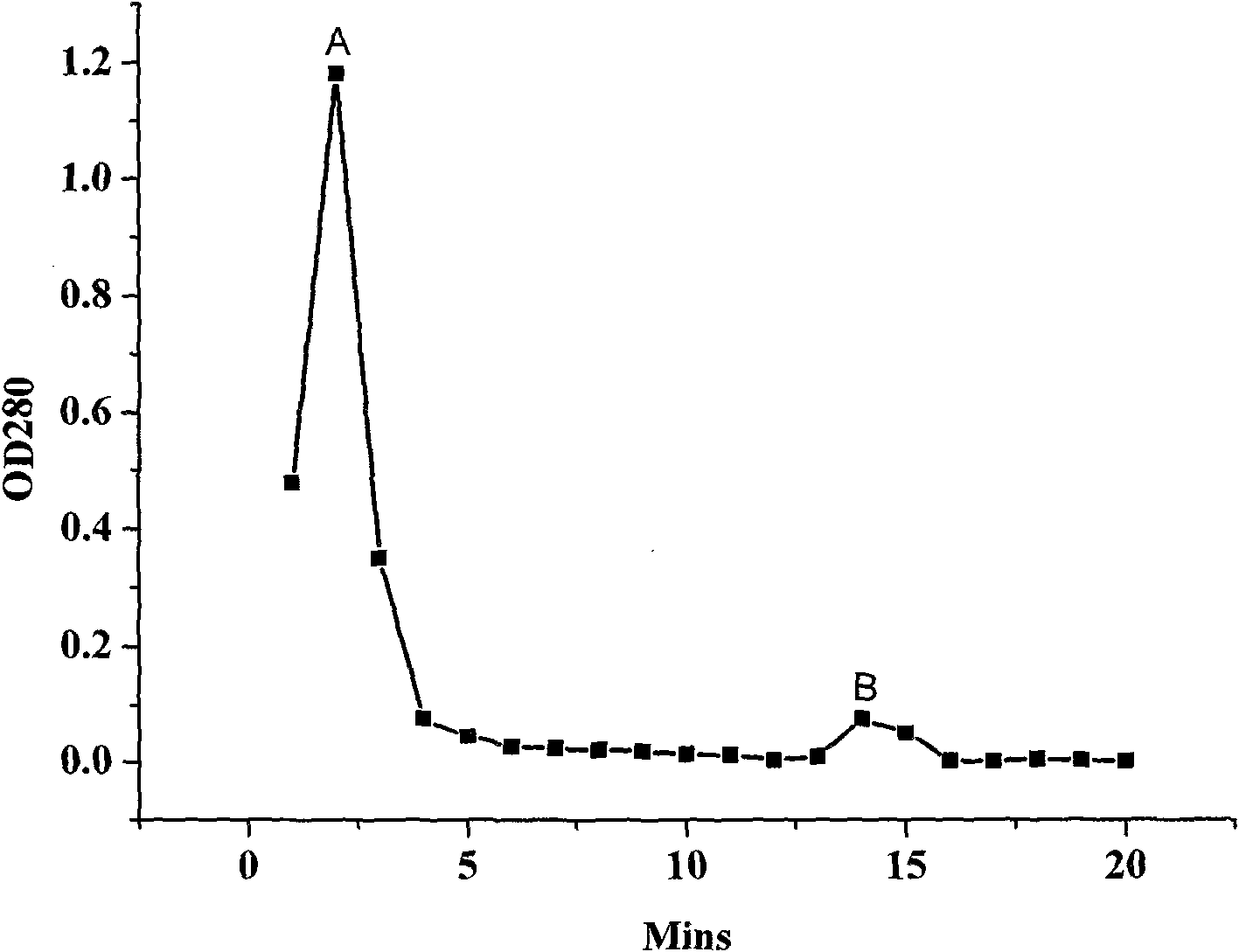
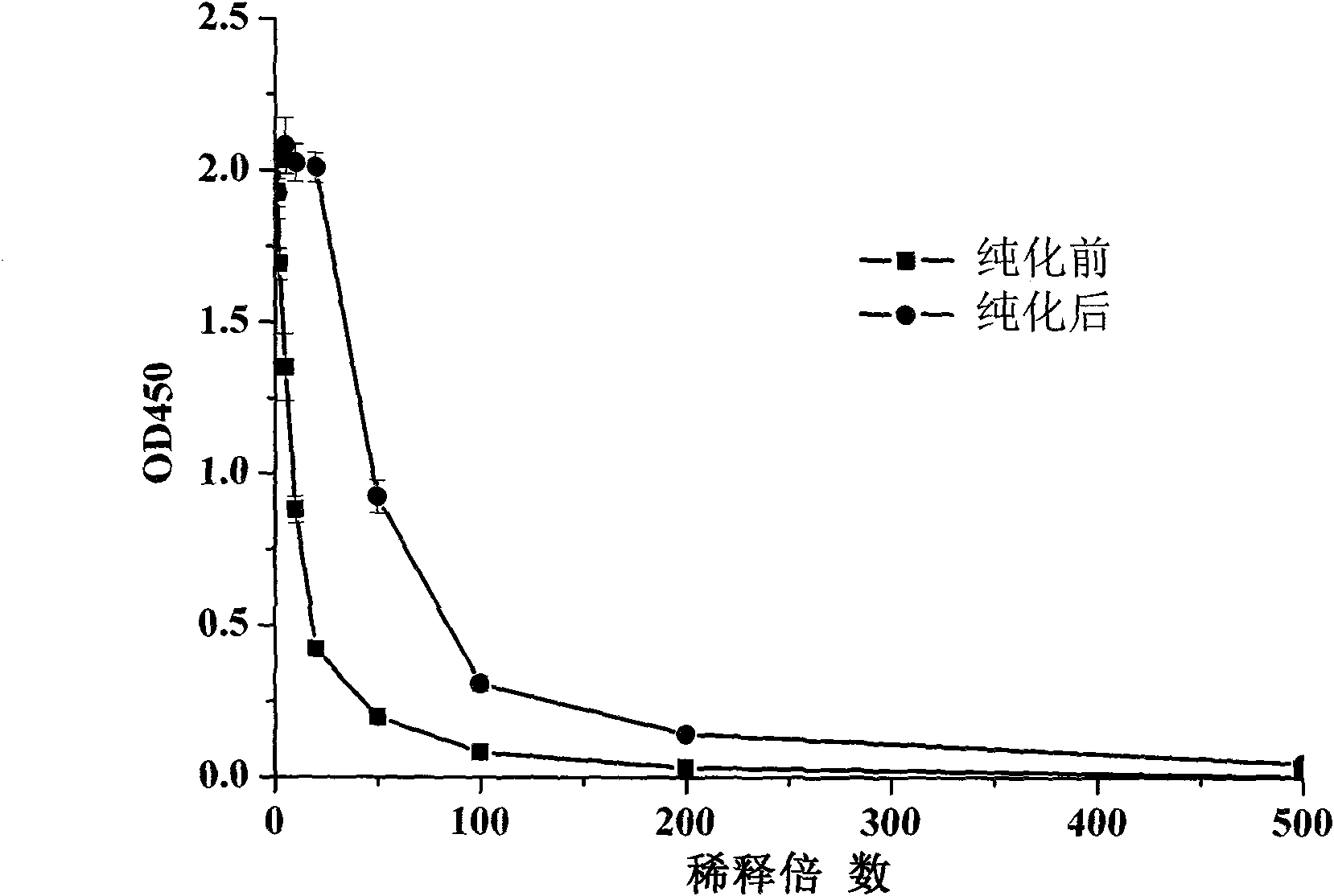
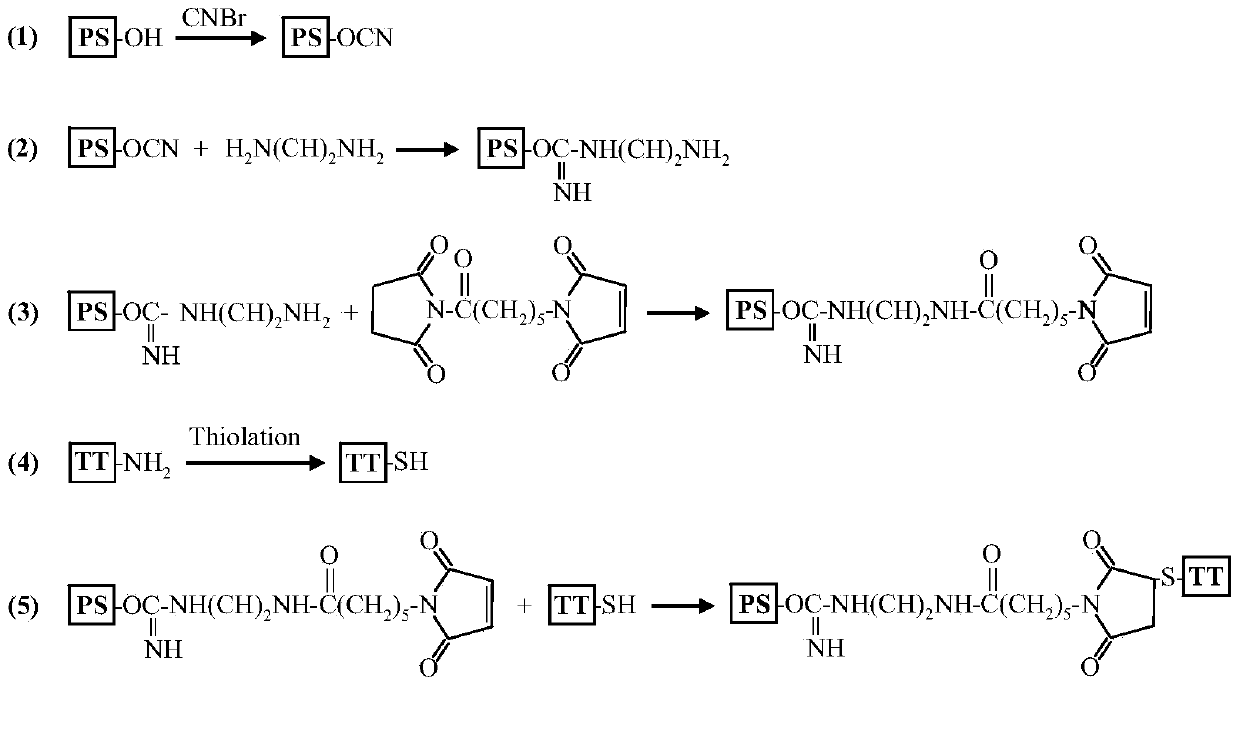
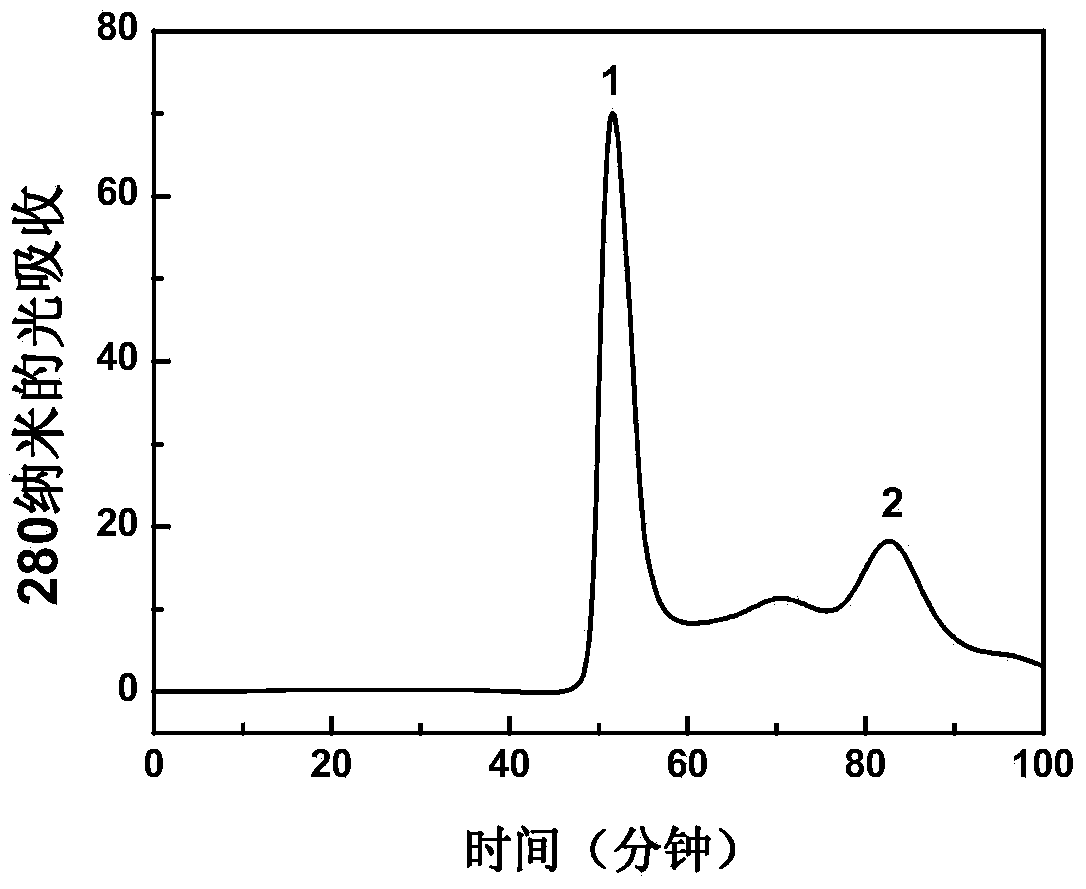
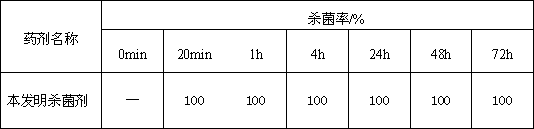
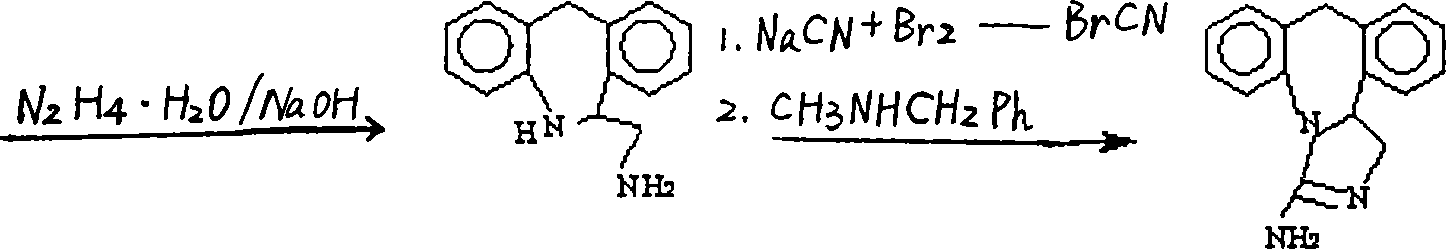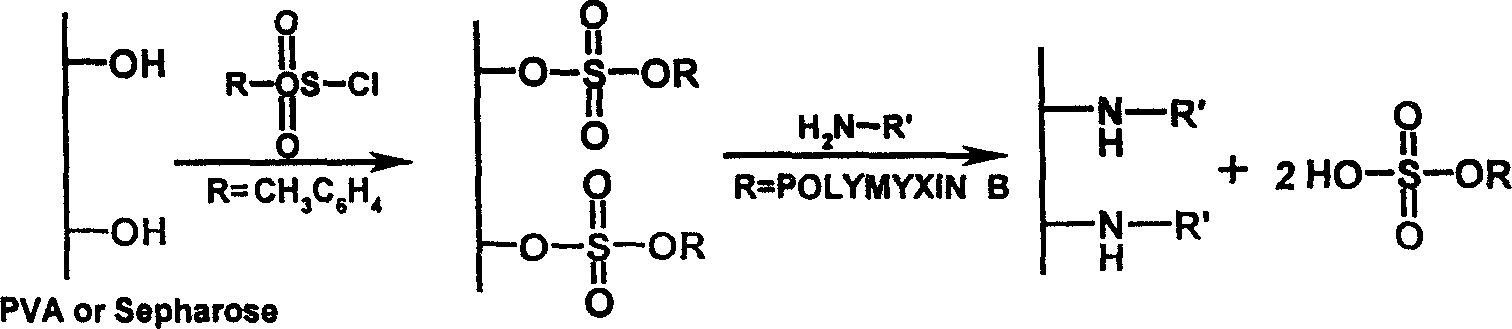Patents
Literature
87 results about "Cyanogen bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides (cuts the C-terminus of methionine), and synthesize other compounds. The compound is classified as a pseudohalogen.
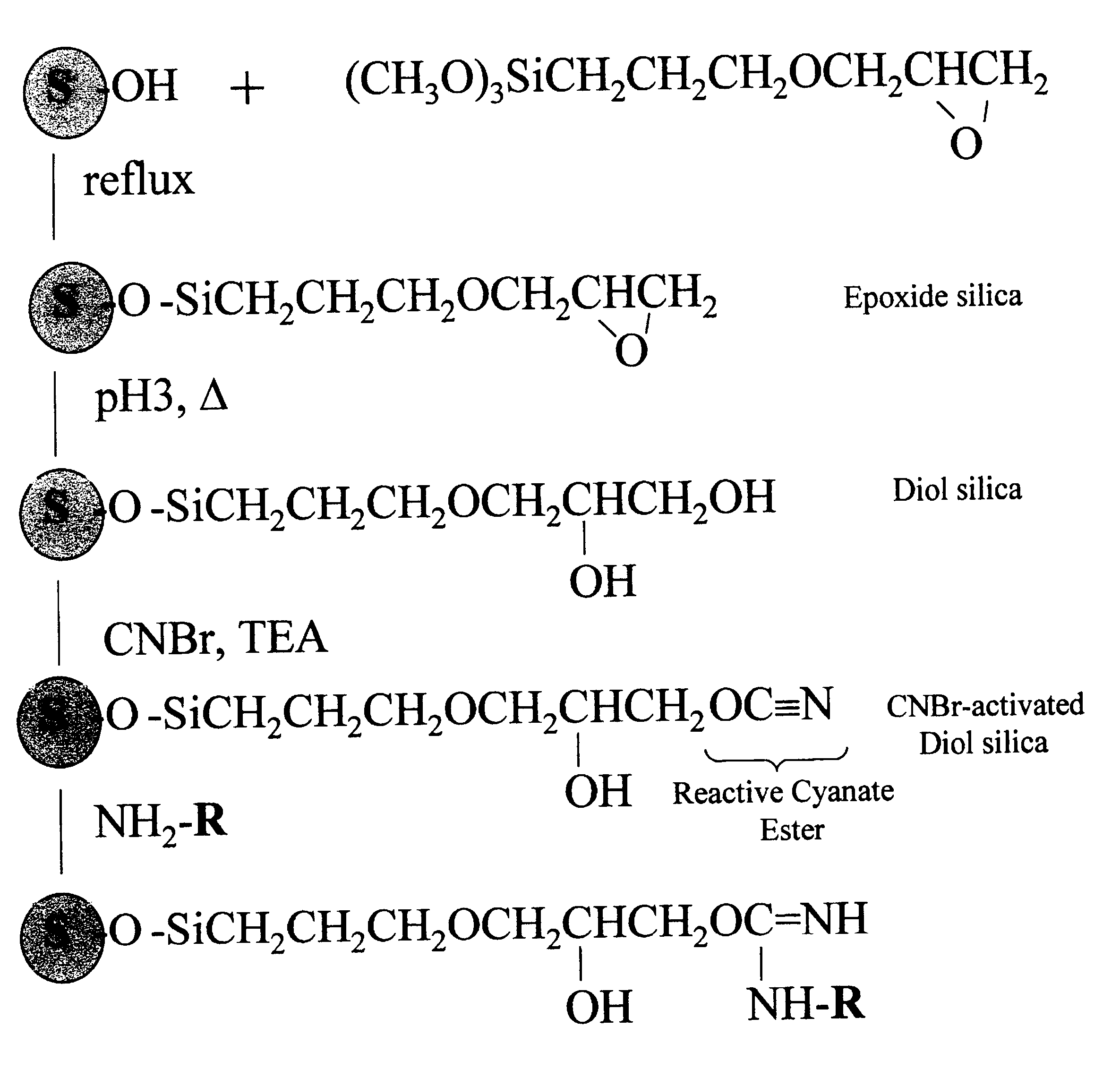
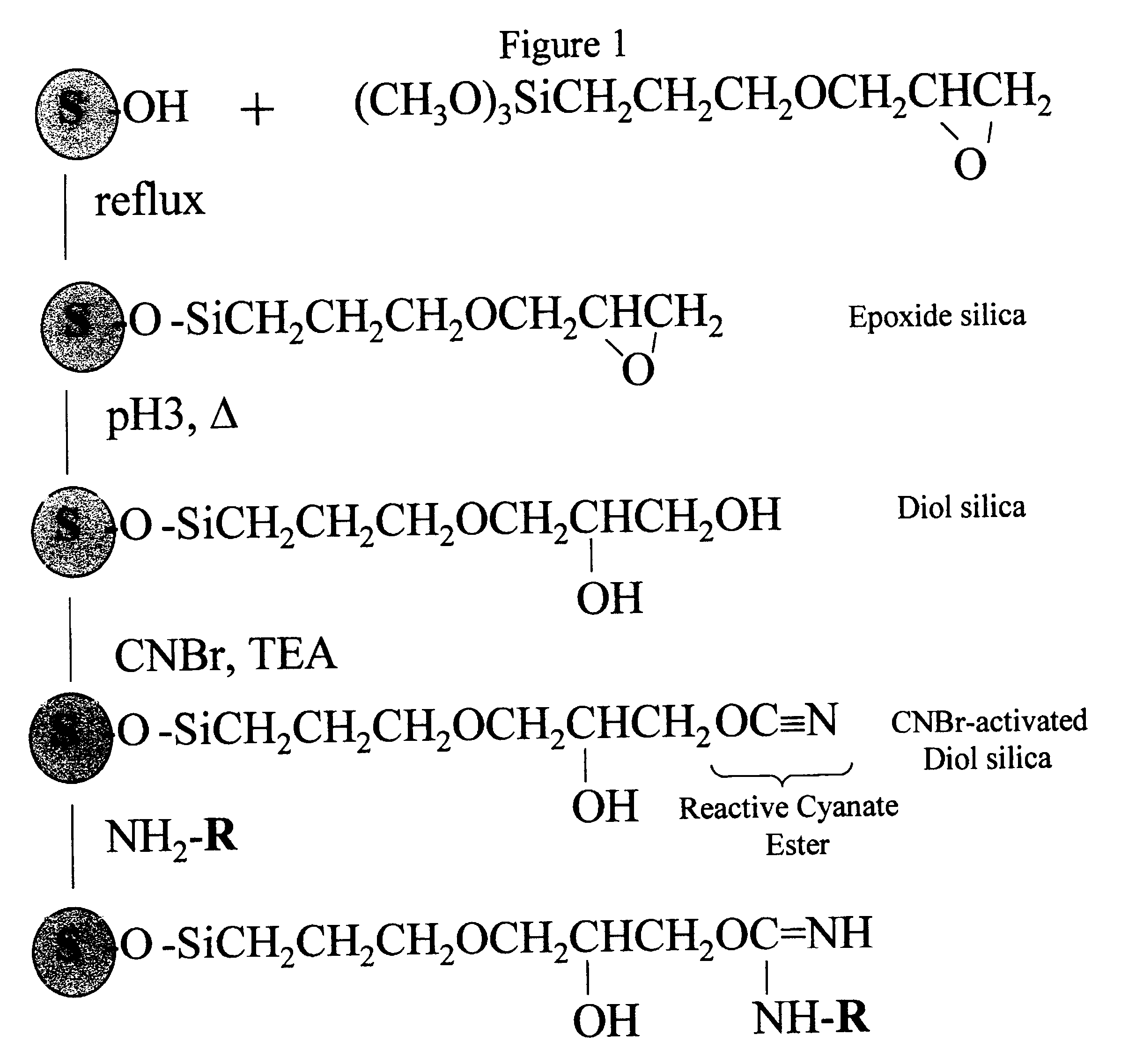
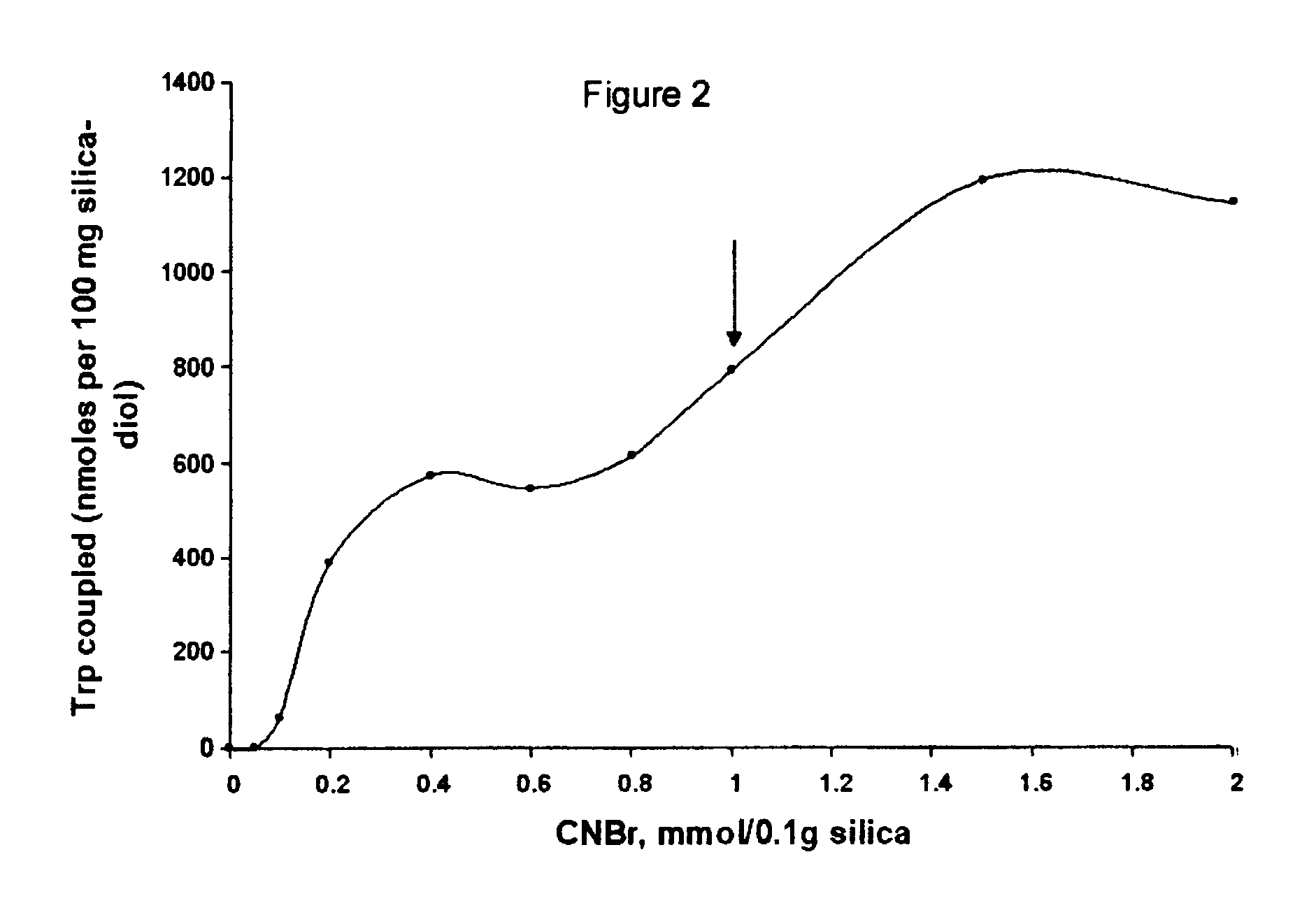
Cyanogen bromide-activation of hydroxyls on silica for high pressure affinity chromatography
InactiveUS6375846B1Excellent chromatographic performanceIon-exchange process apparatusOther chemical processesCyanogen halideCyanogen bromide
The present invention provides for a method to prepare a pressure stable and pH stable medium for use in high pressure (performance) affinity chromatography. The method includes the steps of treating hydroxyalkyl-silica with cyanogen halides or other cyanogen transfer reagents in the presence of an organic base in anhydrous solvents at temperatures in the range of from about -15° C. to about 20° C. for a period of time in the range of from about 1 minute to about 5 minutes, and washing the resulting medium in anhydrous solvent.
Owner:JARRETT HARRY WELLINGTON +1
Preparation method of endotoxin absorbent for blood perfusion
InactiveCN1864755AReduce non-specific adsorptionAvoid instabilityOther blood circulation devicesHaemofiltrationEndotoxin removalBiocompatibility Testing
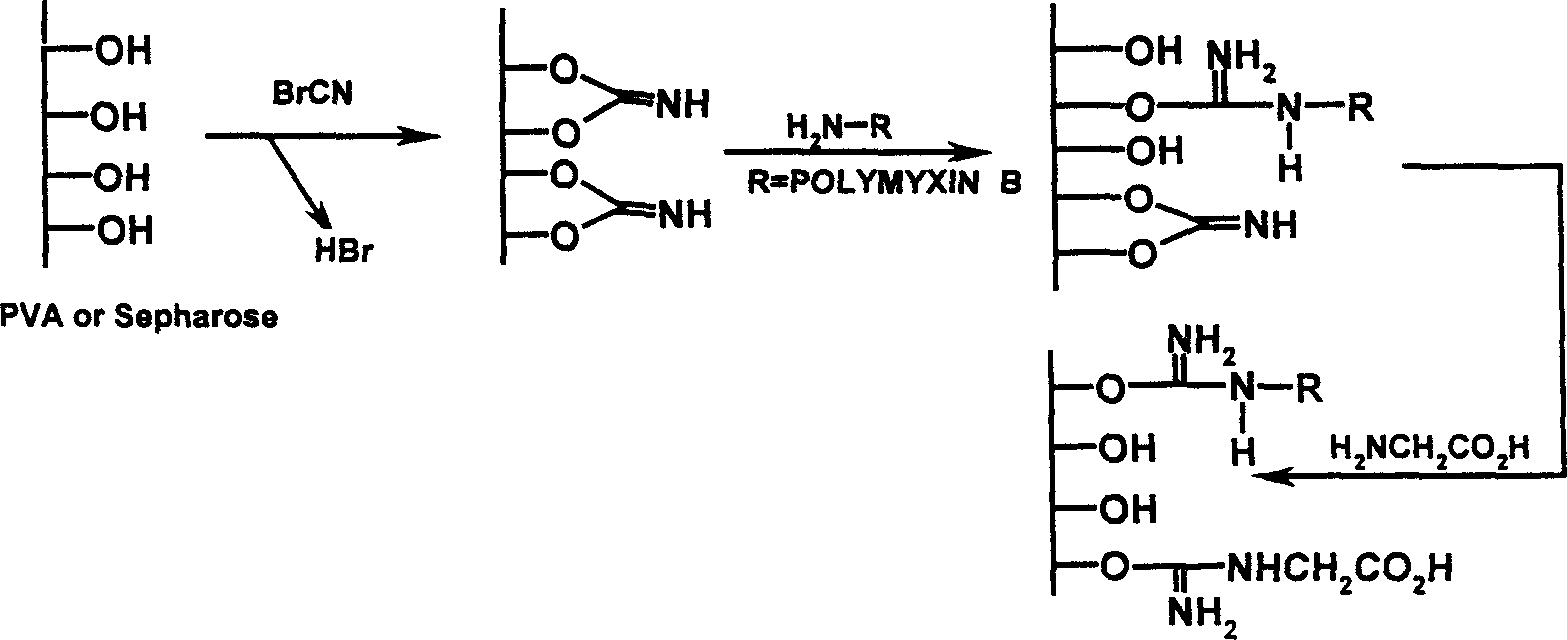
The invention relates to an endotoxin adsorbent, which in detail relates to a method of preparing endotoxin adsorbent for blood perfusion. The method employs spherical agarose gel with good biocompatibility as base material, employs epichlorohydrin, hexamethylene diamine, 1, 1'-carbonyldiimidazole as activating agent, and bonds polymyxin B for endotoxin removal. The invention is characterized in that it overcomes the toxicity of cyanogen bromide and instability of aglycone, improves the safty and reliability of operation, reducesnon-specific adsorption through bonding polymyxin B with 1, 1'-carbonyldiimidazole, and improves biocompatibility of adsorbent and suits for treating endotoxemia. The invention is also characterized by simple experimental procedure and high clearance for endotoxin.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
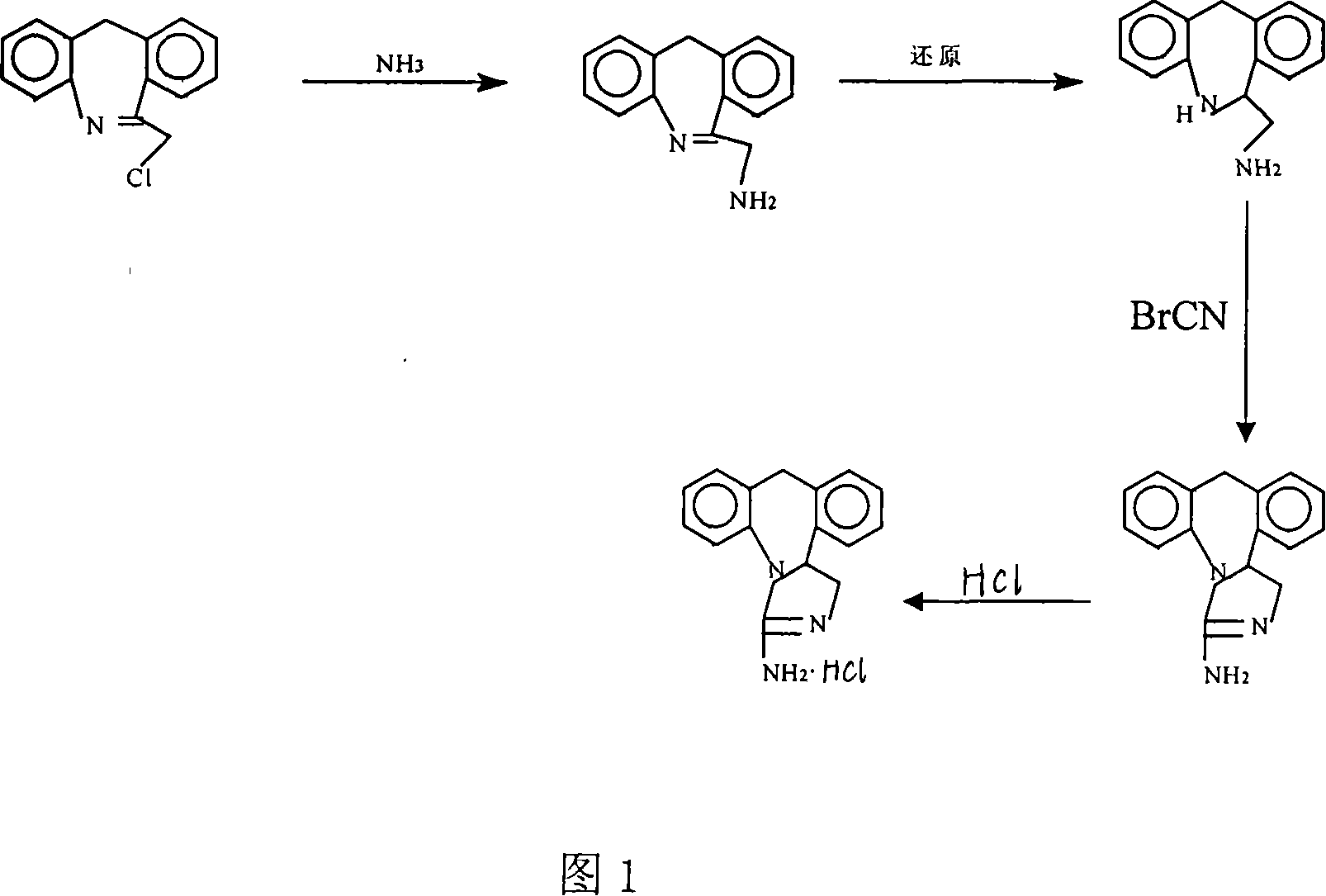
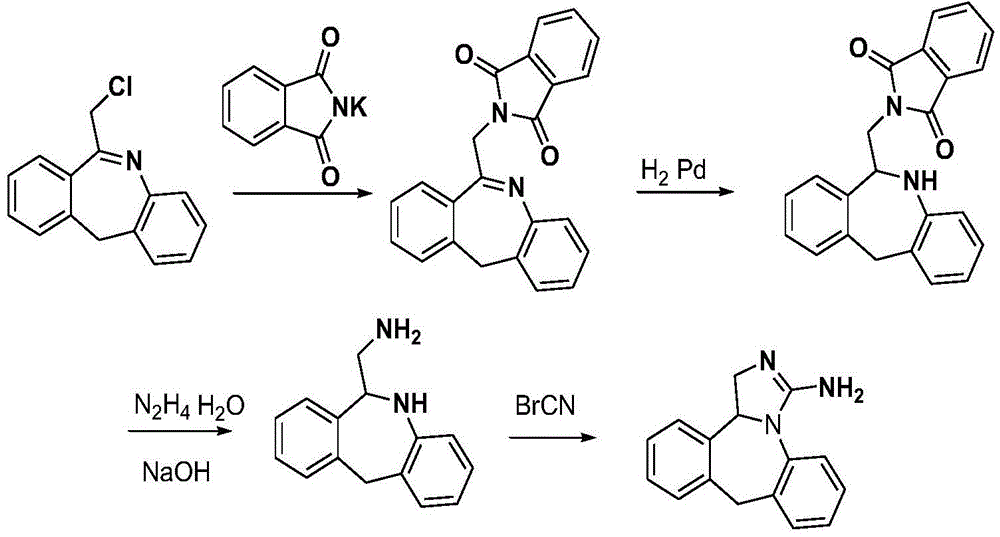
Chemical synthesis method for epinastine
InactiveCN101130544AHigh purityEasy to makeOrganic chemistryImmunological disordersChemical synthesisCyanogen bromide
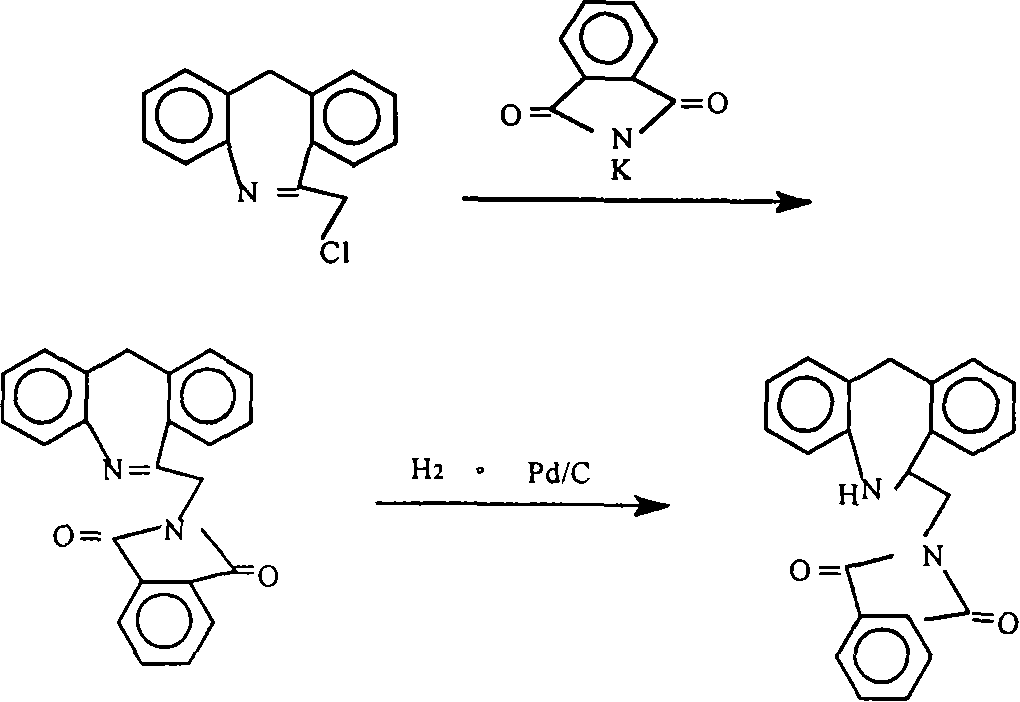
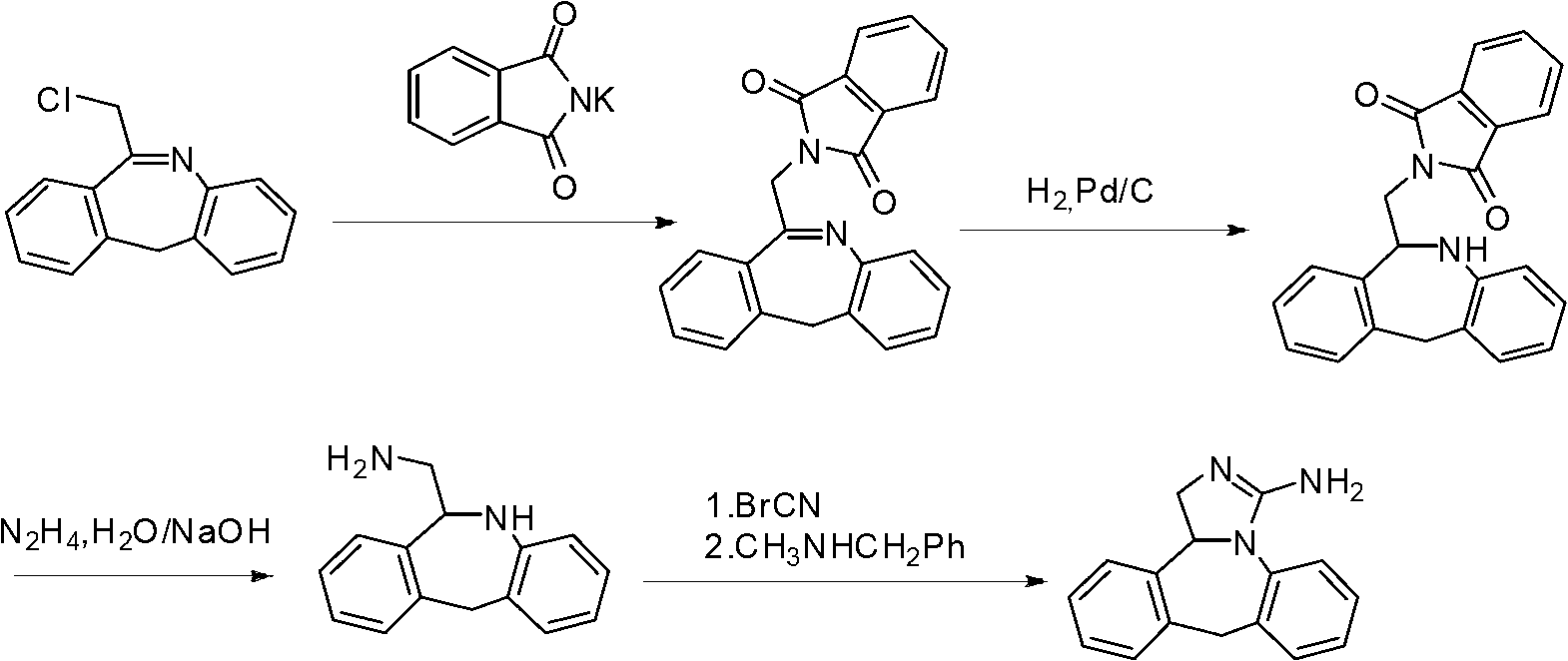
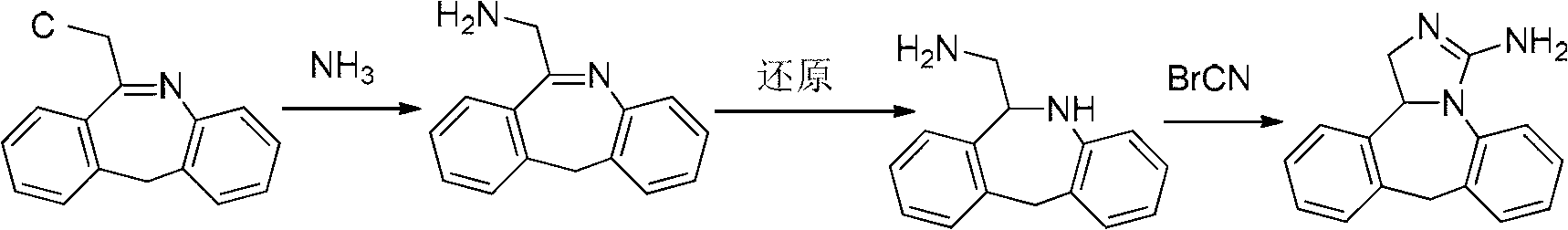
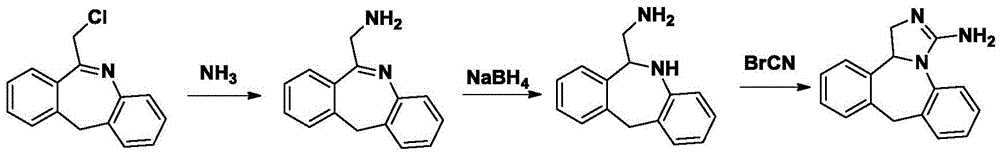
The invention discloses a new chemical synthesizing method of yipisidin, which comprises the following steps: ammonifying 6-chloromethyl-11-dihyrogen-dibenz [b,e] aza to generate 6-aminomethyl-11-dihydrogen-dibenz [b,e] aza; reducing the 6-aminomethyl-11-dihydrogen-dibenz [b,e] aza into 6-aminomethyl-6,11-dihydrogen-5H-dibenz [b,e] aza; generating the product through cyanogen bromide to loop. The invention simplifies the making method with little by-product, which improves the receiving rate by 69% with high purity (HPLC. 99. 0%) for industrial manufacturing.
Owner:HANGZHOU LONGSHAN CHEM CO LTD
A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process
InactiveCN105031634AKeep healthyAvoid pollutionAntibacterial agentsCarrier-bound antigen/hapten ingredientsConjugate vaccineVaccine manufacturing
The invention discloses an A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process. The problem that highly-toxic chemical reagents, such as cyanogen bromide, are used in the vaccine manufacturing process in the prior art and accordingly are harmful to protection of human health and environment is solved. The A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process comprises the following steps of 1 cultivating Neisseria meningitidis, 2 utilizing the cultivated Neisseria meningitidis to extract crude polysaccharide, 3 purifying the crude polysaccharide to prepare refined Neisseria meningitidis polysaccharide and 4 activating and deriving the refined polysaccharide. The A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process has the advantages that the extraction rate of the polysaccharide from a capsule is higher, the precipitation rate of the polysaccharide is higher, the recovery rate and purity of the refined polysaccharide are high, the derivation effect is better and the like.
Owner:CHENGDU OLYMVAX BIOPHARM
Endotoxin adsorptive material, preparing and use thereof
An endotoxic adsorpting method for treating endotoxemia and anaphylactic reaction caused by endotoxemia is prepared through activating the hydroxy group of agarose gel or DVA as carrier by cyanogen bromide or epoxy chloropropane or sulfuryl chloride, and adding polymyxin solution.
Owner:浙江科锐生物科技有限公司
Synthesis method of epinastine
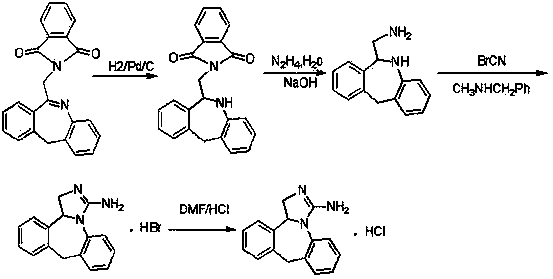
The invention discloses a synthesis method of epinastine. The synthesis method is implemented by taking 2-aminobenzophenone as a raw material and comprises the following steps of: reacting the 2-aminobenzophenone with a silane agent to obtain 2-benzylaniline; then, carrying out acylation reaction on the 2-benzylaniline and 2-chloroacetyl chloride to obtain N-(2-benzyl phenyl)-2-chloroacetamide; carrying out acidamide dehydration and cyclization on the N-(2-benzyl phenyl)-2-chloroacetamide under the action of a dehydrating agent to obtain 6-(chloromethyl)-11H-dibenzo[b,e] azepine; carrying out azidation reaction on the 6-(chloromethyl)-11H-dibenzo[b,e] azepine to obtain 6-(azido-methytbiphenyl)-11H-dibenzo[b,e] azepine; carrying out reduction on the 6-(azido-methytbiphenyl)-11H-dibenzo[b,e] azepine to obtain 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] azepine; and finally, carrying out cyclization on the 6-(aminomethyl)-6,11-dihydro-1H-dibenzo[b,e] azepine and cyanogen bromide to obtain the epinastine. The synthesis method disclosed by the invention avoids the application of expensive and flammable lithium aluminium hydride and aluminium hydride as well as hypertoxic sodium cyanide, so that the operation is safer in industrial production, and the cost is reduced. The method is simple in process and high in yield, requires mild conditions, and is suitable for industrialized production.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Preparation method of epinastine hydrochloride and intermediate thereof
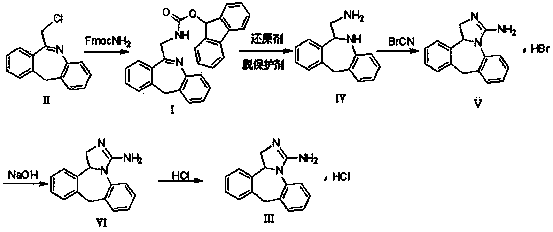
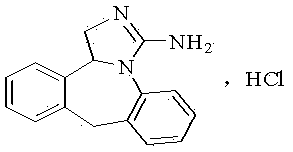
The invention relates to a method preparing an epinastine hydrochloride intermediate and epinastine hydrochloride. The method comprises the following steps: reacting 6-chloromethyl-11-dihydro-dibenzo[b,e]azepine and an amino reagent under protection to generate an amino-protective compound; carrying out reduction reaction and deprotection reaction to generate 6-aminomethyl-6,11-dihydro-dibenzo[b,e]azepine; and finally, carrying out cyanogen bromide cyclization on the 6-aminomethyl-6,11-dihydro-dibenzo[b,e]azepine, neutralizing with alkali, and reacting with hydrochloric acid to generate the epinastine hydrochloride. The preparation method has the advantages of accessible reaction raw materials, fewer byproducts and high product purity, and is suitable for industrial production.
Owner:YAOPHARMA CO LTD +1
Acetylcholinesterase chemiluminescence bioreactor, and preparation method and application thereof
InactiveCN101968448AIncrease enzyme activityHigh recovery rateMicrobiological testing/measurementChemiluminescene/bioluminescenceCarbamatePesticide residue
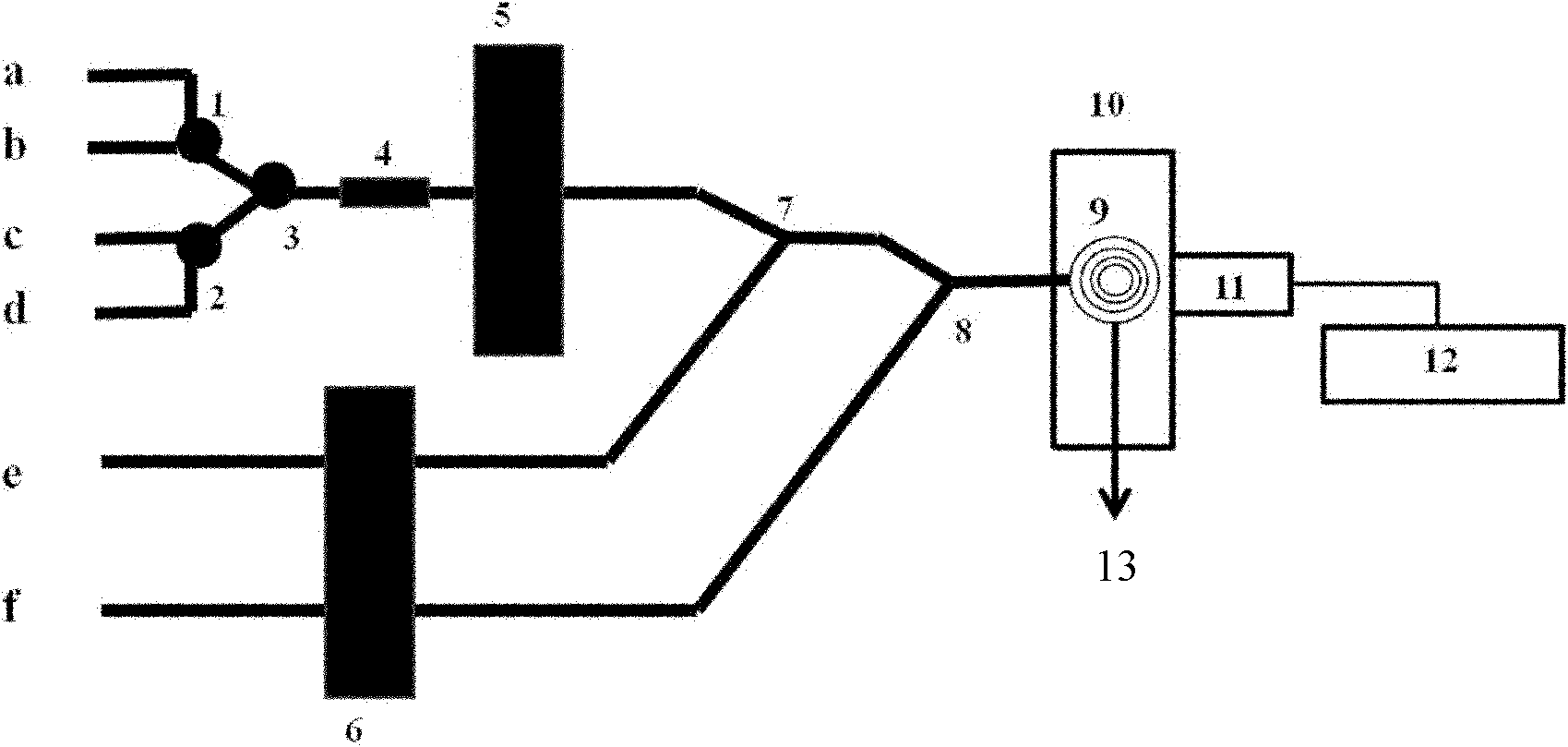
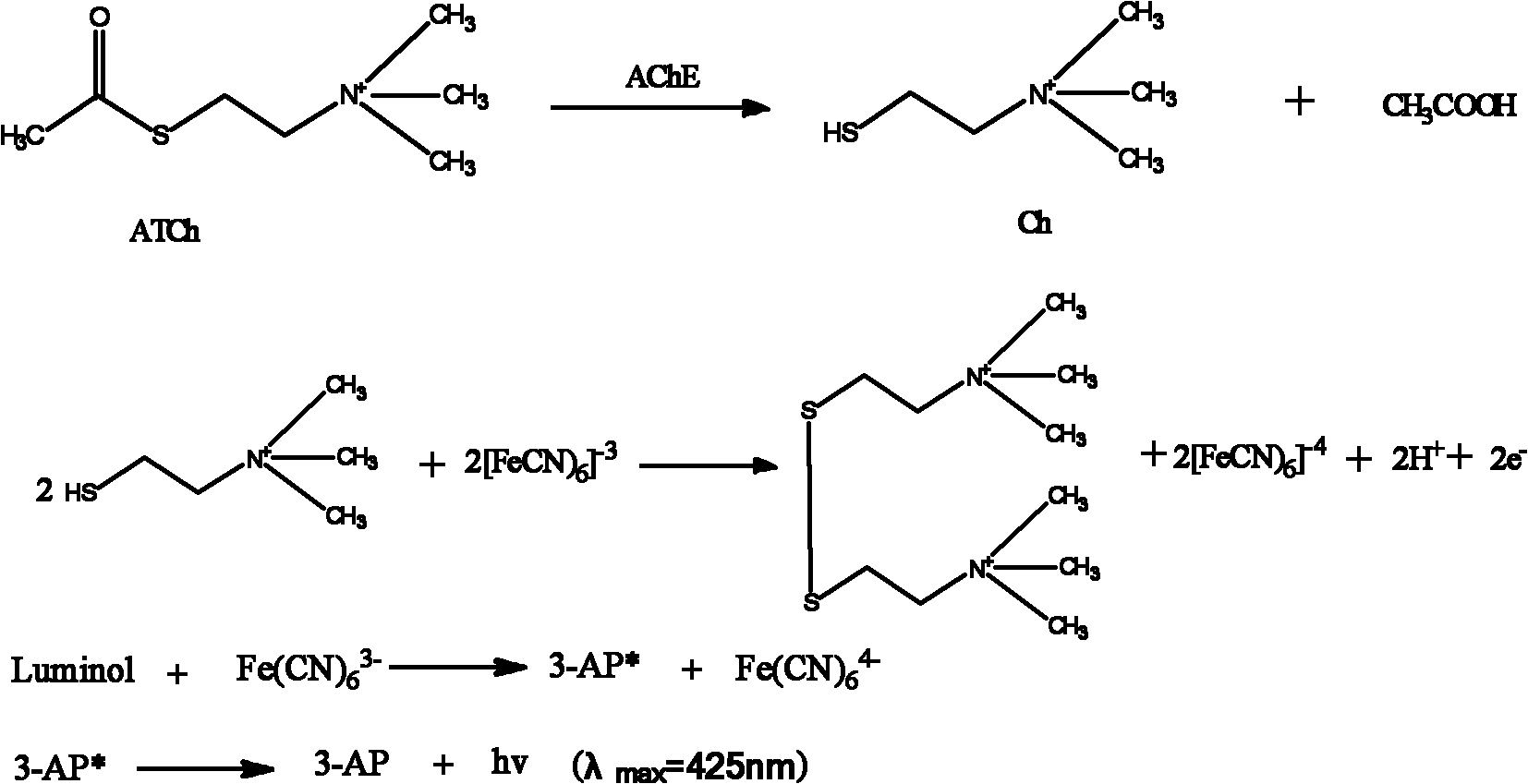
The invention discloses an acetylcholinesterase chemiluminescence bioreactor, and a preparation method and application thereof, and belongs to the technical fields of analysis of pesticide residues and biotechnology. The reactor is a glass tube of which the two ends are sealed by 300-mesh bolting silk, wherein immobilized acetylcholinesterase is held in the glass tube; and the immobilized acetylcholinesterase is purified crucian muscle acetylcholinesterase which is immobilized on cyanogen bromide-activated (CNBr) sepharose 4B. The application of the reactor is characterized by comprising the following steps of: establishing the acetylcholinesterase chemiluminescence bioreactor; and detecting organic phosphorus and carbamate type pesticides. The crucian muscle acetylcholinesterase (AChE) extracted in the invention has high enzyme activity, obvious purification effect and high immobilized enzyme activity recovery rate; the detection limit of the organic phosphorus type pesticides and the carbamate type pesticides is low; the analysis time is short; and the enzyme reactor can be repeatedly used.
Owner:HUAZHONG AGRI UNIV
Mammalian-derived peptides for the treatment of microbial infection
The present invention provides compositions useful as antimicrobial agents which include mammalian hemoglobin, the alpha and beta chains of hemoglobin free of heme, fragments of the alpha and beta chains that result from cyanogen bromide cleavage of the alpha and beta chains, and synthetic peptides derived therefrom. The compositions exert antimicrobial activity against both bacteria and fungi that is comparable to known antimicrobial peptides from human neutrophils, cathepsin G and azurocidin. Sensitive organisms include Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa, Gram-positive bacteria such as Staphylococcus aureus and Streptococcus faecalis, and the fungus Candida albicans. Methods for preparing the compositions also are provided.
Owner:THERAGEM
Preparation method of deoxynivalenol immunoaffinity column
InactiveCN103801110AReduce exposureOther chemical processesPreparing sample for investigationSpecific adsorptionPretreatment method
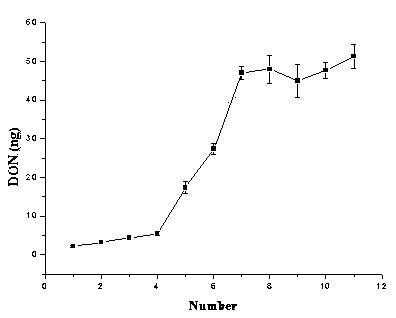
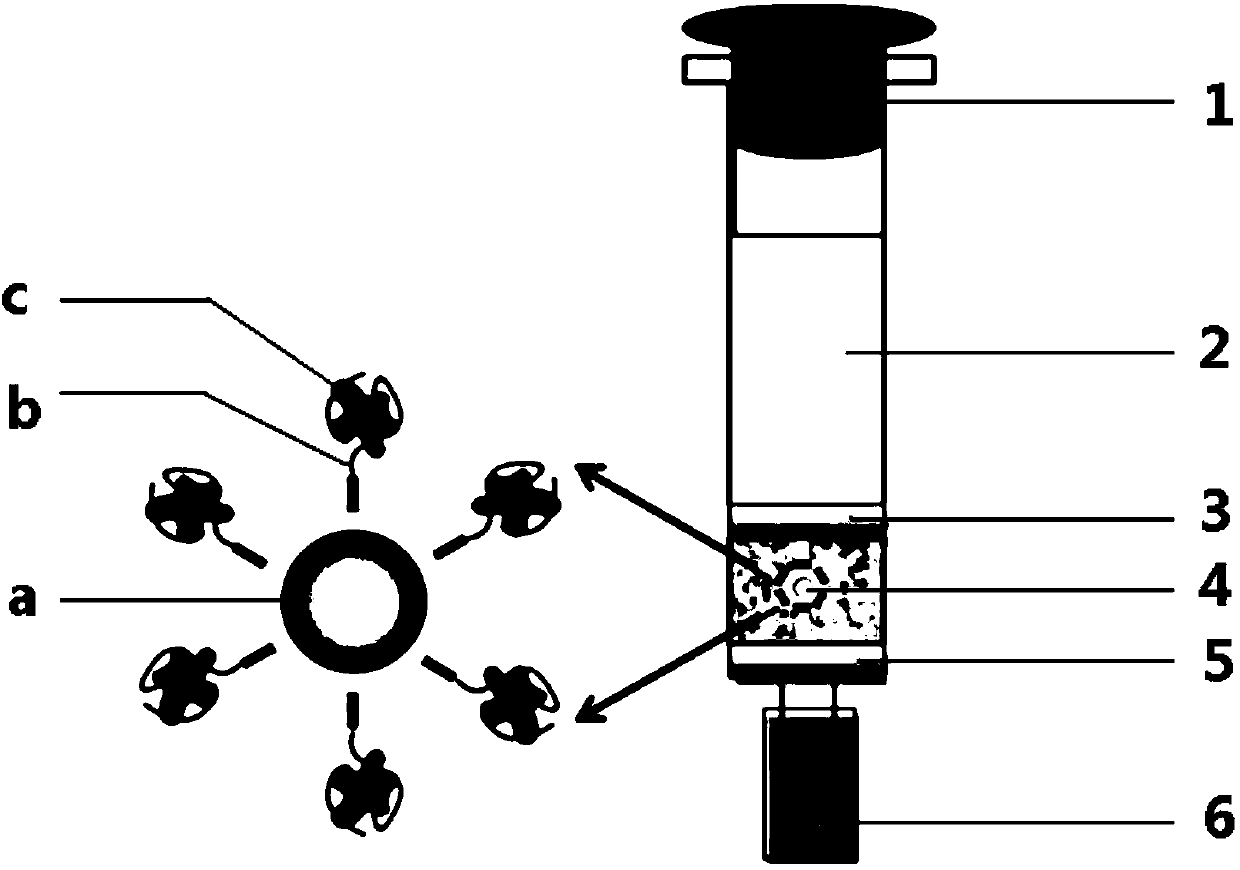
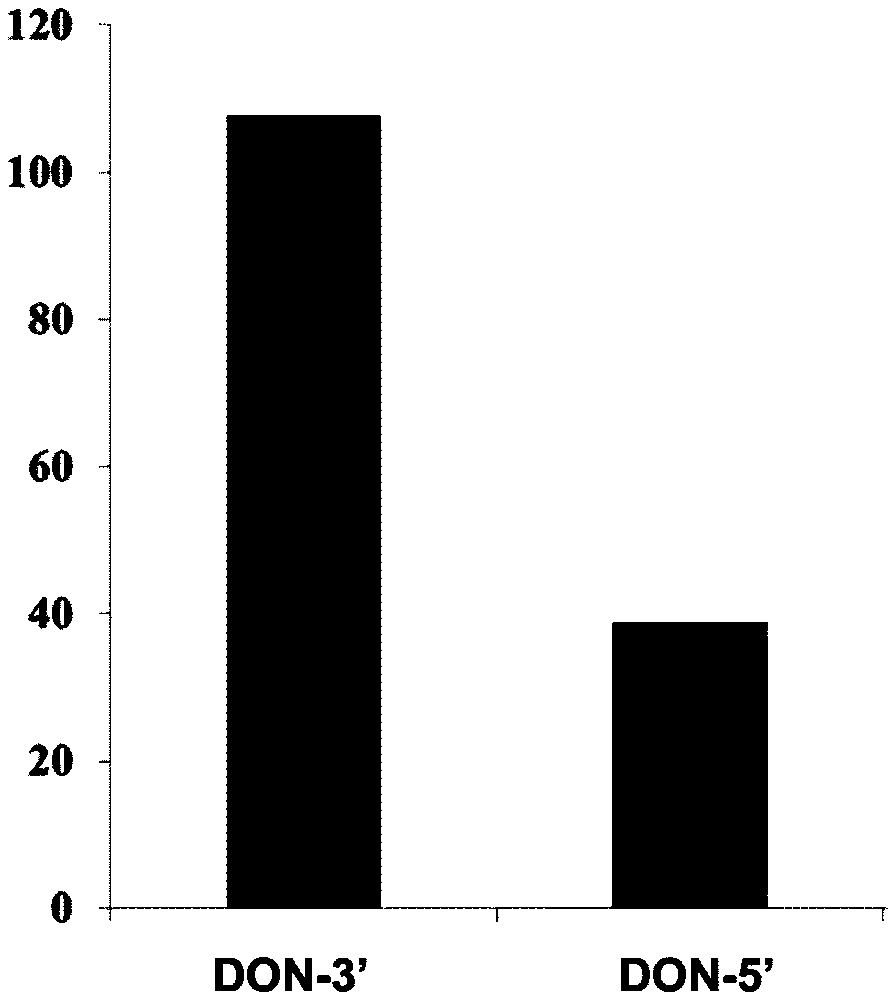
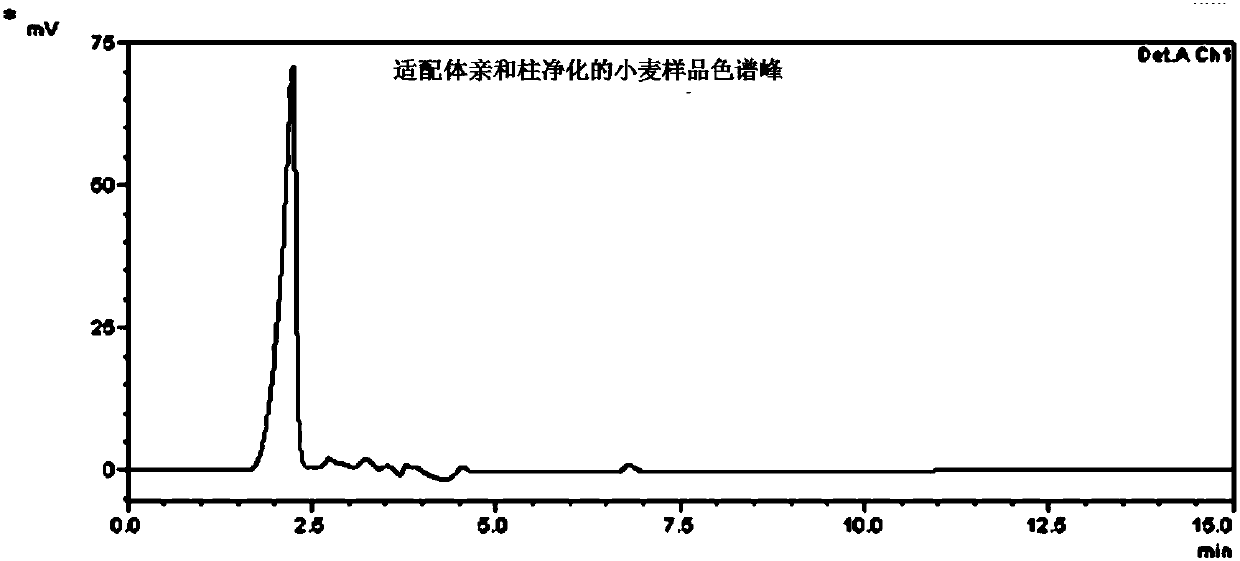
The invention discloses a preparation method of an immunoaffinity column based on deoxynivalenol monoclonal antibodies. An affinity adsorbent obtained through coupling of monoclonal antibodies obtained through cell fusion and cyanogen bromide activated agarose 4B is filled in a solid phase extraction tube to obtain the immunoaffinity column capable of specific adsorption of deoxynivalenol. The immunoaffinity column prepared by the invention can specifically combine with deoxynivalenol, has a maximal binding capacity of about 240ngDON, and reaches recovery rate of no less than 90% for three times of repeated usage. The invention can be used in the pretreatment method of detection of deoxynivalenol residues in cereals and cereal products.
Owner:JIANGSU WISE SCI & TECH DEV
Liquid phenolic-type cyanate resin suitable for RTM (resin transfer molding) process
The invention discloses a liquid phenolic-type cyanate resin suitable for RTM (resin transfer molding) and a preparation method of the liquid phenolic-type cryanate resin. Thermoplastic phenolic resin with low molecular weight is taken as a matrix, and reacts with cyanogen bromide to prepare the liquid cyanate resin under base catalysis. A material with good heat resistance is obtained from the phenolic-type cyanate resin disclosed by the invention by thermocuring and catalytic curing, and the phenolic-type cyanate resin is suitable for being used as a heat-resistant composite material matrix resin.
Owner:BEIHANG UNIV
Immunoaffinity gel detection column for detecting enrofloxacin and preparation method thereof
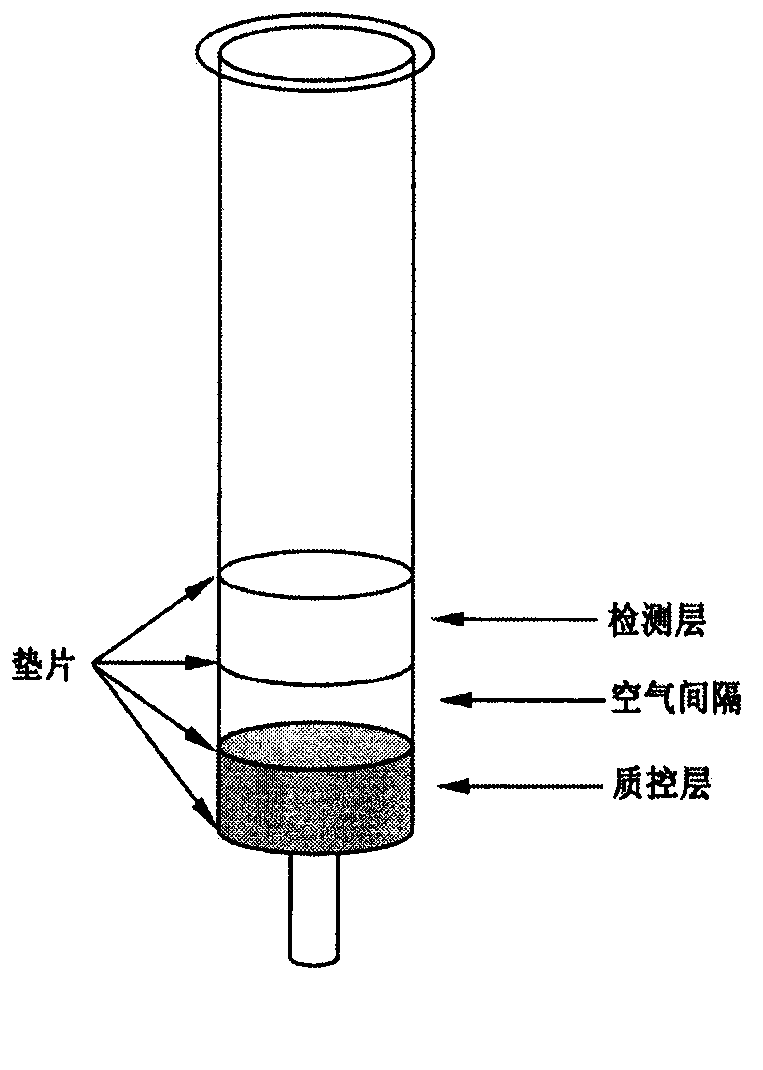
InactiveCN105388284AInhibit migrationHigh selectivitySolid sorbent liquid separationSeparation devicesCyanogen bromideSimple sample
The invention provides a preparation method of a visualized immunoaffinity gel detection column for rapidly detecting enrofloxacin in food. The method comprises the steps of with sepharose gel as a solid vector, coupling an enrofloxacin antibody with sepharose gel activated by cyanogen bromide to prepare an antibody gum serving as a detection layer; coupling an HRP antibody with the sepharose gel activated by cyanogen bromide to prepare an HRP antibody gum serving as a quality control layer; and putting the HRP antibody gum into 1mL of solid-phase extraction column to prepare the immunoaffinity detection column. The invention develops a novel immunoaffinity gel column detection product for rapidly, qualitatively and semi-quantitatively detecting enrofloxacin residues in food, and the detection limit is 5mu g / L. The immunoaffinity gel column detection product has the following outstanding advantages of high specificity, good sensitivity, simple sample pretreatment, short detection consumed time, high accuracy, simple and convenient operation and no assistance of large-size instruments.
Owner:TIANJIN UNIV OF SCI & TECH
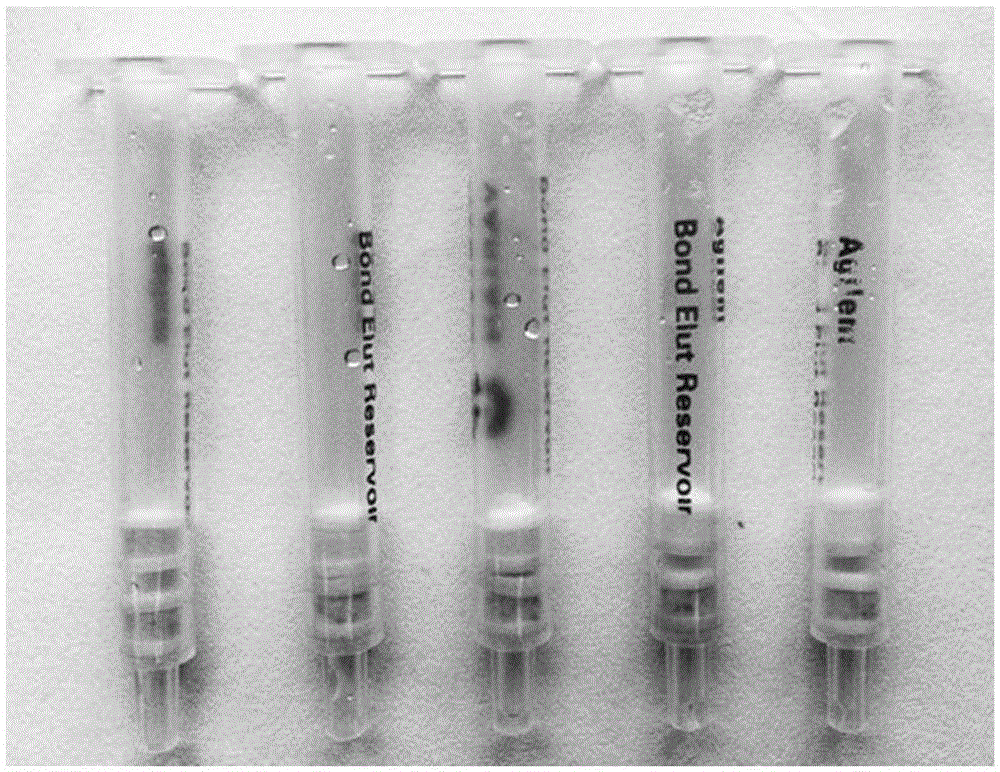
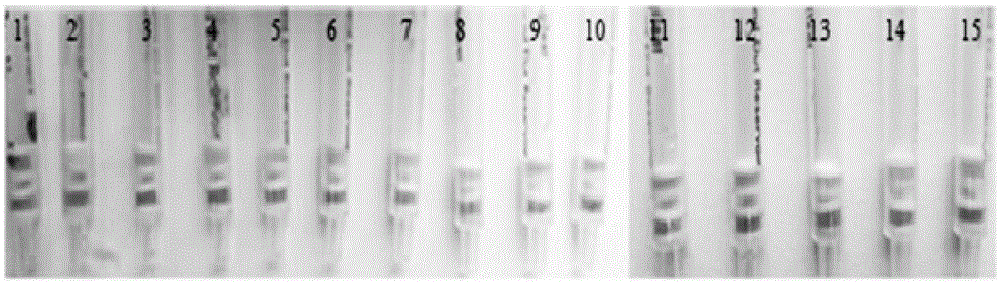
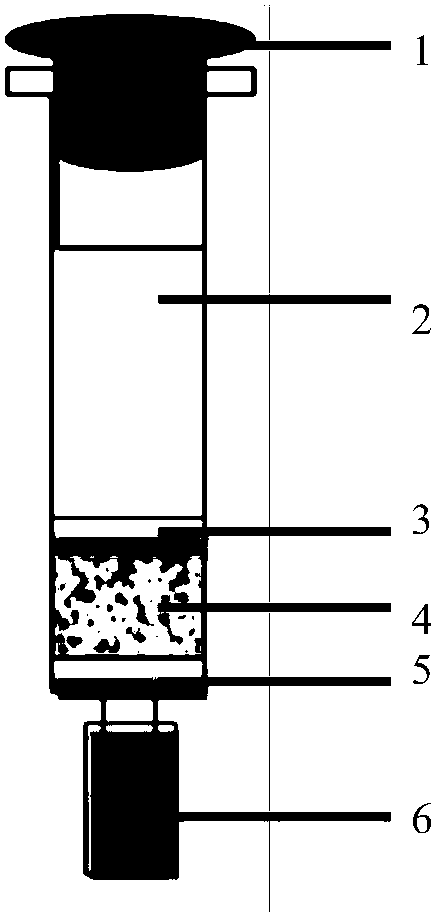
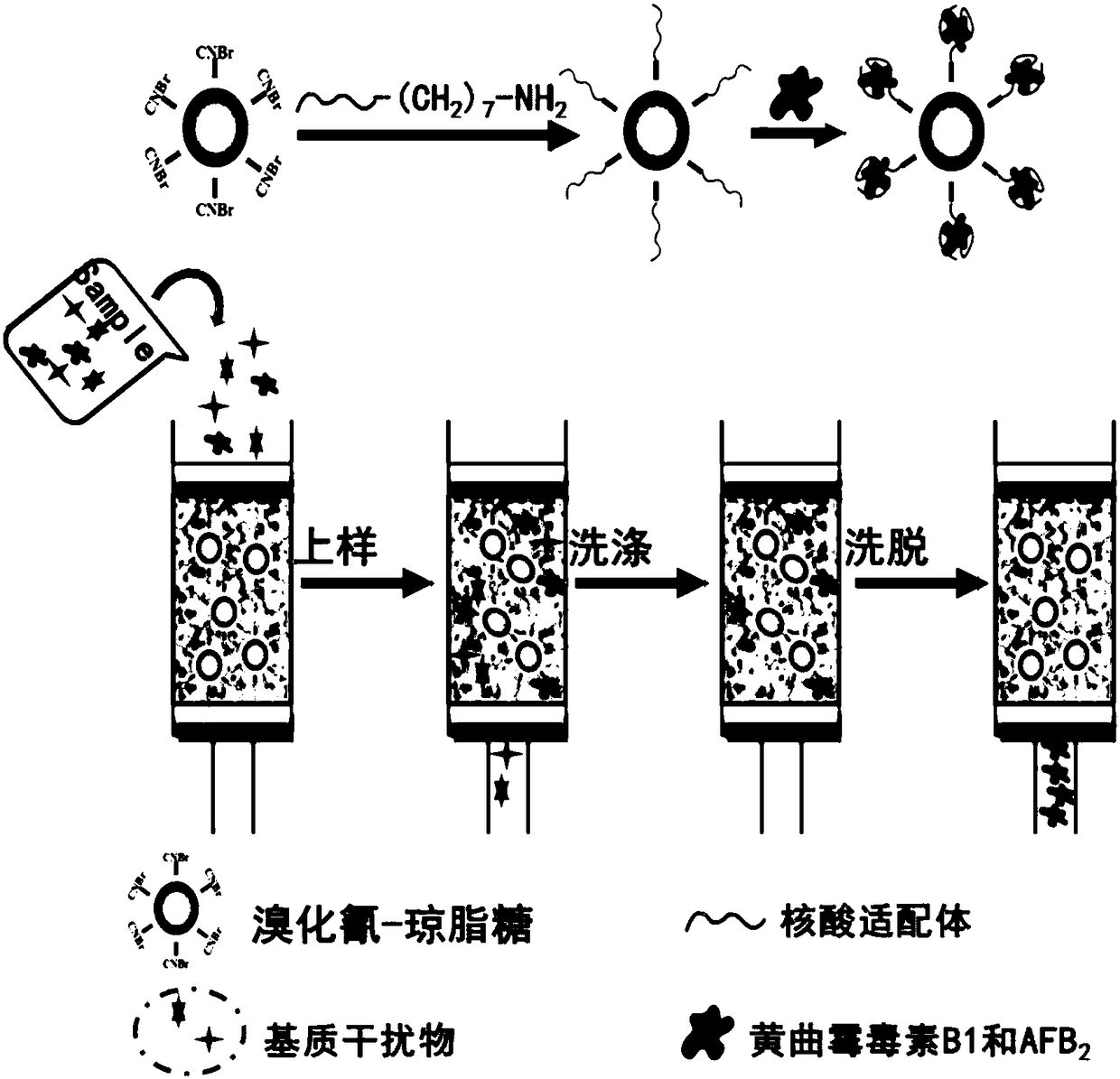
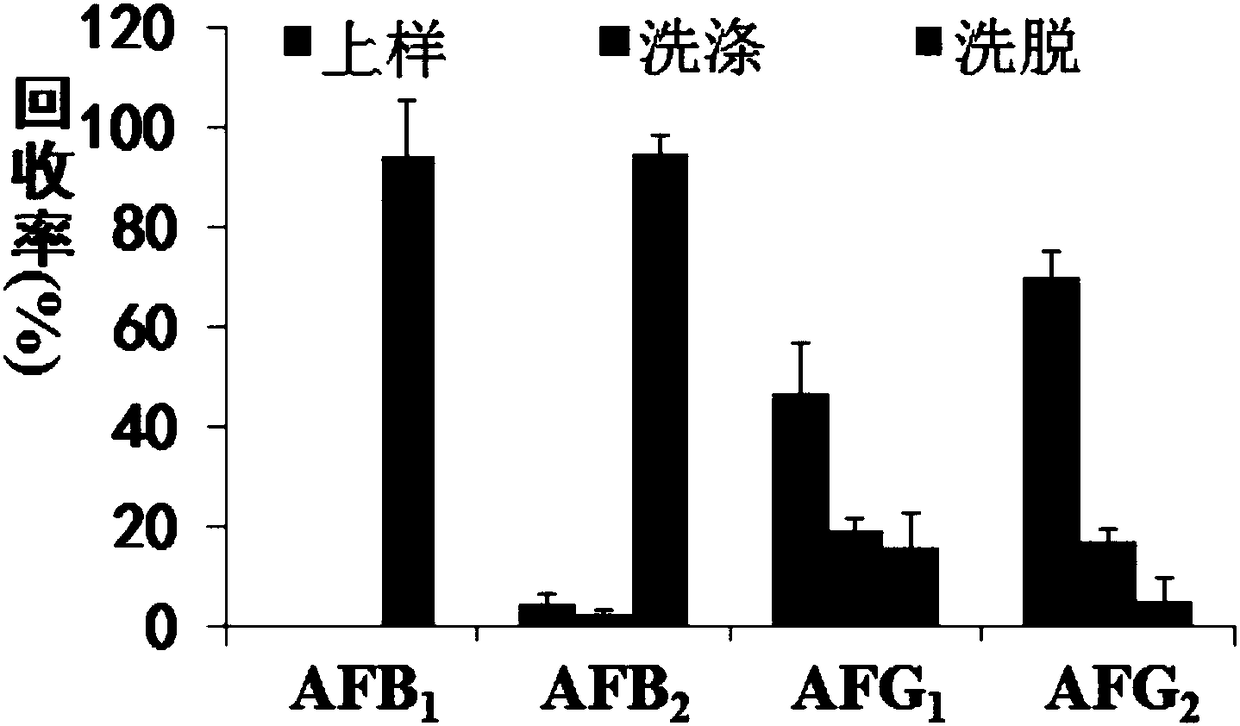
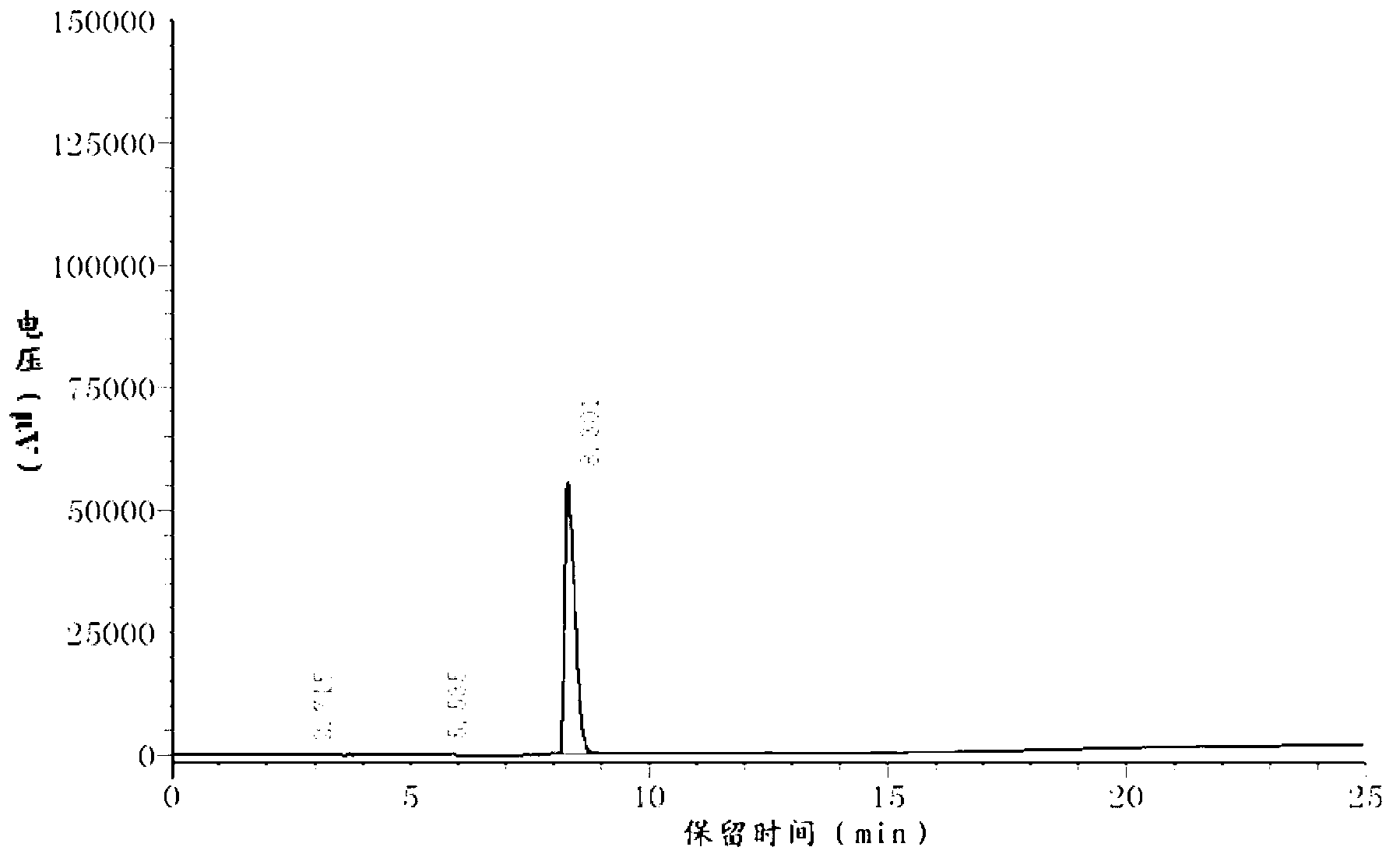
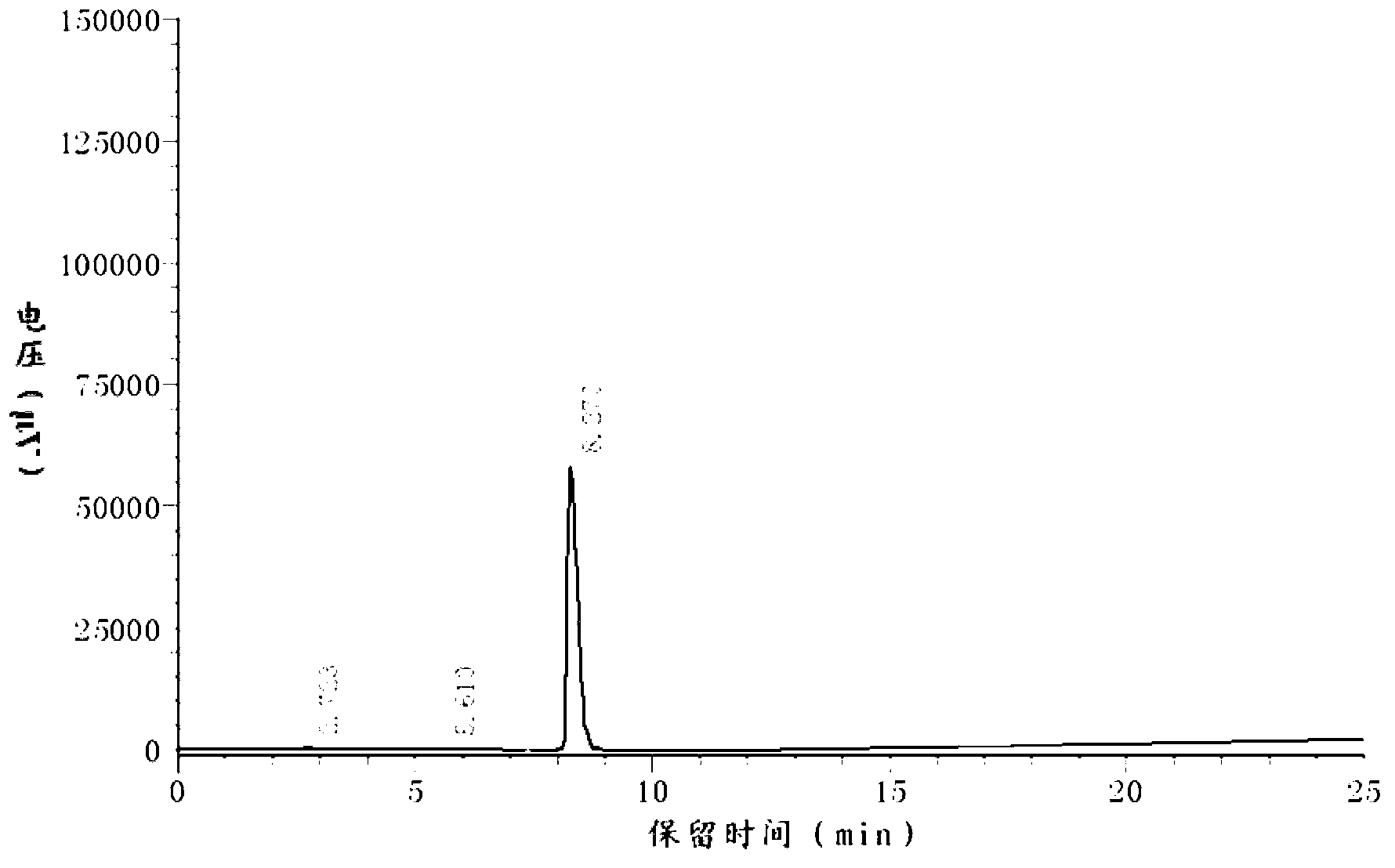
Aflatoxin B1 and B2 aptamer affinity column and preparation method and use thereof
The invention provides an aflatoxin B1 and B2 aptamer affinity column and a preparation method thereof. The affinity column uses a cyanogen bromide-modified agarose as a carrier, then aptamers for high affinity and high specificity recognition of aflatoxin B1 and B2 and the carrier are covalently coupled, and the coupled aflatoxin B1 and B2 aptamer complex carrier fills the affinity column. The affinity column is mainly used for purification of aflatoxin B1 and B2 in multiple samples such as foods, feed, milk, blood samples and traditional Chinese medicines and is conducive to later high-performance liquid chromatography and fluorescence detection of aflatoxin B1 and B2 in the sample.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
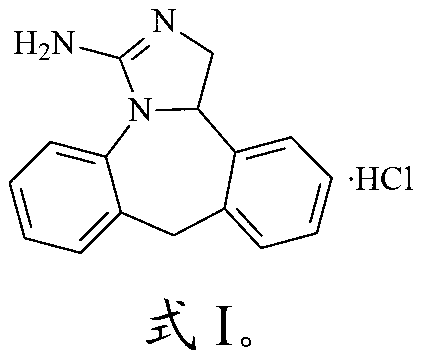
Preparation method of epinastine hydrochloride
ActiveCN103172638ANo residueImprove securityOrganic chemistryEpinastine HydrochlorideOrganic solvent
The invention relates to the technical field of medicine, in particular to a preparation method of epinastine hydrochloride. The preparation method comprises the following steps: performing substitution reaction of a compound with a structure as shown in formula (II) and a compound with a structure as shown in formula (III) in an organic solvent, so as to produce a compound with a structure as shown in formula (IV); cyclizing the compound with the structure as shown in formula (IV) in the organic solvent in the presence of p-toluene sulphonic acid, so as to produce a compound with a structure as shown in formula (V); and then salifying the compound with the structure as shown in formula (V), so as to produce a compound with the structure as shown in formula (I). Thus, cyanogens bromide which is used in the traditional preparation method is prevented from being used in the cyclizing reaction, and therefore the toxicity of the preparation method is reduced. The operation of the preparation method is safer and more environment-friendly.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Immune affinity chromatography purification method for specific egg yolk immunoglobulins
InactiveCN101817880AImprove binding efficiencyEfficient removalEgg immunoglobulinsImmunoglobulins against bacteriaSpecific adsorptionCyanogen bromide
The invention discloses an immune affinity chromatography purification method aiming at specific egg yolk immunoglobulins (IgY) against listeria monocytogenes. The method comprises the following steps of: combining listeria monocytogenes bacteria cells serving as specific adsorption materials and Sepharose4B filler subjected to amino activation and cyanogen bromide activation to form a solid phase carrier; during purification, combining the specific IgY antibody to a chromatographic column through specific adsorption with the listeria monocytogenes bacteria cells combined on the chromatographic column, and making non-specific antibody outflow along with eluent; after no non-specific antibody outflows, dissociating the antibody and the antigen combined on the chromatographic column by using dissociating buffer solution; and detecting the adsorption and dissociating conditions of the specific antibody by using a method for measuring ultraviolet absorption, and detecting the purification effect of the antibody by using an indirect enzyme-linked immunosorbent assay (ELISA). The method has simple operation, and can be applied to effective separation and purification of the specific IgY.
Owner:OCEAN UNIV OF CHINA
Meningitis polysaccharide conjugate vaccine and preparing method thereof
InactiveCN103690944AImproving immunogenicityRelieve painAntibacterial agentsCarrier-bound antigen/hapten ingredientsConjugate vaccineEpitope
The invention describes a method for preparing a meningitis polysaccharide conjugate vaccine. The meningitis polysaccharide conjugate vaccine is developed based on the method. The method comprises the following steps: (1) activating meningococcus polysaccharides by cyanogen bromide, then deriving the meningococcus polysaccharides which are activated by the cyanogen bromide by ethanediamine, finally deriving the polysaccharides by a reagent which is of a structure of succinimidyl ester-R-maleimide; (2), sulfhydrylating carrier proteins; (3) combining the derived meningococcus polysaccharides with the sulfhydrylated carrier proteins. As conjugation bridges between the meningococcus polysaccharides and the carrier proteins are very long, the spatial shielding effect of the carrier proteins on the antigenic epitopes of the polysaccharides is reduced, and the original immunization property of the polysaccharide conjugate vaccine is improved.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Beta-glucan modified meningitis polysaccharide conjugate vaccine and preparation method thereof
ActiveCN104548090AImproving immunogenicityReduce the number of vaccinationsAntibacterial agentsCarrier-bound antigen/hapten ingredientsCyanogen bromideMeningococcal meningitis
The invention relates to a beta-glucan modified meningitis polysaccharide conjugate vaccine and a preparation method thereof. The preparation method of the beta-glucan modified meningitis polysaccharide conjugate vaccine comprises the following steps: (1) activating meningococcus polysaccharide by cyanogen bromide, and then deriving by adopting adipic dihydrazide; (2) combining a derived meningococcus polysaccharide derivative with carrier protein; (3) activating beta-glucan; and (4) modifying polysaccharide-protein conjugate by the activated beta-glucan. By virtue of the steps, a novel and efficient meningitis polysaccharide conjugate vaccine can be prepared and can be used for preventing infection caused by epidemic cerebrospinal meningitis Neisseria gonorrhoeae.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
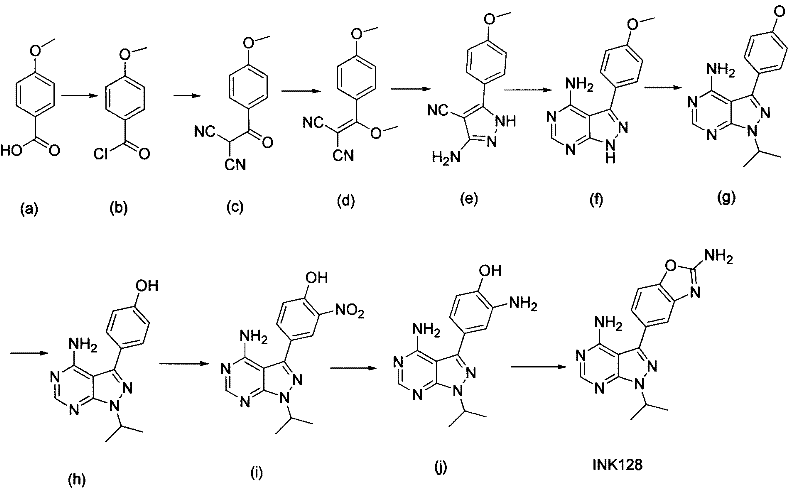
New synthesis process of new antineoplastic drug INK128
The invention discloses a new synthesis process of a new antineoplastic drug INK128, which comprises the following steps: acylating the initial raw material p-methoxybenzoic acid, reacting with malononitrile, methylating to cyclize with hydrazine hydrate to obtain 3-amino-5-(4-methoxyphenyl)-pyrazolyl-4-nitrile, purifying by crystallization, heating to cyclize with formamide to obtain the target mother cycle, alkylating, nitrifying, deprotecting, reducing, and finally, cyclizing with cyanogen bromide.
Owner:CGENETECH (SUZHOU CHINA) CO LTD
Vomitoxin aptamer affinity column and preparation method and application thereof
PendingCN107807034AReduce processing costsComponent separationPreparing sample for investigationAptamerCyanogen bromide
The invention relates to a vomitoxin aptamer which particularly is 5'-GCCCGGATCGAGTTGATTTCAAGCGCATGAAGGCTA-CCCCCCC-NH2-3'. The invention relates to a vomitoxin aptamer affinity column which comprisesa column pipe and a CNBr (cyanogen bromide) activated sepharose, the bottom of the column pipe is filled with the CNBr activated sepharose, and a 3'-terminal amino-modified vomitoxin aptamer is coupled on the surface of the CNBr activated sepharose. The invention further relates to a preparation method of the vomitoxin aptamer affinity column. The affinity column takes high-affinity and specificity vomitoxin aptamer as a recognition element, the vomitoxin aptamer is connected on a cyanogen bromide activated sepharose solid-phase vector, washed, closed and packed to prepare the affinity column.Agricultural products and products prepared from the agricultural products are treated by the aptamer affinity column and used for subsequent detection, vomitoxin can be effectively enriched and purified, and the affinity column has the advantages that the affinity column is high in selectivity and good in stability, false positive of detection is reduced, and sensitivity is improved.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
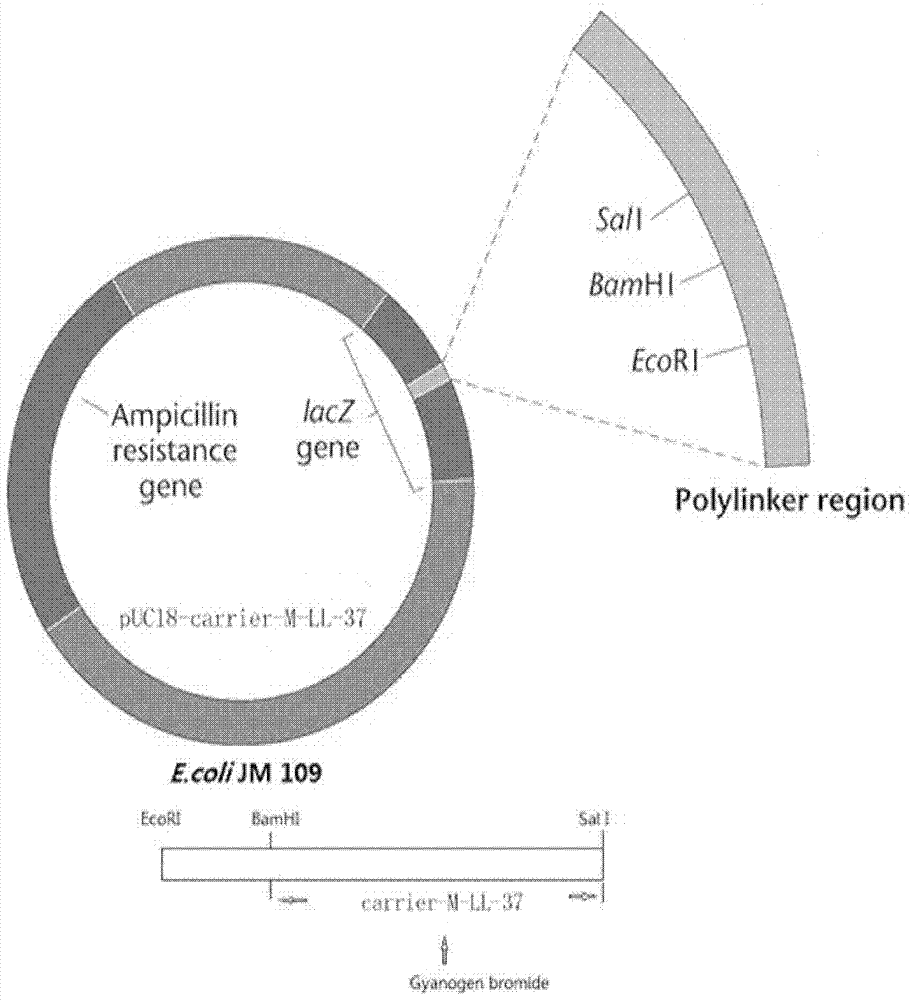
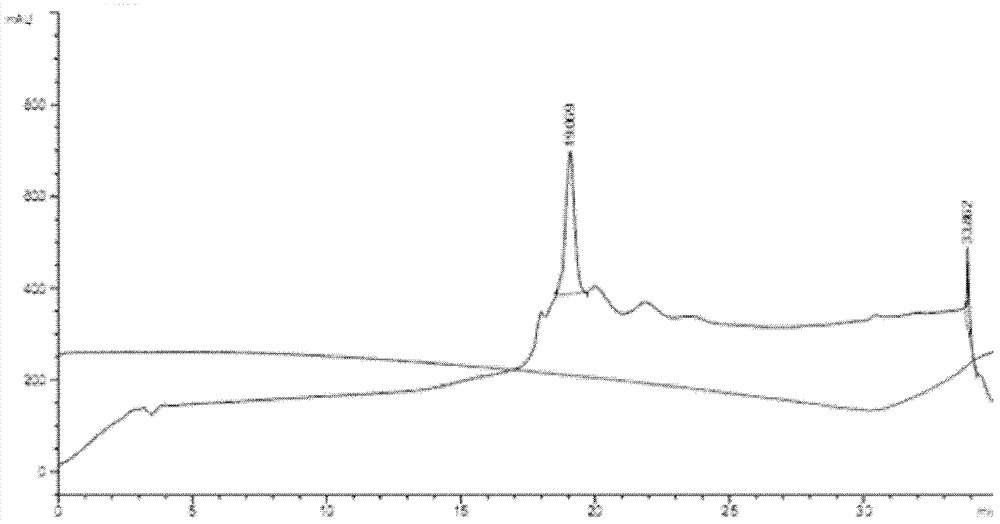
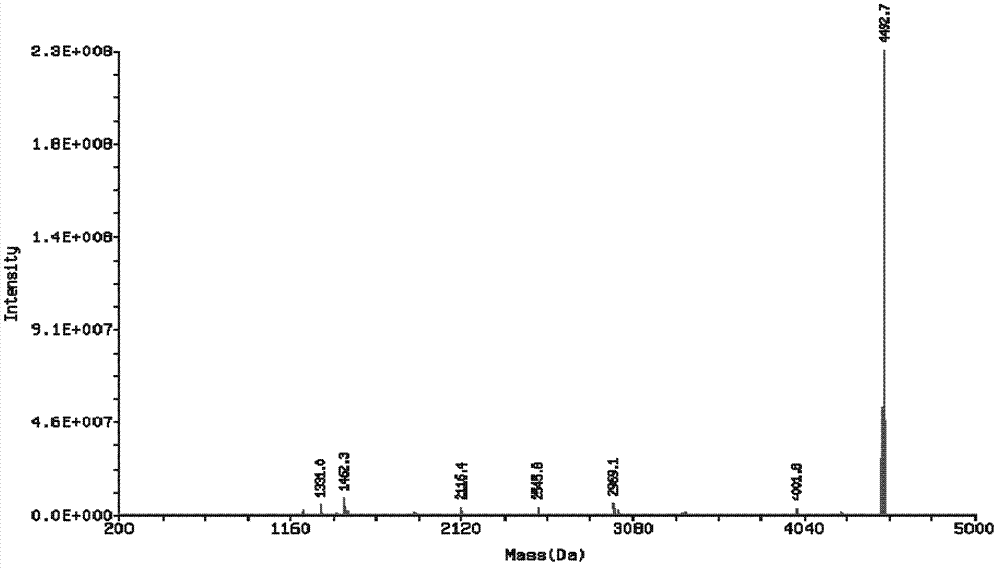
Genetic engineering expression and preparation methods of cecropin LL-37 and application thereof
InactiveCN103361341AEffective infectionSimple processAntibacterial agentsAntimycoticsEscherichia coliDisease
The invention relates to genetic engineering expression and preparation methods of cecropin LL-37 and application thereof. The preparation method comprises the steps of synthesizing a DNA (Deoxyribonucleic Acid) sequence coding the cecropin LL-37, and connecting the DNA sequence to a suitable expression vector for expression and purification, wherein the DNA sequence coding the cecropin LL-37 adopts Escherichia coli preferred codons, and the LL-37 expressed in an Escherichia coli fusion and expression system accounts for about 20% of the total protein of a thallus; and cutting off the purified fusion protein by using cyanogen bromide so as to obtain the target polypeptide LL-37, and obtaining LL-37 powder with the purity higher than 90% through HPLC (High-Performance Liquid Chromatography). The LL-37 of low concentration can play a significant bactericidal effect in the aspect of human preparations for external use, serves as a drug for external use and is applied to the treatment of diseases, such as skin infection. The methods have the advantages that the cecropin LL-37 is expressed and prepared in the form of fusion protein, the process is simple, and the yield of fermentation liquor per cubic liter can reach 20 mg, so that the methods have significance in industrial batch production.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
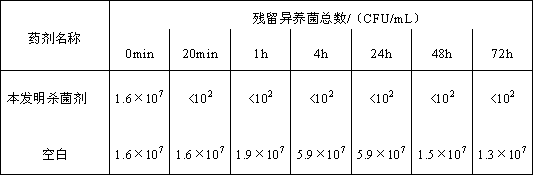
Bactericide used for reverse osmosis membrane and preparation method thereof
ActiveCN102794112AGood material compatibilityImprove compatibilityBiocideSemi-permeable membranesBronopolCyanogen bromide
The invention discloses a bactericide used for a reverse osmosis membrane and a preparation method thereof. The bactericide is prepared by compounding the following components in percentage by weight: 20-30 percent of cyanogen bromide propanamide, 5-10 percent of bronopol, 40-60 percent of solvent, 1-10 percent of auxiliary agent and the balance of deionized water. The preparation method comprises the following steps of: adding the solvent into a reaction kettle at room temperature; adding the cyanogen bromide propanamide and the bronopol and stirring for 50-60 minutes; adding the auxiliary agent and the deionized water and stirring for 20-30 minutes; and standing still for 10 minutes. The bactericide used for the reverse osmosis membrane can be used for quickly penetrating through a microbial cell membrane and acting on certain protein groups to ensure that the normal oxidation and reduction of cells are stopped so as to cause cell death, and the bactericide used for the reverse osmosis membrane has the advantages of quick and efficient performance and convenience in use.
Owner:安徽精高水处理有限公司
Chloramphenicol immunoaffinity gel detection column
InactiveCN103675256ASimple visualizationSensitive visualizationBiological testingPeroxidaseSolid phase extraction
The invention relates to an immunoaffinity gel detection column for visually fast detecting residual chloramphenicol in food. The chloramphenicol immunoaffinity gel detection column belongs to the field of immunology, enzymology and analytic chemistry. Cyanogen bromide-activated agarose gel is respectively coupled with a chloramphenicol antibody and a hydrogen peroxidase (HRP) antibody to prepare chloramphenicol antibody glue and HRP antibody glue; the cyanogen bromide-activated agarose gel is confined by confining liquid to prepare confining glue. The confining glue and the chloramphenicol antibody glue are mixed to prepare a detection layer, the confining glue and the HRP antibody glue are mixed to prepare a quality control layer, the quality control layer is filled in a 1ml solid phase extraction column, the working conditions of all steps in the detection process can be determined, and a novel immunoaffinity gel detection column for fast qualitatively and semi-quantitatively detecting residual chloramphenicol in food can be researched, with the detection limit of 1mug / L. When the product detects the residual chloramphenicol in food samples, no organic solvents and complicated pretreatment, as well as the assistance of large instruments are not required, the chloramphenicol immunoaffinity gel detection column has good usability and accuracy, and can meet the requirement for visual fast detection.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
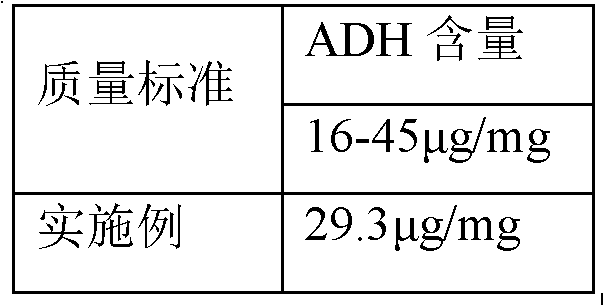
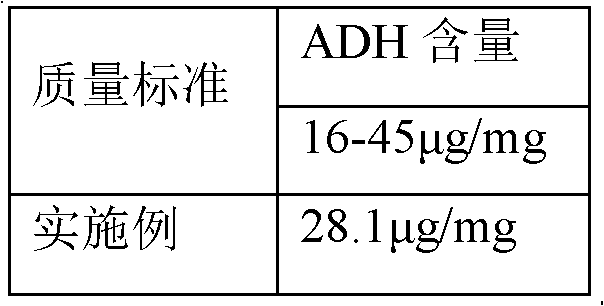
Process for activating Haemophilus influenzae type b (Hib) polysaccharide conjugate vaccine
ActiveCN102626515AAvoid harmImprove securityAntibacterial agentsCarrier-bound antigen/hapten ingredientsTetrafluoroborateFreeze-drying
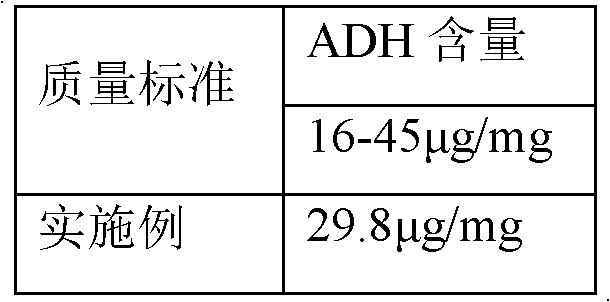
The invention discloses a process for activating a Haemophilus influenzae type b (Hib) polysaccharide conjugate vaccine, which comprises the following steps of: A, preparing Hib polysaccharide; B, dissolving 1-Cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) by using acetonitrile into a solution; C, preparing the Hib polysaccharide into a solution; D, adding the CDAP solution to the Hib polysaccharide solution, and stirring for 2-5 minutes at the room temperature; E, dissolving adipic dihydrazide (ADH) by using NaHCO3 into a solution, adding the ADH solution to a mixed solution, and stirring for 0.5-2 hours at the room temperature; and F, collecting eluent of the void volume from a loading solution after the reaction is ended at a SephadexG-25 gel chromatography column balanced in advance by water for injection, and performing freeze drying to obtain a Hib polysaccharide-ADH derivative. The process for activating the Hib polysaccharide conjugate vaccine has the beneficial effects that the quality index of the prepared Hib polysaccharide-ADH can reach the industrial standard, and moreover, the safe and nontoxic CDAP is adopted to serve as an activating agent instead of cyanogen bromide which is greatly harmful to human and environment, and therefore, the safety is enhanced, and the harm to the human and the environment are avoided.
Owner:CHENGDU OLYMVAX BIOPHARM
Preparation method and application of purified avidin medium based on 6B agarose microsphere
InactiveCN110075818AIncrease the scope of applicationHigh purityOther chemical processesSolid sorbent liquid separationCyanogen bromidePhosphate
The invention provides a preparation method of a purified avidin medium based on a 6B agarose microsphere. The preparation method comprises the following steps of: 1) activating the 6B agarose microsphere through cyanogen bromide to form activated agarose microsphere, 2) allowing the activated agarose microsphere and an amino compound to give a coupling reaction to form an amino modified agarose microsphere, 3) allowing the amino modified agarose microsphere and amino biotin capable of being specifically bound with avidin to give a coupling reaction under the action of a coupling agent, and 4)adding into a PBS (phosphate buffer solution) for flushing to form the purified avidin medium after the reaction is finished. According to the preparation method, the surface of 6B agarose activatedby cyanogen bromide is subjected to amination modification by the stable amino compound; chemical stability and tolerance of a product are improved; an application scope of the agarose microsphere isexpanded; at the same time, the amino biotin capable of being dissociated sufficiently in a weakly acidic environment is adopted as a ligand for specific binding of the avidin; and the purity and purification efficiency of the avidin are improved.
Owner:武汉菲恩生物科技有限公司
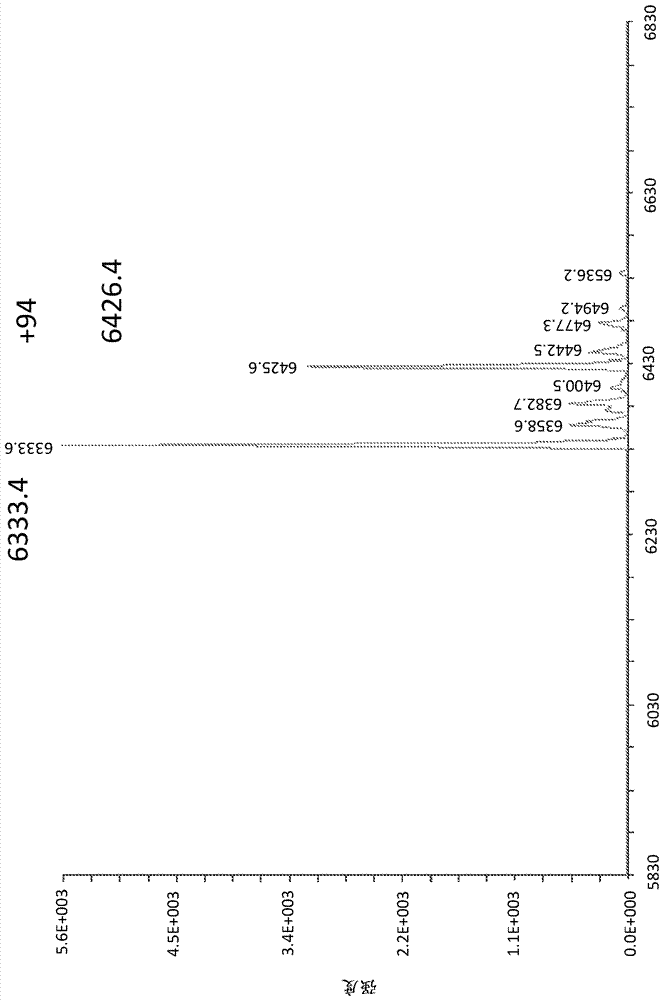
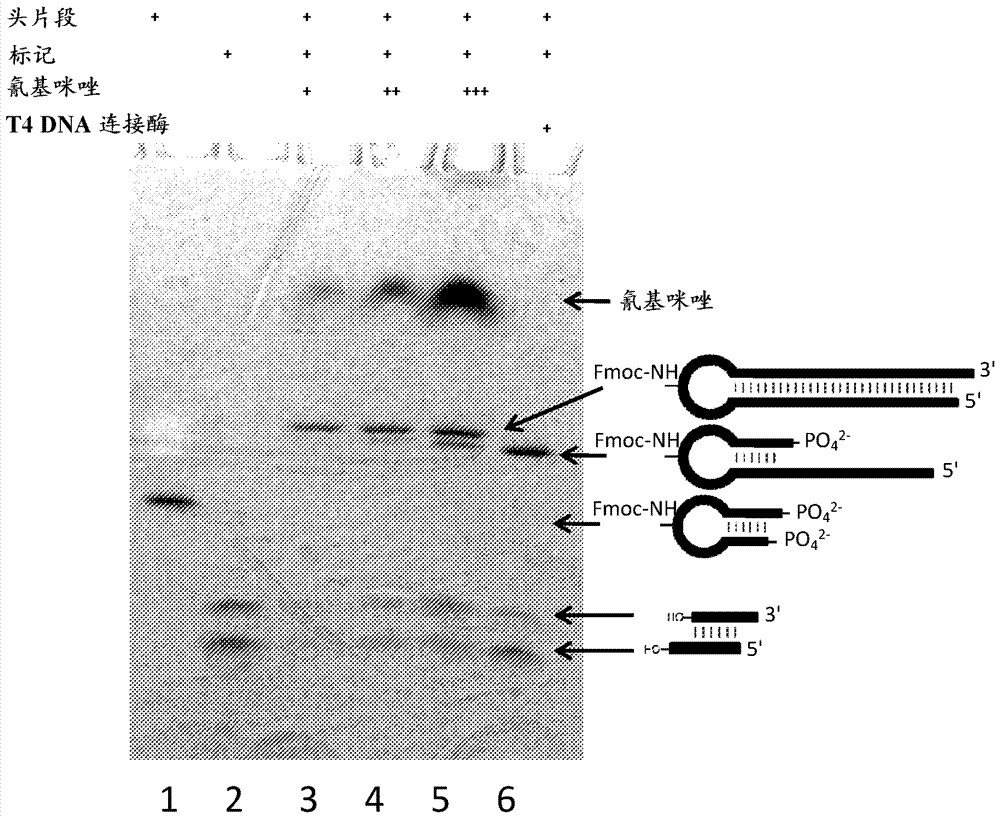
Methods for tagging dna-encoded libraries
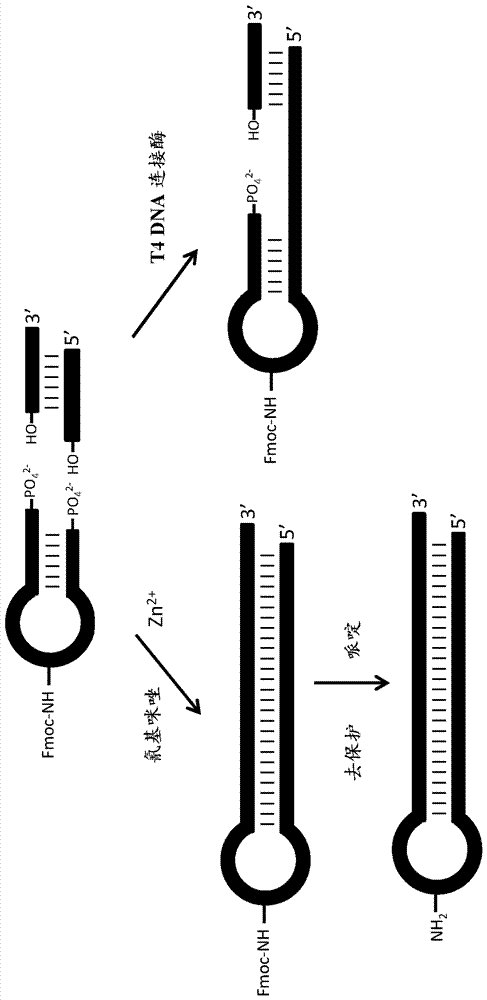
ActiveCN107428795AReservation ConvenienceChemical linkage achievedSugar derivativesSugar derivatives preparationChemical ligationCyanogen bromide
The present invention relates to methods for producing encoded chemical entities. In particular, the oligonucleotides and methods can include encoded chemical entities having wild-type linkages formed through chemical ligation techniques. One strategy that can be utilized that simultaneously takes advantage of chemical ligation as a means to encode chemical history, while also retaining the ability of polymerases to directly recover tag sequence and association information, is to perform chemical ligation in a manner that generates wildtype phosphodiester linkages. Such methods generally utilize condensing agents such as cyanogen bromide or similar along with 5'-phosphate and 3'-hydroxyl oligonucleotides in a double-stranded or templated context. Similarly cyanogen bromide has also been shown to chemically ligate pairs of substrate oligonucleotides that are 5'-hydroxyl and 3'-phosphate. However, these methods suffer from poor efficiency making them ill-suited for use in an iterative process such as tagging DNA-encoded libraries.
Owner:X CHEM
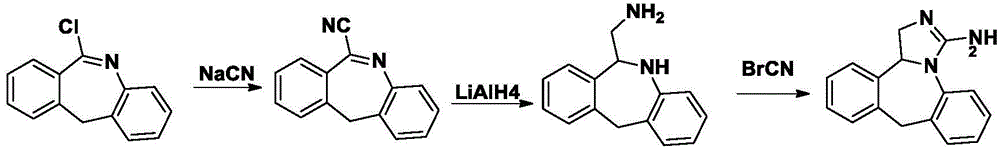
Method for synthesizing epinastine
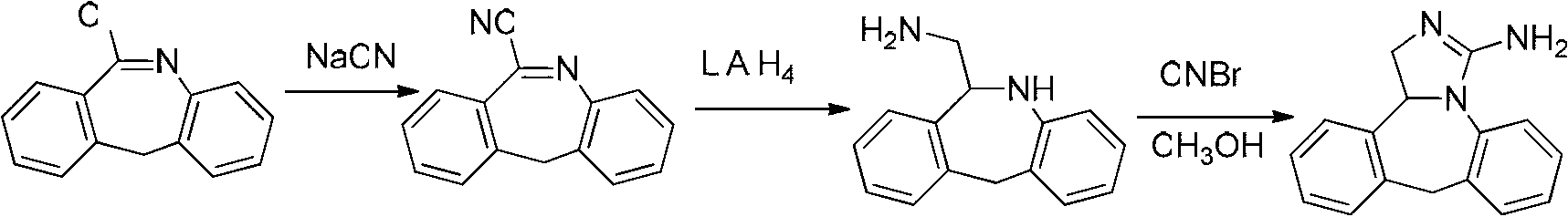
The invention discloses a method for synthesizing epinastine. The method comprises the following steps: (1) reacting 6-halomethylmorphanthridine with hexamine in an organic solvent, thereby obtaining a 6-halomethylmorphanthridine quaternary ammonium salt; (2) dissolving the 6-halomethylmorphanthridine quaternary ammonium salt in the organic solvent to carry out an acid hydrolysis reaction, thereby obtaining 6-halomethylmorphanthridine hydrochloride; (3) reducing the product 6-halomethylmorphanthridine hydrochloride obtained in the step (2) by using a reducing agent, thereby obtaining 6-aminomethyl-6,11-dihydro-5H-dibenzo[b,e]aza-cycloheptatrien; and (4) adding cyanogen bromide to carry out a ring-closure reaction, thereby obtaining the epinastine. According to the synthetic method disclosed by the invention, use of high-price and flammable lithium aluminum hydride or aluminum hydride is avoided, use of virulent sodium cyanide is avoided, and the security risk and production cost are effectively reduced. The method disclosed by the invention is simple in synthetic process, the reaction conditions are mild, the product is high in yield and high in purity, and industrial production is facilitated.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Process for producing peptide
InactiveCN1509336ACell receptors/surface-antigens/surface-determinantsBacteriaCyanogen bromideTarget peptide
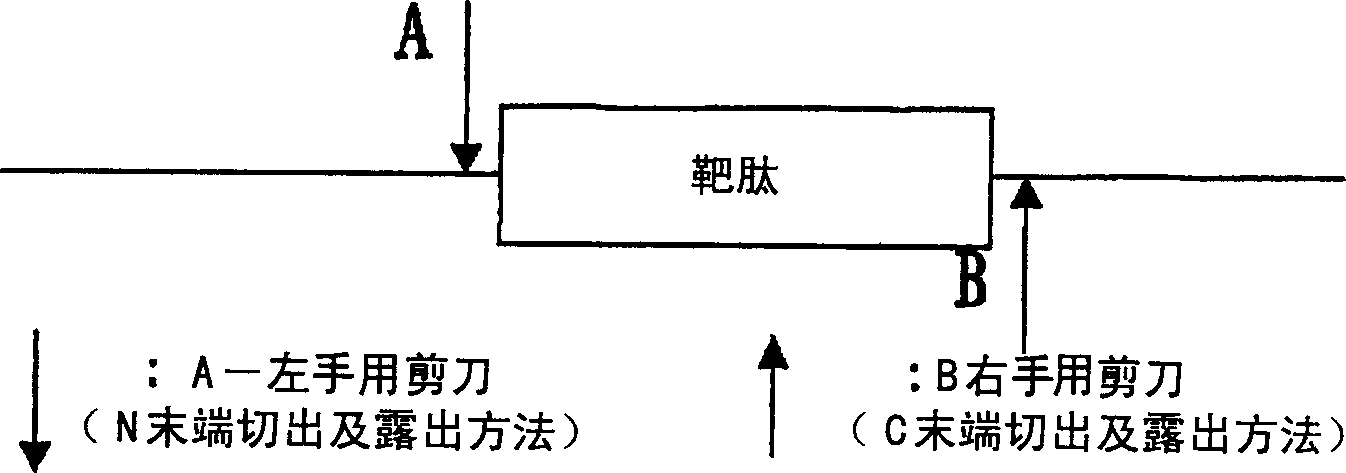
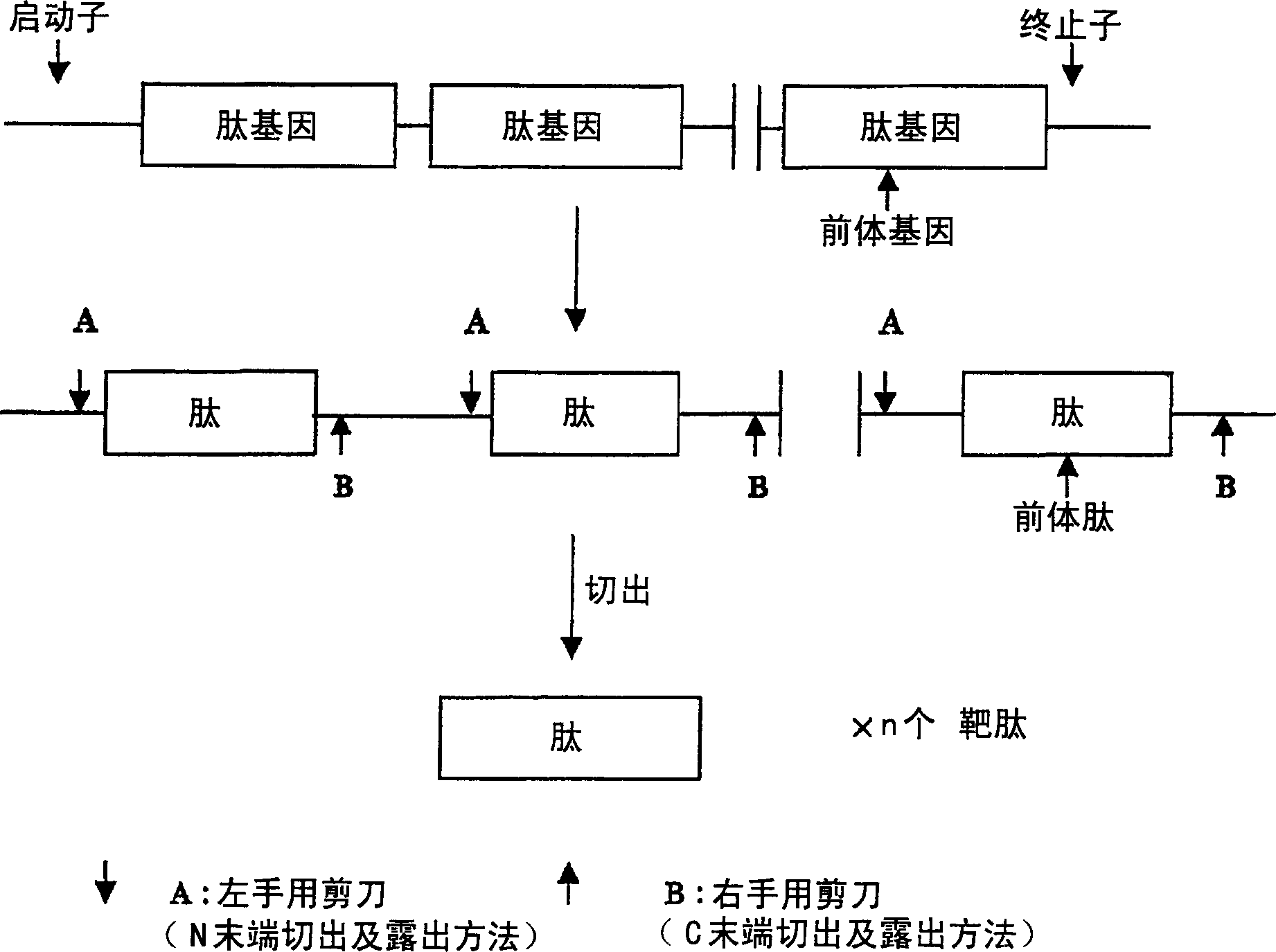
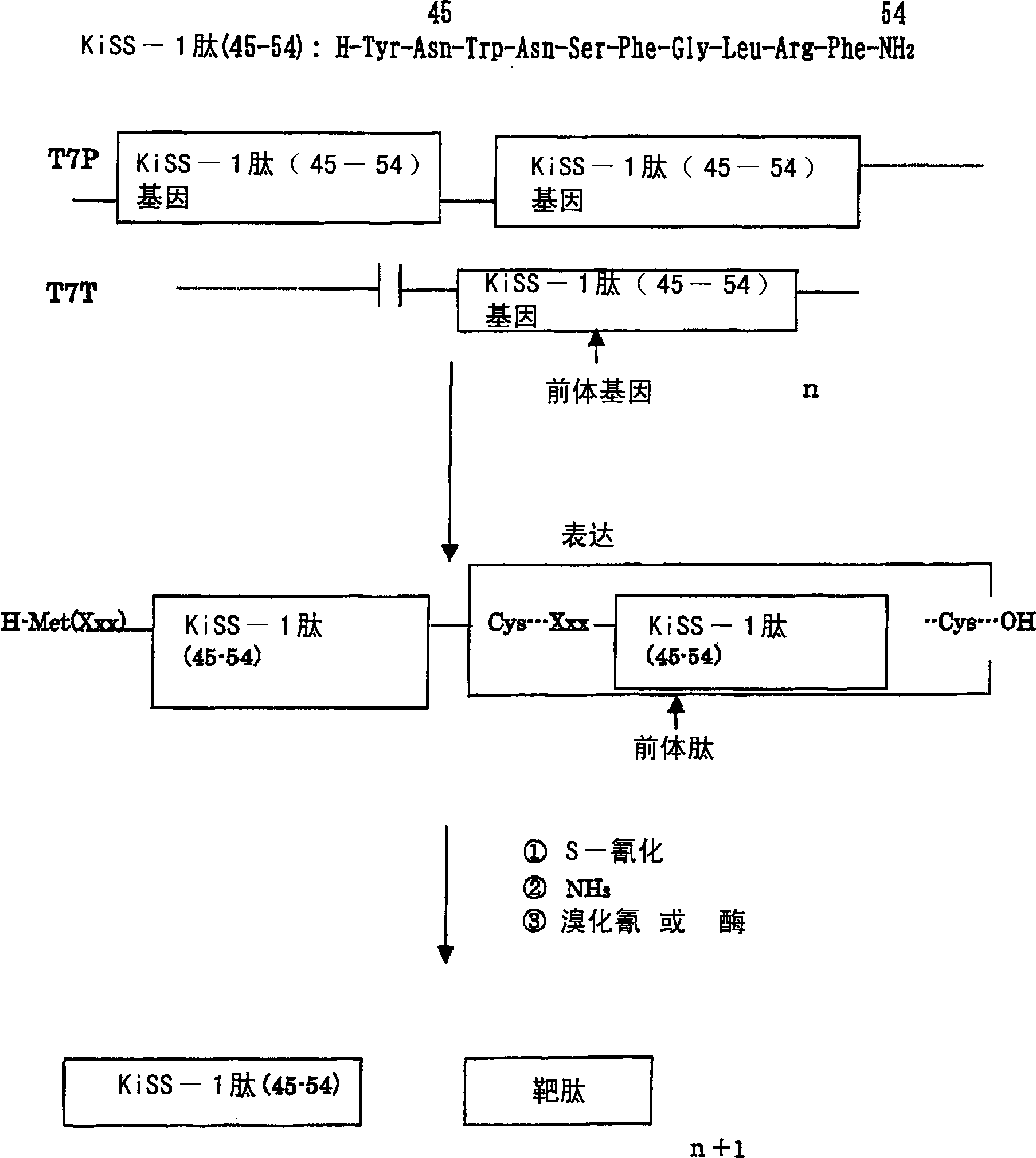
An object of the present invention is to provide a method that can efficiently and mass-produce a target peptide by using a gene recombination method. The method of the present invention based on the combination of right-hand scissors (S-cyanation reaction) and left-hand scissors (cyanogen bromide treatment, enterokinase, factor Xa treatment, etc.) to cut out the target peptide and tandem repeat method can be used for large-scale synthesis of gene-based Peptides of recombinant technology, especially low molecular weight peptides.
Owner:SHIMADZU SEISAKUSHO CO LTD
Lenvatinib synthesis method
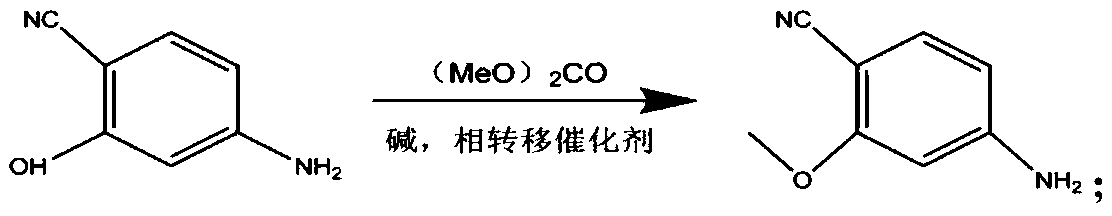
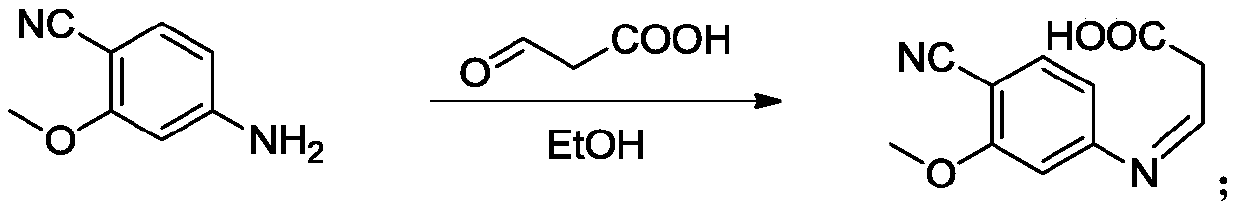
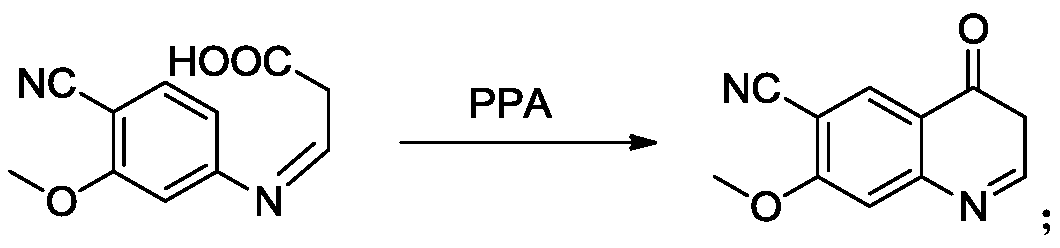
ActiveCN109734661ALower requirementMild reaction conditionsOrganic chemistryLenvatinibAlkyl transfer
The invention provides a lenvatinib synthesis method. The method includes: taking 4-cyano-3-hydroxyaniline as a starting material, subjecting to dimethyl carbonate methylation and oximation through reaction with malonaldehydic acid at the room temperature, performing cyclization under the PPA condition to form 6-cyano-7-methoxy-4-quinolinone, forming 6-cyano-7-methoxy-4-chlorolinone under the action of thionyl chloride, performing cyano hydrolyzing synthesis of 6-formamido-7-methoxy-4-chloroquine which is an intermediate of lenvatinib under an acidic condition, subjecting 4-hydroxy-2-chloroaniline and cyanogen bromide to low-temperature reaction to form 4-hydroxy-2-chlorocyanogen amine, and subjecting 4-hydroxy-2-chlorocyanogen amine and bromopropane to ritter reaction to synthesize 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropyl urea which is another key intermediate of lenvatinib; finally, subjecting the two intermediates including 6-formamido-7-methoxy-4-chloroquine and 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropyl urea to alkylation reaction in an alkaline environment to obtain lenvatinib. By the scheme, mild reaction conditions, avoidance of highly toxic reagents, environmentally friendliness and the like are realized.
Owner:IANGSU COLLEGE OF ENG & TECH
Immunoaffinity gel detection column for detecting gentamicin and preparation method thereof
The invention provides a preparation method of an immunoaffinity gel detection column capable of visually and rapidly detecting gentamicin residues in food. The method comprises the steps that agarose gel is used as a solid-phase carrier, a gentamicin antibody and the agarose gel which is activated through cyanogen bromide are coupled to prepare antibody gel used as a detection layer, an HRP antibody and the agarose gel which is activated through the cyanogen bromide are coupled to prepare HRP antibody gel used as a quality control layer, the product is placed into 1mL of solid phase extraction column, and the immunoaffinity gel detection column is prepared. The novel immunoaffinity gel detection product which detects the gentamicin residues in the food rapidly, qualitatively and semiquantitatively is developed, and the detection limit is 40 micrograms / L. The immunoaffinity gel detection column has the outstanding advantages that specificity is high, and sensitivity is good; sample pretreatment is simple; detection time is short and accuracy is high; operation is easy and convenient, and assisting of large instruments is not needed.
Owner:TIANJIN UNIV OF SCI & TECH
Immunoaffinity gel detection column for detecting furaltadone metabolite
The invention provides a preparation method of an immunoaffinity gel detection column capable of visually and rapidly detecting furaltadone metabolite (AMOZ) in food. The method comprises the steps that agarose gel is used as a solid-phase carrier, an AMOZ antibody and the agarose gel which is activated through cyanogen bromide are coupled to prepare antibody gel used as a detection layer, an HRP antibody and the agarose gel which is activated through the cyanogen bromide are coupled to prepare HRP antibody gel used as a quality control layer, the product is placed into 1mL of solid phase extraction column, and the immunoaffinity gel detection column is prepared. The novel immunoaffinity gel detection product which detects AMOZ residues in food rapidly, qualitatively and semiquantitatively is developed, and the detection limit of the AMOZ in animal derived food is 3 micrograms / kg. The immunoaffinity gel detection column has the outstanding advantages that specificity is high, and sensitivity is good; sample pretreatment is simple; detection time is short and accuracy is high; operation is easy and convenient, and assisting of large instruments is not needed.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com