Beta-glucan modified meningitis polysaccharide conjugate vaccine and preparation method thereof
A technology combining vaccine and meningitis, applied in the field of biomedicine, can solve the problem of no patent application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
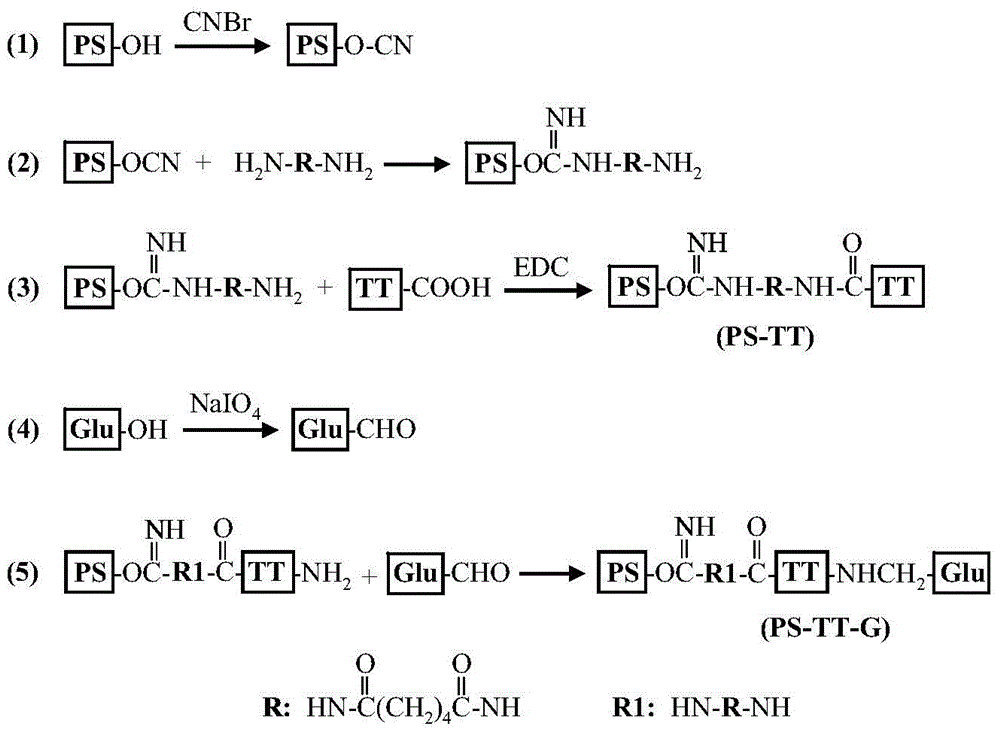
[0019] Example 1: Preparation and separation and purification of β-glucan-modified meningitis polysaccharide protein-conjugated vaccine
[0020] (1) Preparation of meningitis polysaccharide-carrier protein conjugate vaccine (PS-TT)
[0021] Dissolve 5 mg of meningococcal capsular group Y polysaccharide (PS) in 1.25 ml of normal saline, adjust the pH of the solution to 10.8 with 0.5 mol / L of sodium hydroxide, add 10 microliters of 50% (w / v) cyanogen bromide, reacted for 30 minutes. During the activation process, as the pH decreased continuously, the pH value of the solution was maintained at 10.8 with 0.5 mol / liter of sodium hydroxide. After the activation, the pH value of the solution was adjusted to 8.5 with 0.5 mol / liter of hydrochloric acid, followed by adding 0.15 milliliters of adipic hydrazide solution with a concentration of 100 mg / ml, reacting overnight at room temperature ( figure 1 ). Subsequently, it was dialyzed three times for 12 hours in 20 mM phosphate buffe...
Embodiment 2
[0028] Example 2: Characterization of β-glucan modified polysaccharide conjugate vaccines
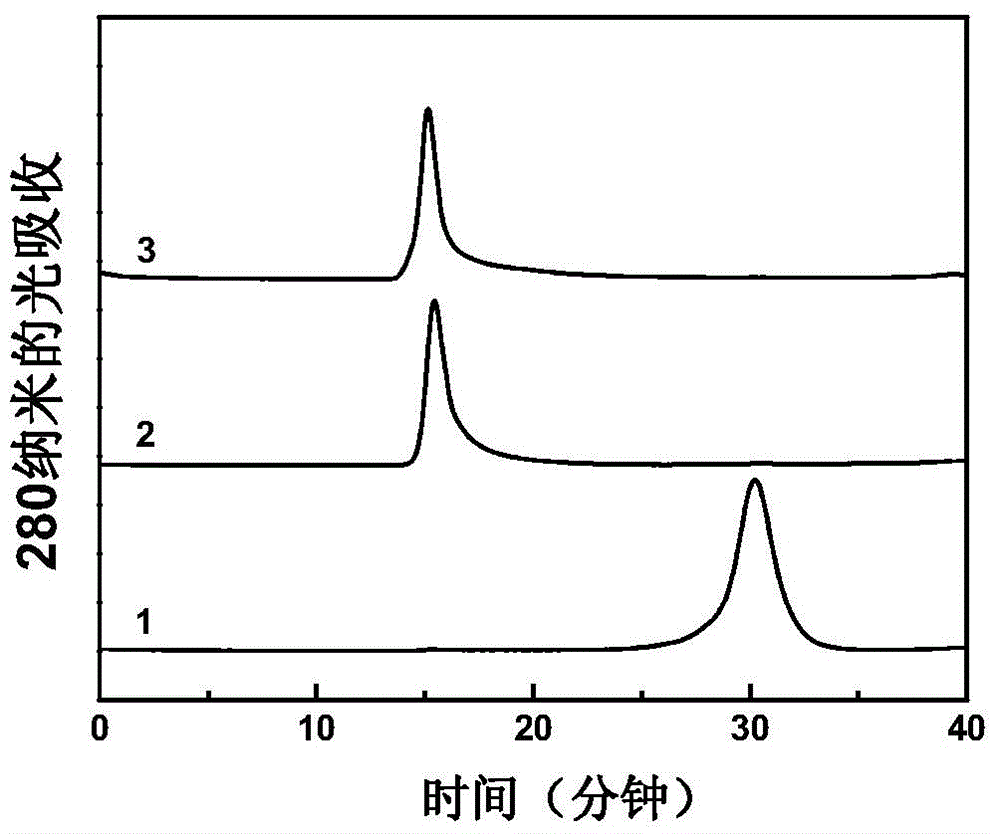
[0029] The purified product was identified with a Superose 6 gel filtration column (1.0 cm×30 cm), the eluent was 20 mM phosphate buffer (pH 7.4), and the flow rate was 0.5 ml / min. Such as figure 2 As shown, compared with the carrier protein TT, the peak time of PS-TT and PS-TT-G was significantly earlier. This indicates that the molecular weight of the carrier protein increases significantly after binding to the meningitis capsular polysaccharide.
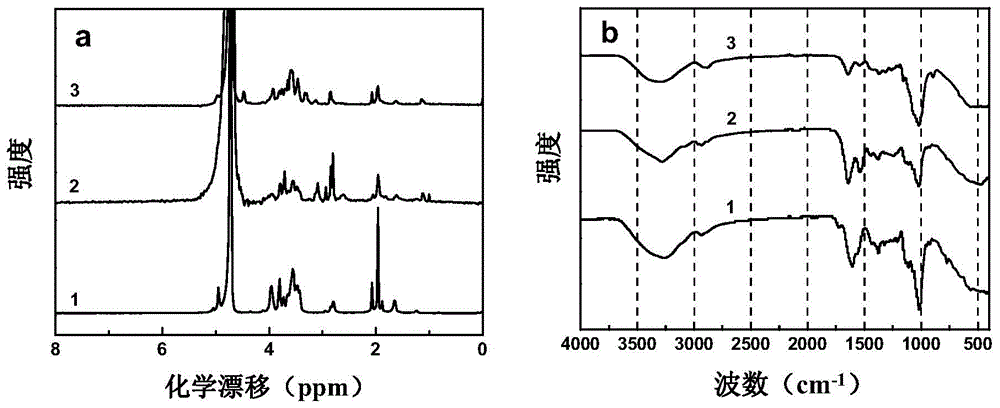
[0030] use 1 H-NMR detection of polysaccharide conjugated vaccines. Group Y polysaccharides consist of →4-O-α-D-glucose p-(1→6)-β-D-sialic acid-2→repeating units, such as image 3 As shown in a, there are clear dextran terminal carbon proton peaks and sialic acid H3eq / ax resonance peaks at chemical shifts 3.3-4.2, and water peaks appear at 4.7ppm. At these positions, PS-TT and PS-TT-G also have the same peaks as PS, and at the same ti...
Embodiment 3
[0032] Example 3: Determination of immunogenicity of β-glucan modified polysaccharide conjugate vaccine
[0033] Take PS-TT, in which the concentration of PS is 10 μg / ml, both in a total volume of 3 ml, and physically mix with 30 μg and 90 μg of β-glucan, respectively. The mixed solutions were set as PS-TT / G1 group and PS-TT / G2 group respectively. Select 30 8-week-old female Blab / C mice weighing 15-22 g. They were randomly divided into 5 groups, namely PS group, PS-TT group, PS-TT-G group, PS-TT / G1 group and PS-TT / G2 group, with 6 mice in each group. Intraperitoneal injection, each injection containing 5 micrograms of polysaccharides, once a week, a total of 3 injections. After 21 days, blood was collected from the orbit. Anti-meningitis polysaccharide IgG, IgG1, IgG2a and IgM in mouse plasma were detected by ELISA.
[0034] (1) Determination of immunogenicity of PS-TT-G
[0035] Such as Figure 4 As shown in a, the IgG antibody titer produced by the first dose in the PS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com