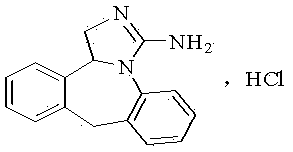
Preparation method of epinastine hydrochloride and intermediate thereof
A technology of epinastine hydrochloride and intermediates, which is applied in the field of preparation of pharmaceutical compounds, can solve the problems of many side reactions, many impurities generated, and easy to be oxidized, and achieve simple preparation methods, high product purity, and few by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
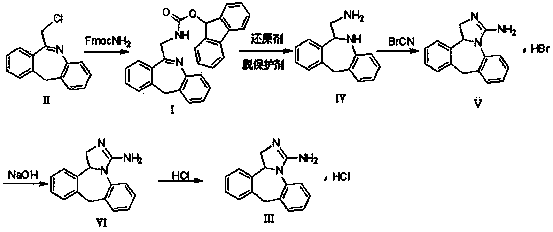
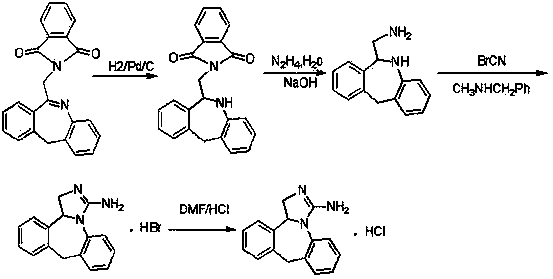
[0037] In the reaction flask, drop in the starting material 6-chloromethyl-11-dihydro-dibenzo[ b,e ] Azepine 10g, 150 milliliters of ethanol, add N - 11.2 g of fluorenylmethoxycarbonyl ammonia, 4.2 g of triethylamine was added dropwise, and reacted at 60 ° C for 10 hours. After the reaction was completed, the solid was removed by filtration, distilled to dryness under reduced pressure, 15 ml of water was added, and extracted with 20 ml of dichloromethane. The solvent is recovered to obtain the product 6- N -Fmoxycarbonylaminomethyl-11-dihydro-dibenzo[ b,e ] Azepine 16.2g (HPLC purity 98.4%, yield 91%).
[0038]
Embodiment 2
[0040] In the reaction flask, drop in the starting material 6-chloromethyl-11-dihydro-dibenzo[ b,e ] Azepine 20g, 280 milliliters of acetonitrile, add N - 22.4 g of fluorenylmethoxycarbonyl ammonia, 8.4 g of triethylamine was added dropwise, and reacted at 80 ° C for 6 hours. After the reaction was completed, the solid was removed by filtration, distilled to dryness under reduced pressure, 30 ml of water was added, extracted with 50 ml of dichloromethane, The solvent is recovered to obtain the product 6- N -Fmoxycarbonylaminomethyl-11-dihydro-dibenzo[ b,e ] Azepine 30.1g (HPLC purity 98.8%, yield 84.6%).
[0041]
Embodiment 3
[0043] In the reaction bottle, drop into raw material 6- N -Fmoxycarbonylaminomethyl-11-dihydro-dibenzo[ b,e] Azapine 15g, methyl alcohol 150 milliliters, sodium borohydride 4.0g room temperature conditions, reacted 24 hours, and reaction was completed, and the control internal temperature was lower than 25 ℃, and 2% dilute hydrochloric acid solution was added dropwise to make its pH value to 5~6, with Dichloromethane 150ml×2 extracted, the organic layer was washed with saturated brine, concentrated to dryness, added 20ml of 18% dimethylamine acetonitrile solution, heated to an internal temperature of 26-30°C, stirred for 0.5 hours, distilled under reduced pressure, added 35ml of acetonitrile was stirred and filtered with suction. After beating with 40ml of isopropyl ether for 10min, filter with suction, add 20ml of purified water to the filter cake, extract with 100ml of dichloromethane×2, wash the organic layer with saturated brine, dry over anhydrous magnesium sulfate, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com