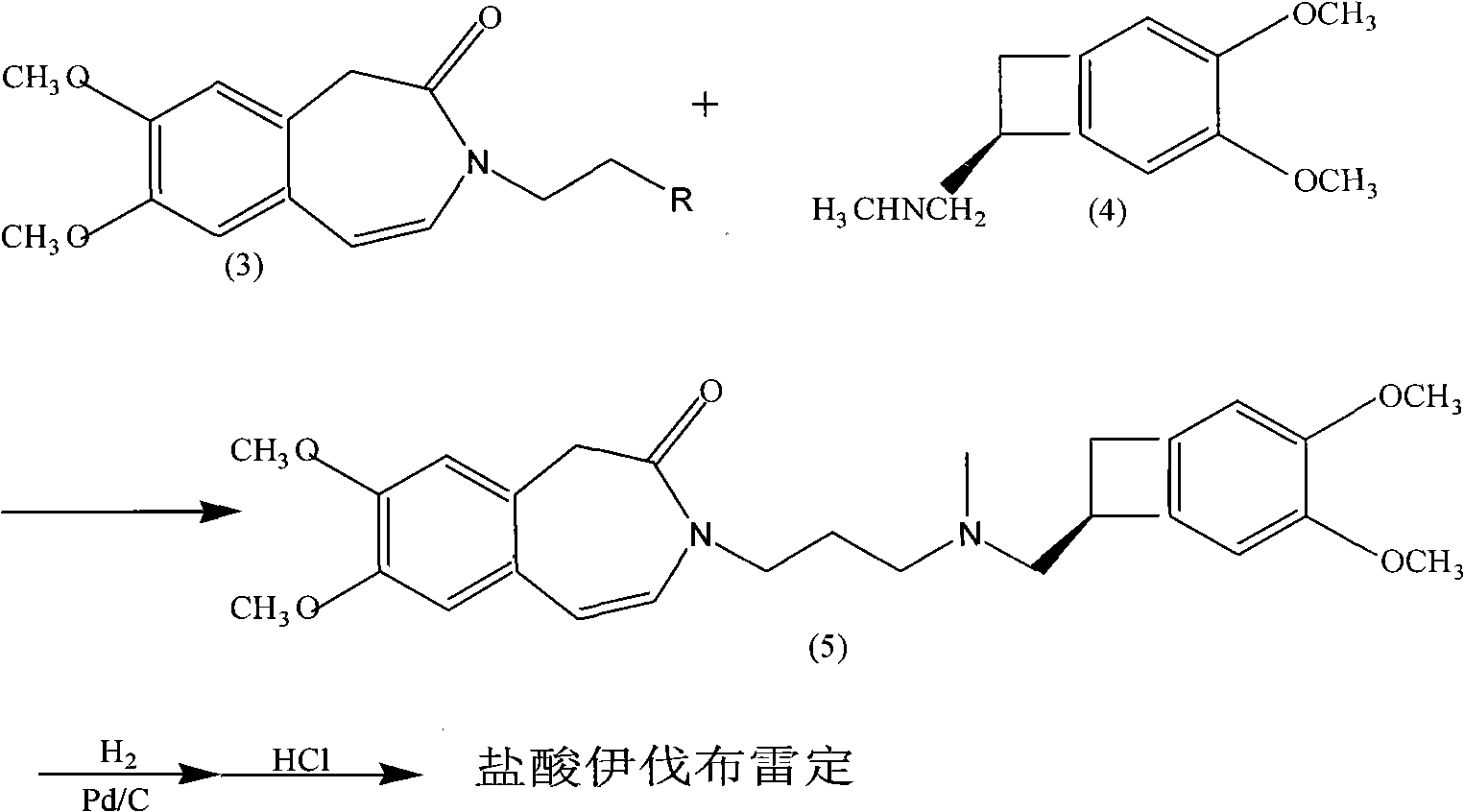
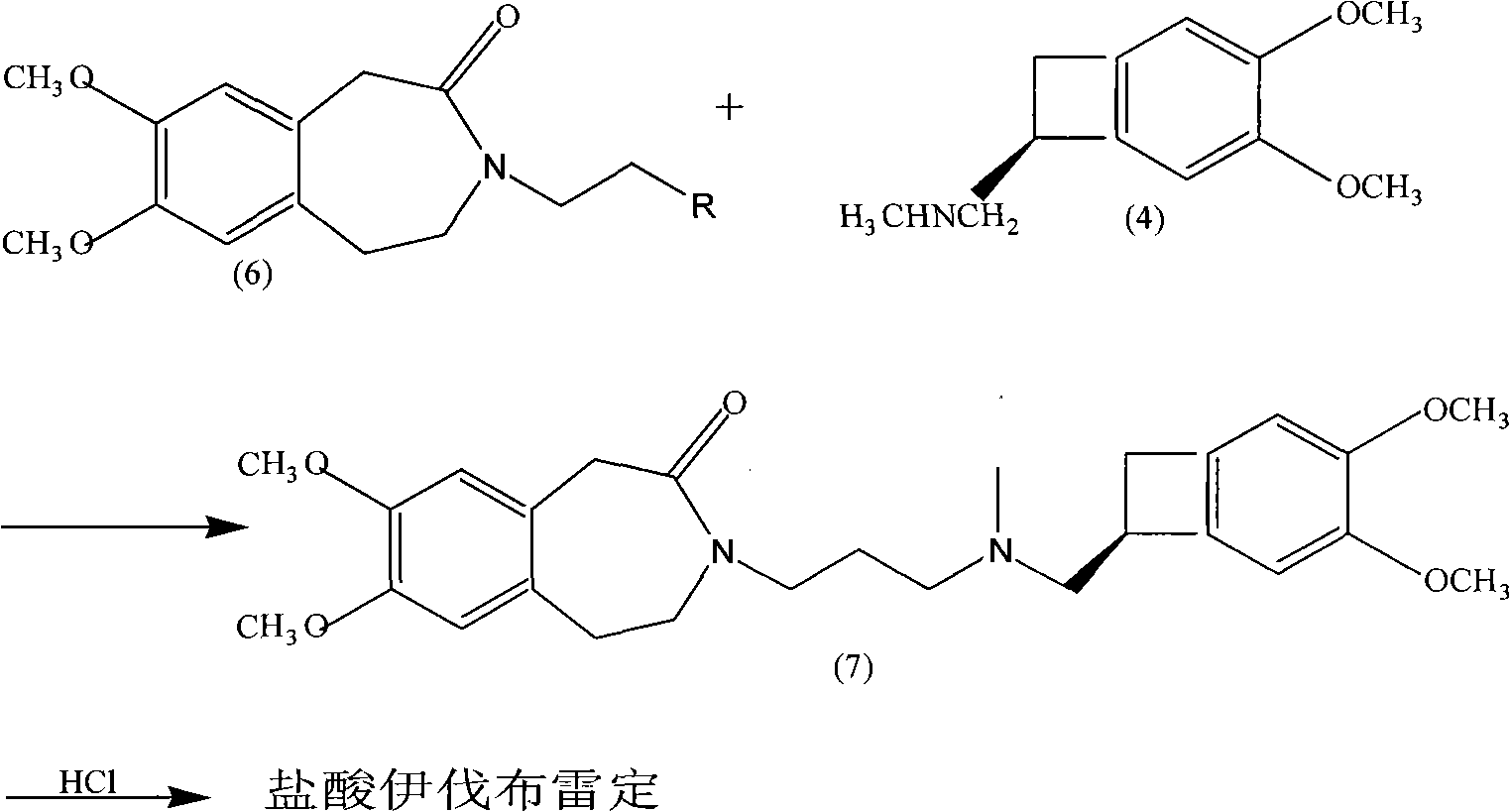
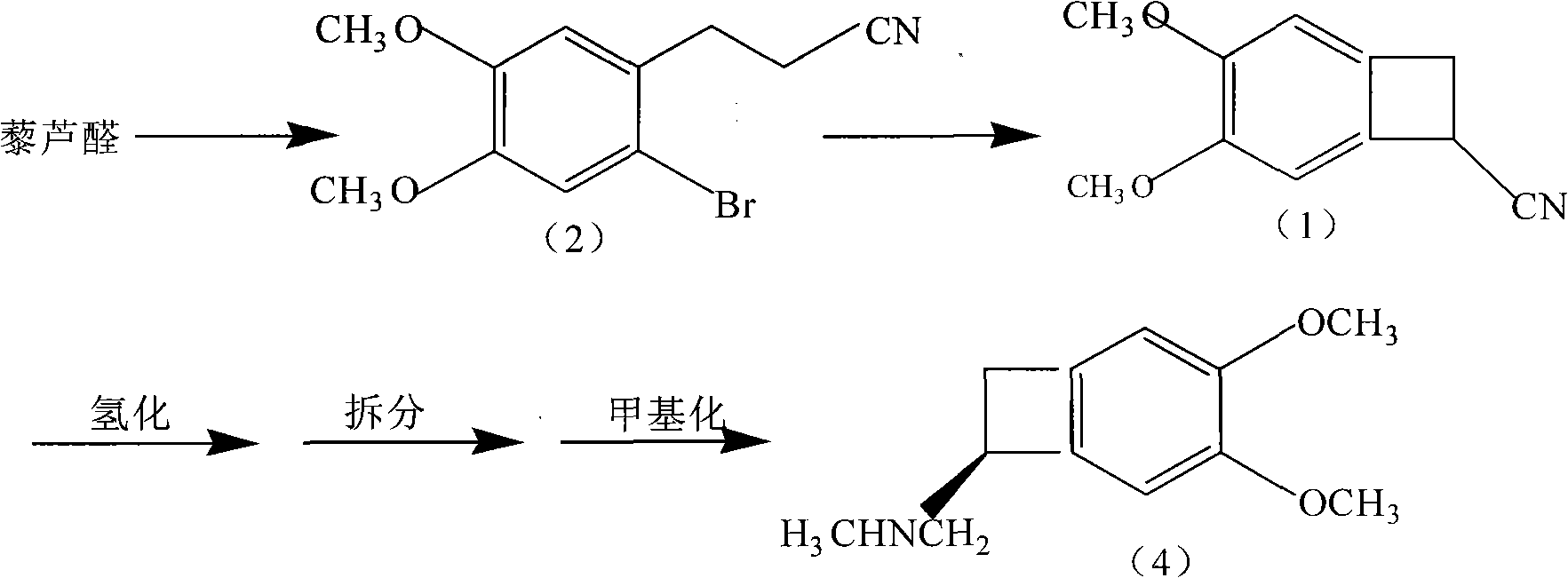
Synthetic method of ivabradine midbody
A technology of ivabradine and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, and can solve the problems of harsh reaction conditions, low yield and purity, and difficult operation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Under the protection of nitrogen, add 70ml of tetrahydrofuran and 60ml of n-butyllithium cyclohexane solution (2.0mol / L) into the reaction flask, stir and cool down to -30°C, then control the temperature at -30°C and add 10ml of diethylamine dropwise , the dropwise addition was completed and the reaction was stirred for 0.5-1h, and 40ml of a tetrahydrofuran solution dissolved in 10g of 3-(2-bromo-4,5-dimethoxyphenyl)propionitrile was added dropwise, and the temperature was controlled at -30°C. After the dropwise addition was completed, Stir the reaction for 0.5-1h, TLC detection (developing agent is petroleum ether: ethyl acetate = 4: 1), after the reaction is complete, dropwise add 60ml of 15% hydrochloric acid to quench the reaction, stir after 5min for liquid separation, and wash the organic layer with water once. Dry with anhydrous magnesium sulfate for 5-8h, filter, and evaporate to dryness to obtain the crude product, which is recrystallized with anhydrous ethanol ...
Embodiment 2
[0034] Under the protection of nitrogen, add 70ml tetrahydrofuran and 80ml cyclohexane solution (1.5mol / L) of lithium diisopropylamide into the reaction flask, stir and cool down to -35°C, then control the temperature at -35°C and add 11ml dropwise After the addition of diethylamine, stir the reaction for 0.5-1h, add dropwise 40ml of tetrahydrofuran solution dissolved in 10g of 3-(2-bromo-4,5-dimethoxyphenyl)propionitrile, control the temperature at -35°C, add dropwise After the completion, stir the reaction for 0.5-1h, TLC detection (developing agent is petroleum ether: ethyl acetate = 4: 1), after the reaction is complete, dropwise add 60ml of 15% hydrochloric acid to quench the reaction, stir for 5min and then separate the liquid, the organic layer is water Wash once, dry with anhydrous magnesium sulfate for 5-8h, filter, and evaporate to dryness to obtain the crude product, which is recrystallized with isopropanol (w / v) 3 times the crude product to obtain the compound 4,5-d...
Embodiment 3
[0036] Under nitrogen protection, add 70ml of n-hexane and 60ml of phenyllithium tetrahydrofuran solution (2.0mol / L) into the reaction flask, stir and cool down to -45°C, then add 12.5ml of diethylamine dropwise at -45°C to control the temperature, After the dropwise addition, stir the reaction for 0.5-1h, add dropwise 40ml of tetrahydrofuran solution dissolved with 10g of 3-(2-bromo-4,5-dimethoxyphenyl)propionitrile, control the temperature at -45°C, after the dropwise addition, stir Reaction for 0.5-1h, TLC detection (developing agent is petroleum ether: ethyl acetate=4:1), after the reaction is complete, dropwise add 60ml of 15% hydrochloric acid to quench the reaction, stir for 5min and separate the liquids, the organic layer is washed once with water, and Dry over anhydrous magnesium sulfate for 5-8 hours, filter, and evaporate to dryness to obtain the crude product, which is recrystallized in absolute ethanol (w / v) twice as large as the crude product to obtain the compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com