrate spins off the solvent under reduced pressure. Add isopropyl ether and beat for 0.5 hours. After suction filtration, the filter cake was air-dried at 60° C. for 8 hours to obtain 2.5 kg of N-(2,6-dimethylphenyl)-1-piperazineacetamide as a white solid, yield: 84%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com