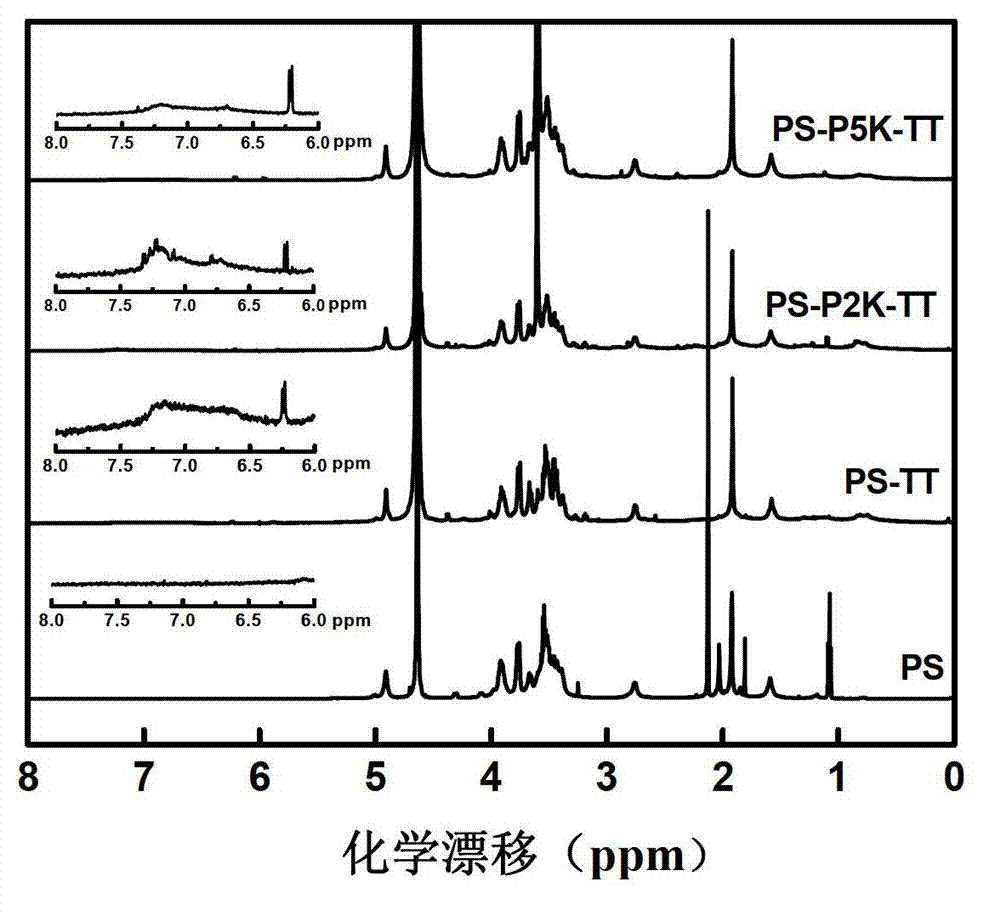
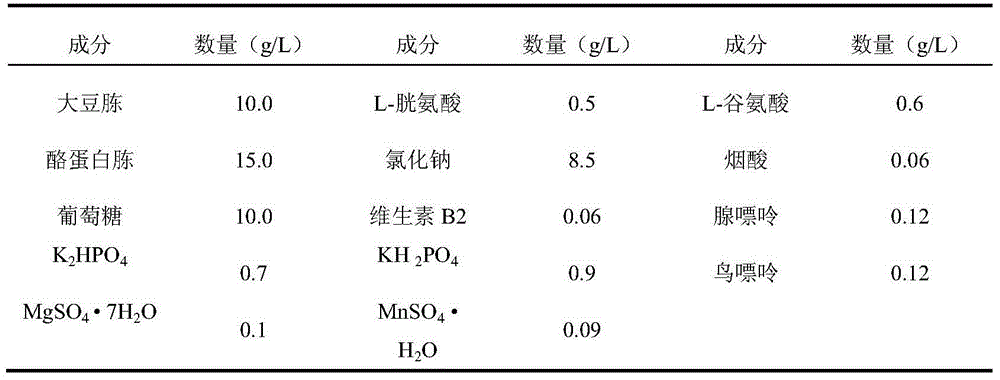
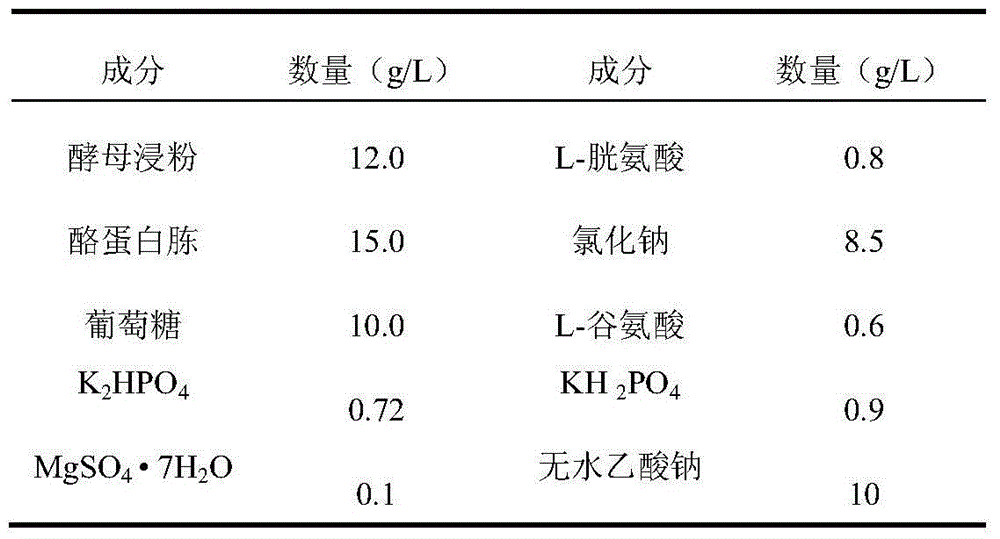
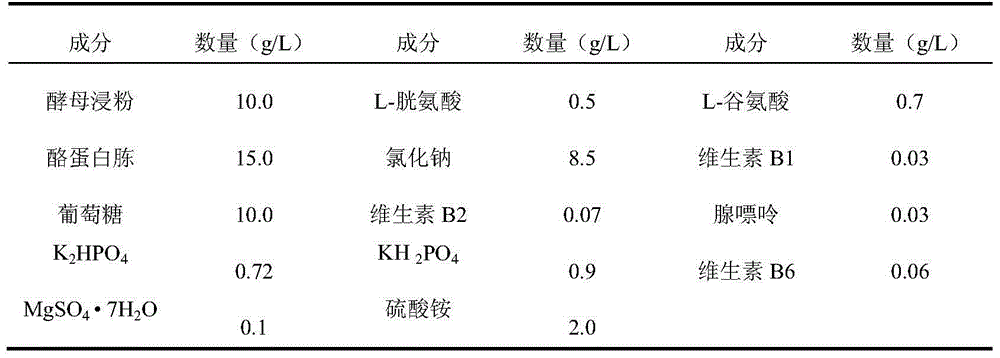
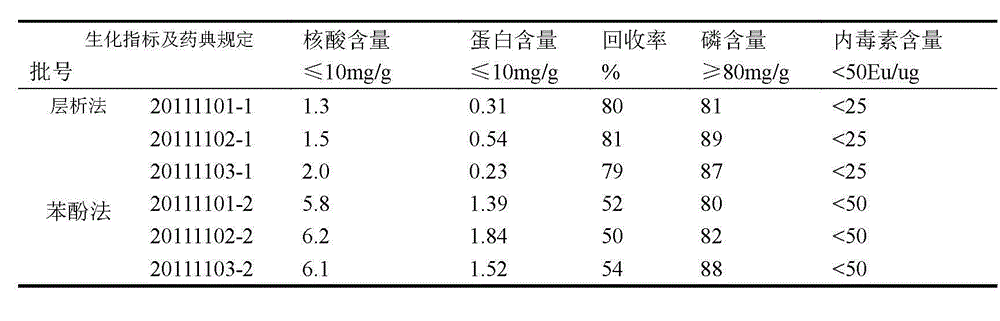
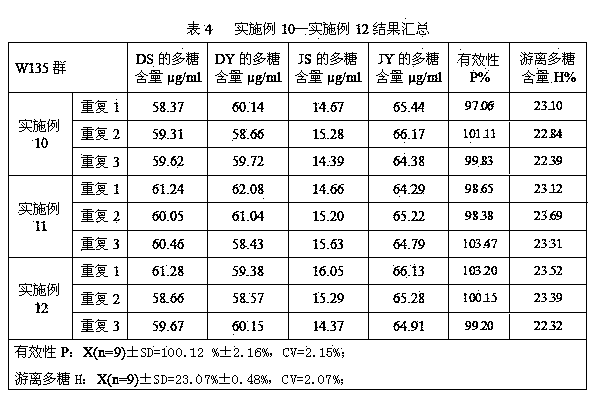
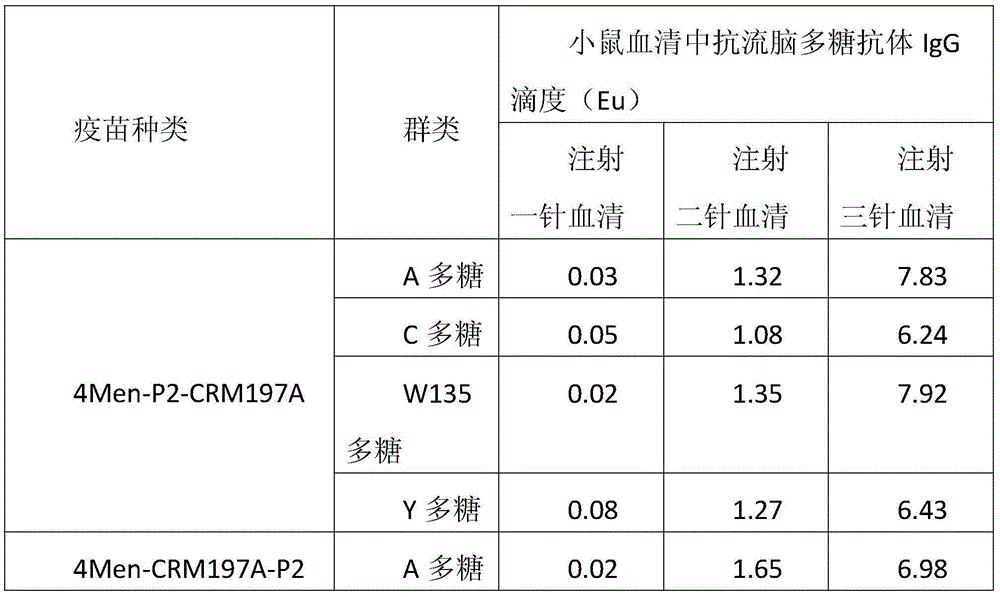
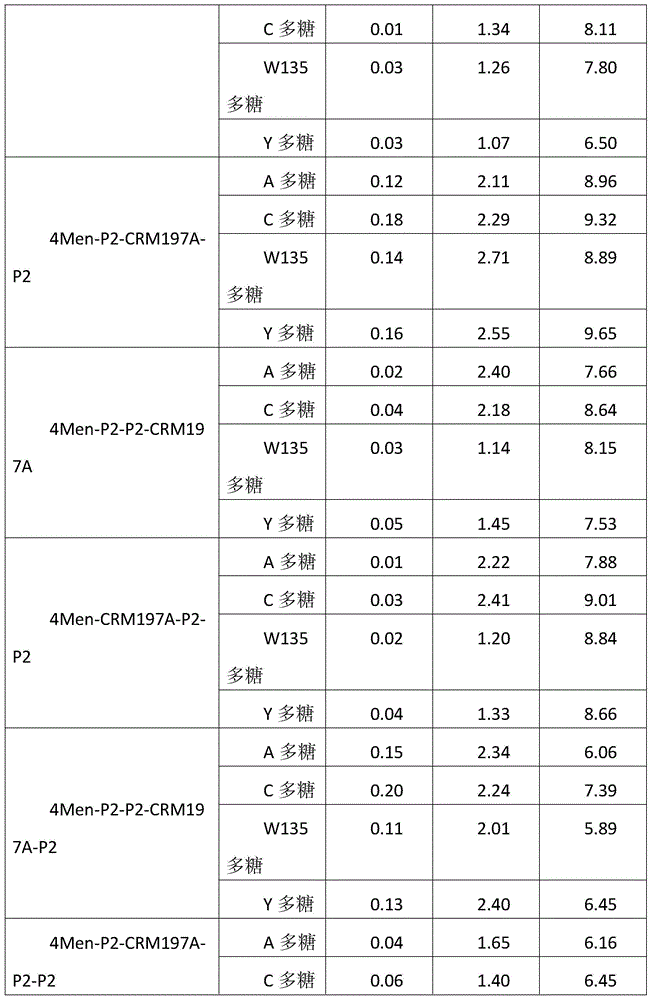
Patents
Literature
43 results about "MENINGOCOCCAL POLYSACCHARIDE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Meningococcal polysaccharide vaccine is used to prevent infection caused by meningococcal bacteria. The vaccine works by exposing you to a small dose of the bacteria or a protein from the bacteria, which causes your body to develop immunity to the disease.
A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process
InactiveCN105031634AKeep healthyAvoid pollutionAntibacterial agentsCarrier-bound antigen/hapten ingredientsConjugate vaccineVaccine manufacturing
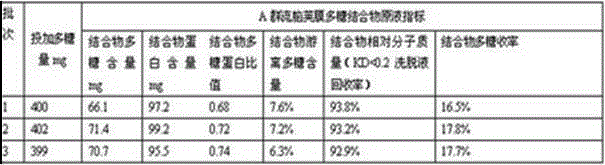
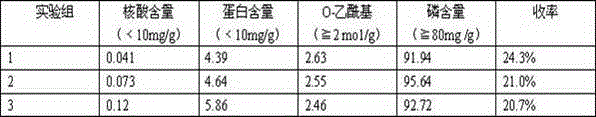
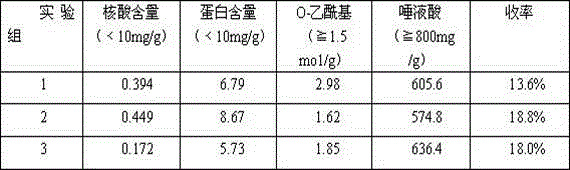
The invention discloses an A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process. The problem that highly-toxic chemical reagents, such as cyanogen bromide, are used in the vaccine manufacturing process in the prior art and accordingly are harmful to protection of human health and environment is solved. The A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process comprises the following steps of 1 cultivating Neisseria meningitidis, 2 utilizing the cultivated Neisseria meningitidis to extract crude polysaccharide, 3 purifying the crude polysaccharide to prepare refined Neisseria meningitidis polysaccharide and 4 activating and deriving the refined polysaccharide. The A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process has the advantages that the extraction rate of the polysaccharide from a capsule is higher, the precipitation rate of the polysaccharide is higher, the recovery rate and purity of the refined polysaccharide are high, the derivation effect is better and the like.
Owner:CHENGDU OLYMVAX BIOPHARM
Vaccines against group neisseria meningitidis and meningococcal combinations thereof
InactiveUS20070020293A1Antibacterial agentsBacterial antigen ingredientsMeningococcal carriageMENINGOCOCCAL POLYSACCHARIDE
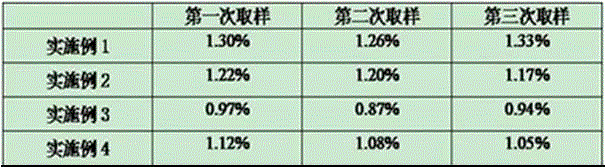
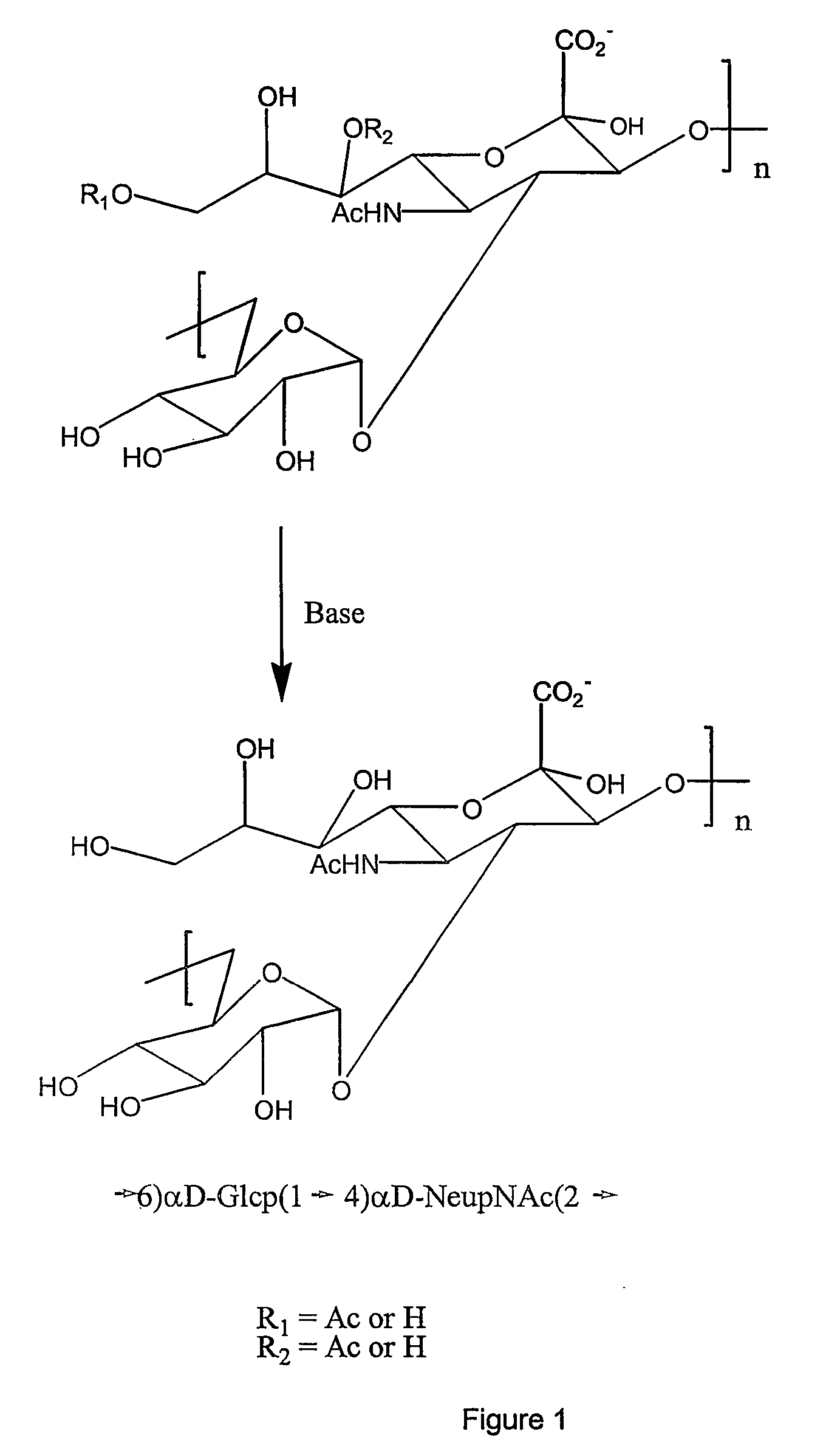
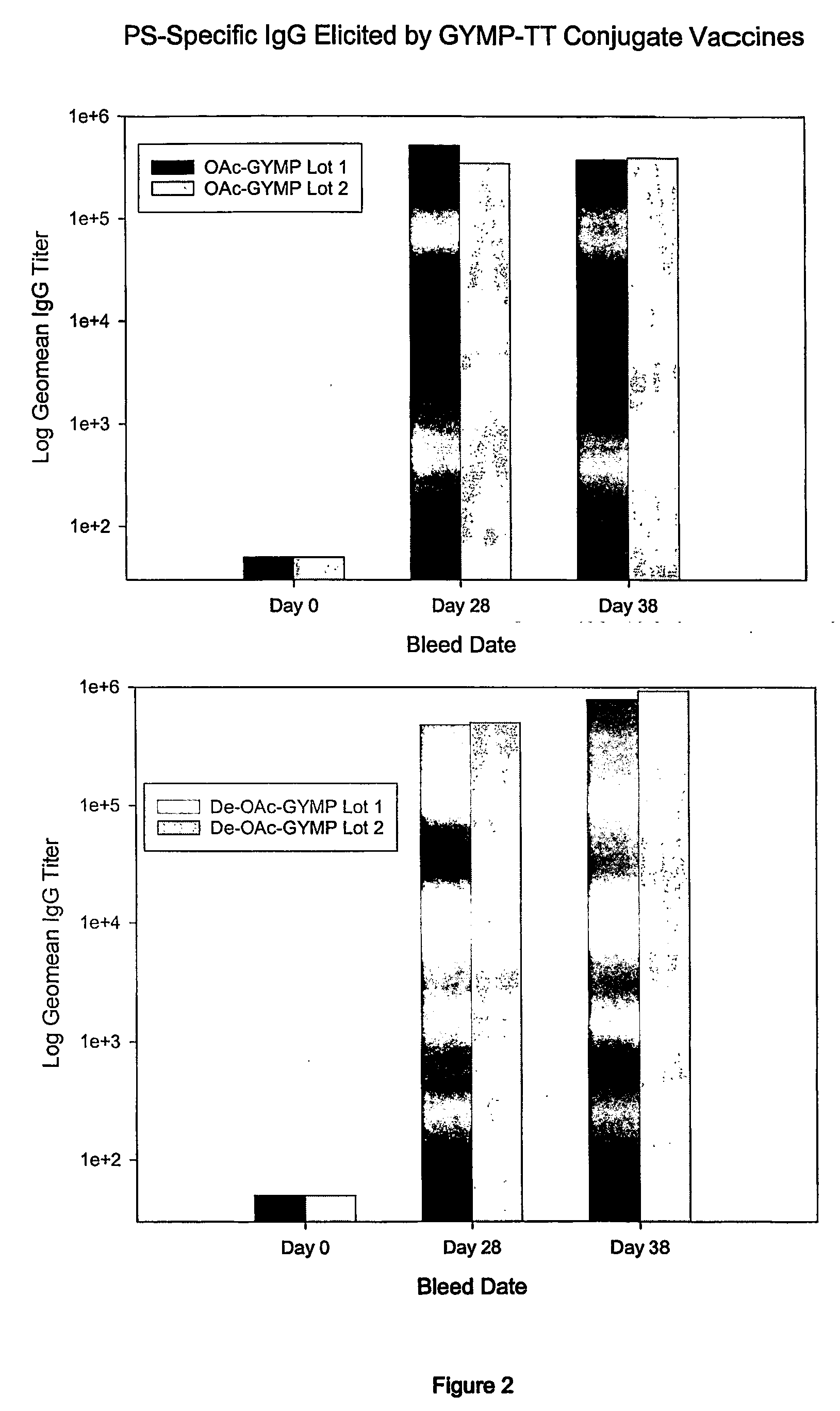
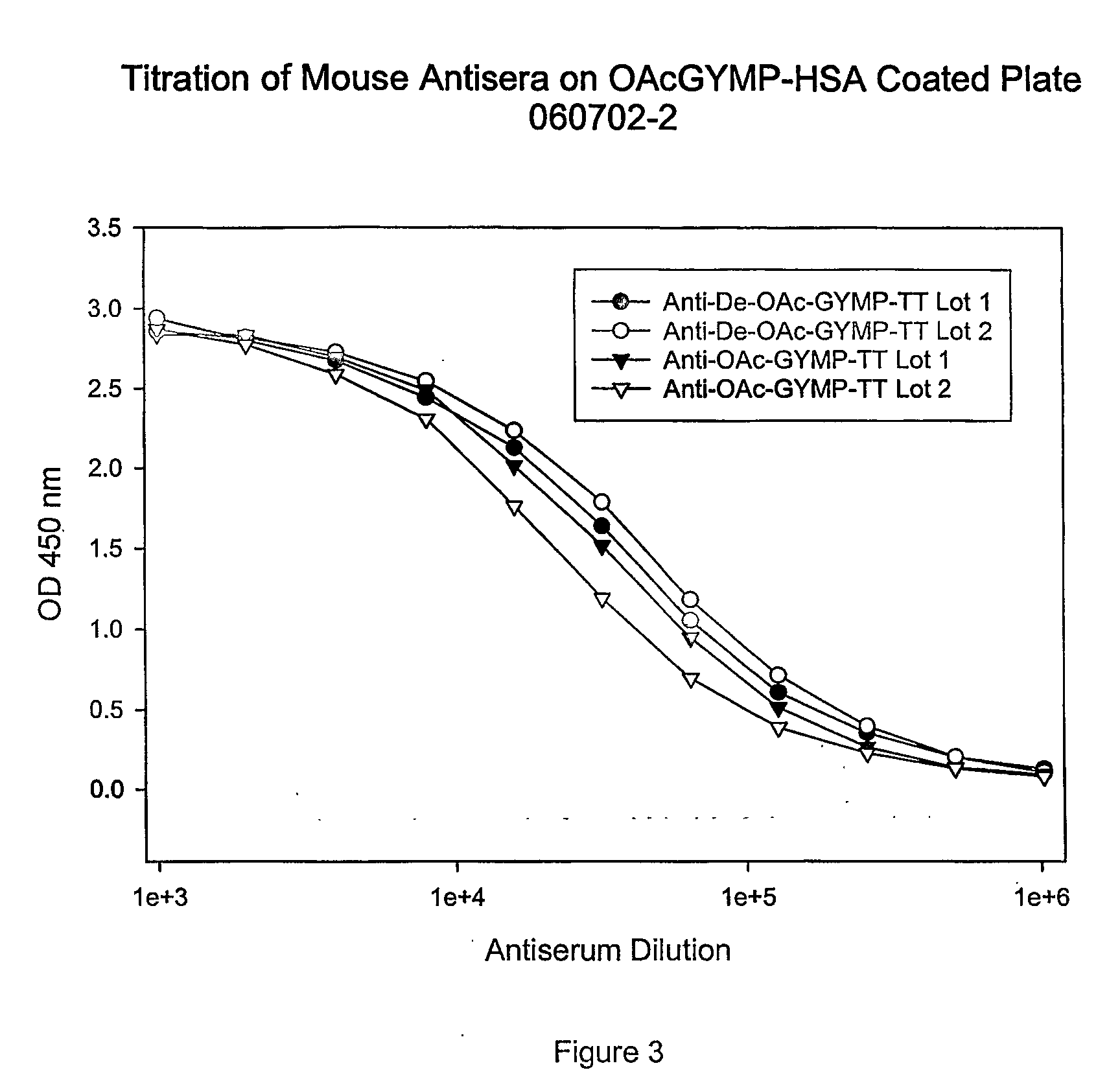
This invention relates to modified meningococcal Y polysaccharides (GYMP), conjugates comprising the modified polysaccharides and a carrier, vaccines for the immunisation of warm-blooded animals, including humans, against Group Y Neisseria meningitidis, and to methods for producing these modified polysaccharides, conjugates and vaccines.
Owner:BAXTER INT INC +1
Multivalent immunogenic composition containing enterovirus antigens
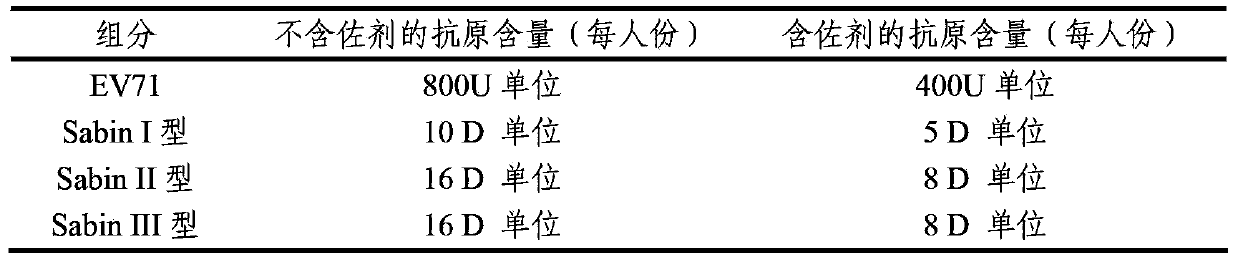
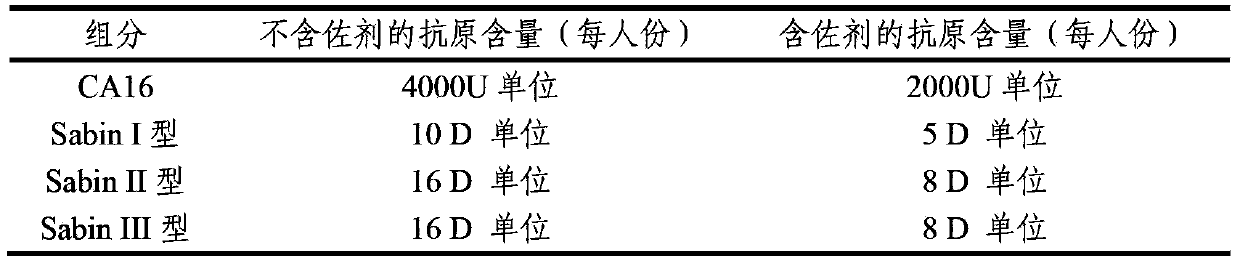
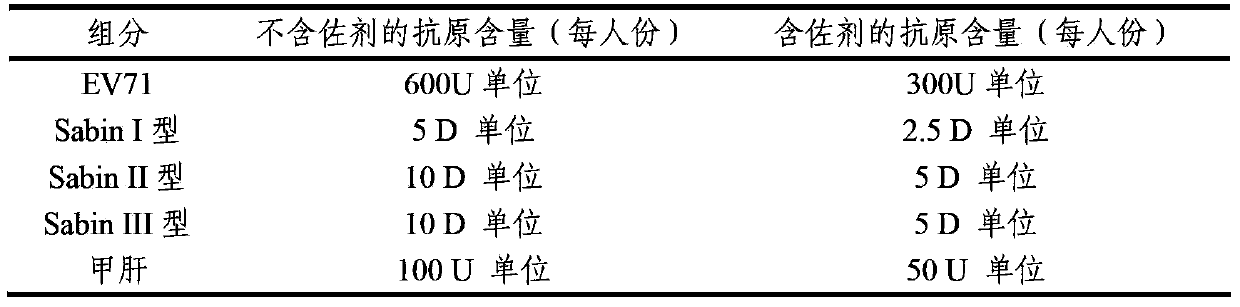
ActiveCN103386126AImproving immunogenicityImprove securityBacterial antigen ingredientsViral antigen ingredientsHepatitis A AntigensTetanus toxoids
The invention provides a multivalent immunogenic composition containing enterovirus antigens. The composition comprises inactivated EV71 antigens and / or inactivated CA16 antigens, and inactivated polio antigens. The composition can further comprise antigens selected from hepatitis A antigens, hepatitis B antigens, acellular pertussis antigens, tetanus toxoid, diphtheria toxoid, Haemophilus influenzae type b capsular polysaccharide, and meningococcal polysaccharide antigens, as well as physiologically acceptable carriers combined with bacterial polysaccharide antigens. The invention also provides a preparation method of the composition. The composition can prevent invasion of a plurality of pathogens simultaneously without interference among the antigens, and the immunogenicity is no less than that of individually activated antigens. With the composition, vaccination processes are significantly simplified, and the vaccination efficiency is improved with reduced costs.
Owner:SINOVAC BIOTECH
Multivalent Meningococcal Polysaccharide-Protein Conjugate Vaccine
InactiveUS20130216571A1Antibacterial agentsBacterial antigen ingredientsConjugate vaccineCarrier protein
Owner:SANOFI PASTEUR INC
Group ABC meningococcus combined vaccine and preparing method thereof
PendingCN106215183AEase the pain of vaccinationEffective preventive effectAntibacterial agentsBacterial antigen ingredientsPericarditisMENINGOCOCCAL POLYSACCHARIDE
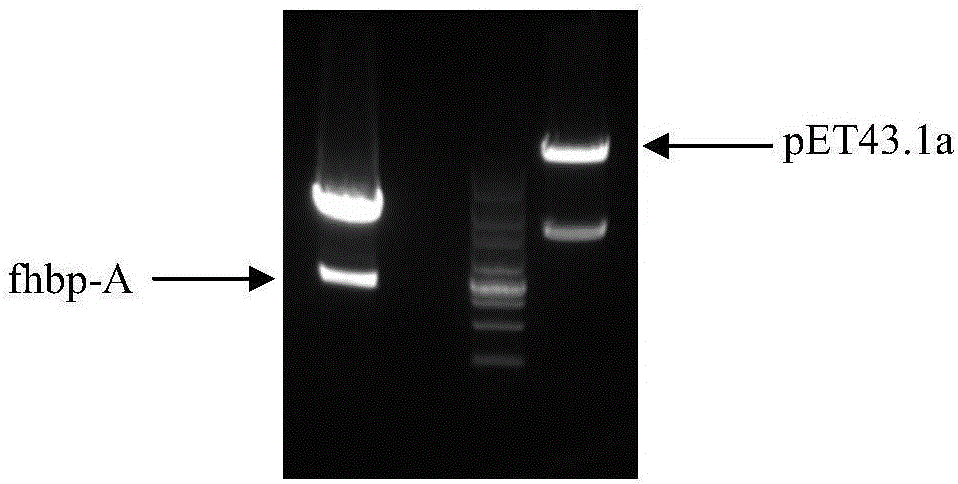
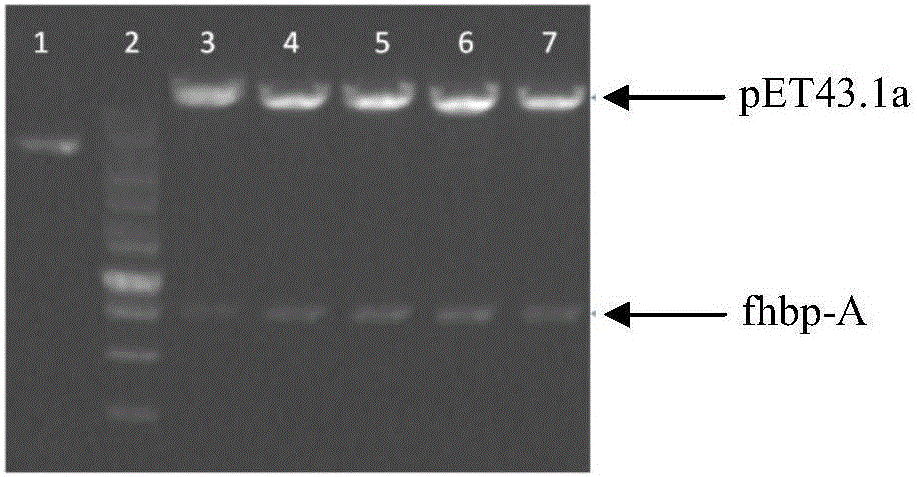
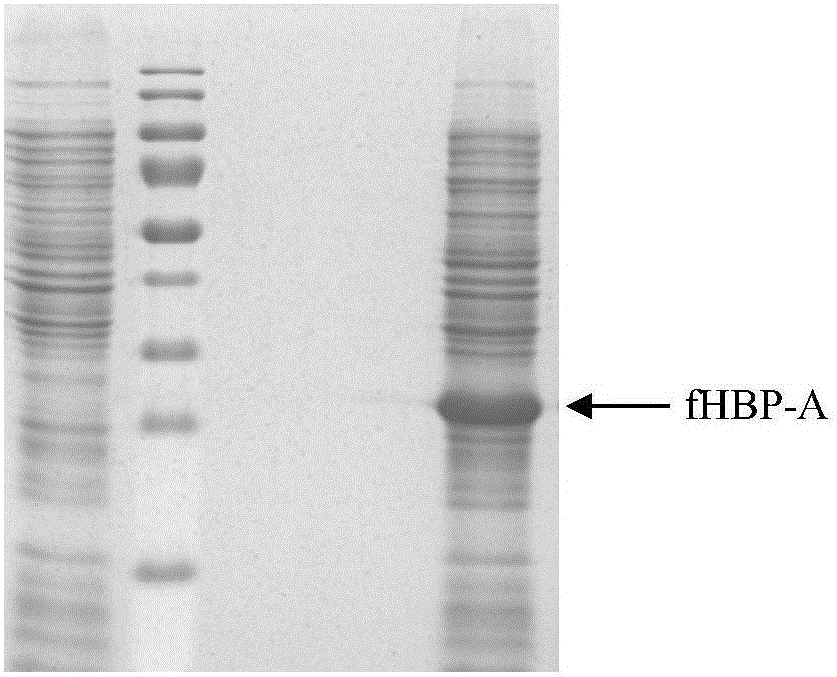
The invention provides a group ABC meningococcus combined vaccine and a preparing method thereof. The group ABC meningococcus combined vaccine is prepared from a group A and group C meningococcus polysaccharide-protein conjugate, and recombinant protein of a human H factor binding protein (fHBP) subgroup A and a human H factor binding protein (fHBP) subgroup B of group B meningococcus. The invention further provides a preparing method of the recombinant fHBP-A protein and fHBP-B protein. The combined vaccine is used for immunity of children 2 or more years old, and used for preventing invasive diseases such as cerebrospinal meningitis, bacteremia, pneumonia and pericarditis caused by group A or group B or group C meningococcus, and providing a better and wider protection effect on meningococcus.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Meningococcal polysaccharide conjugate vaccine treating heterobifunctional reagent as conjugation bridge, and its preparation method
ActiveCN103083652AAvoid self-couplingHigh yieldAntibacterial agentsPharmaceutical non-active ingredientsMENINGOCOCCAL POLYSACCHARIDE CONJUGATEHeterobifunctional reagent
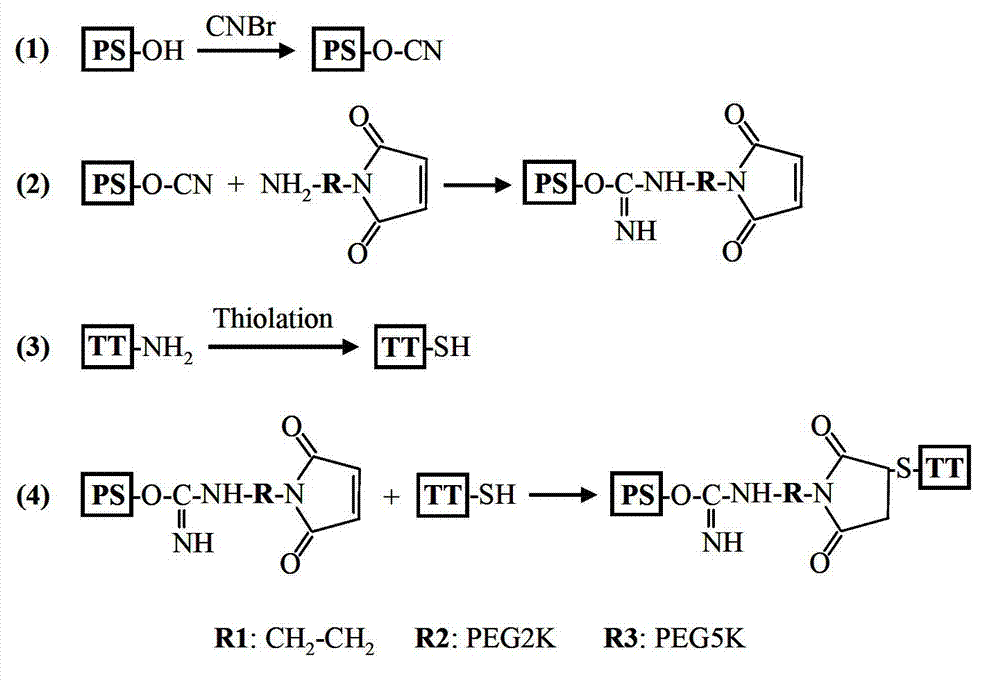
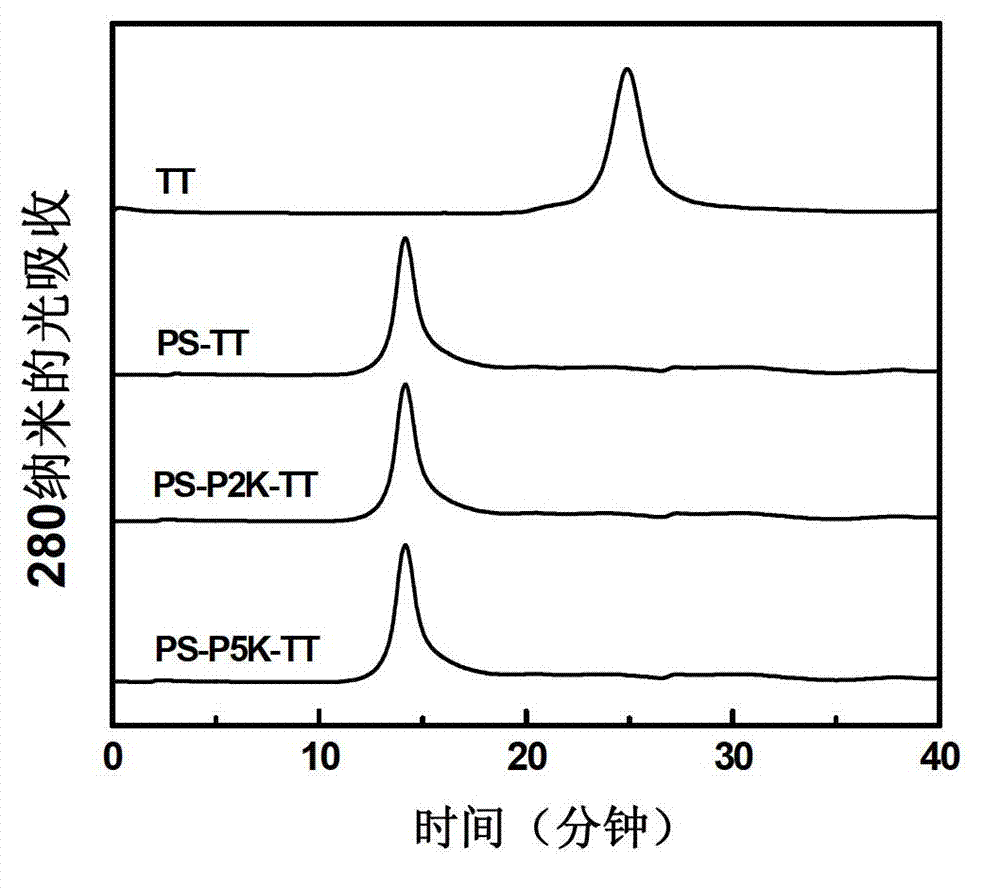
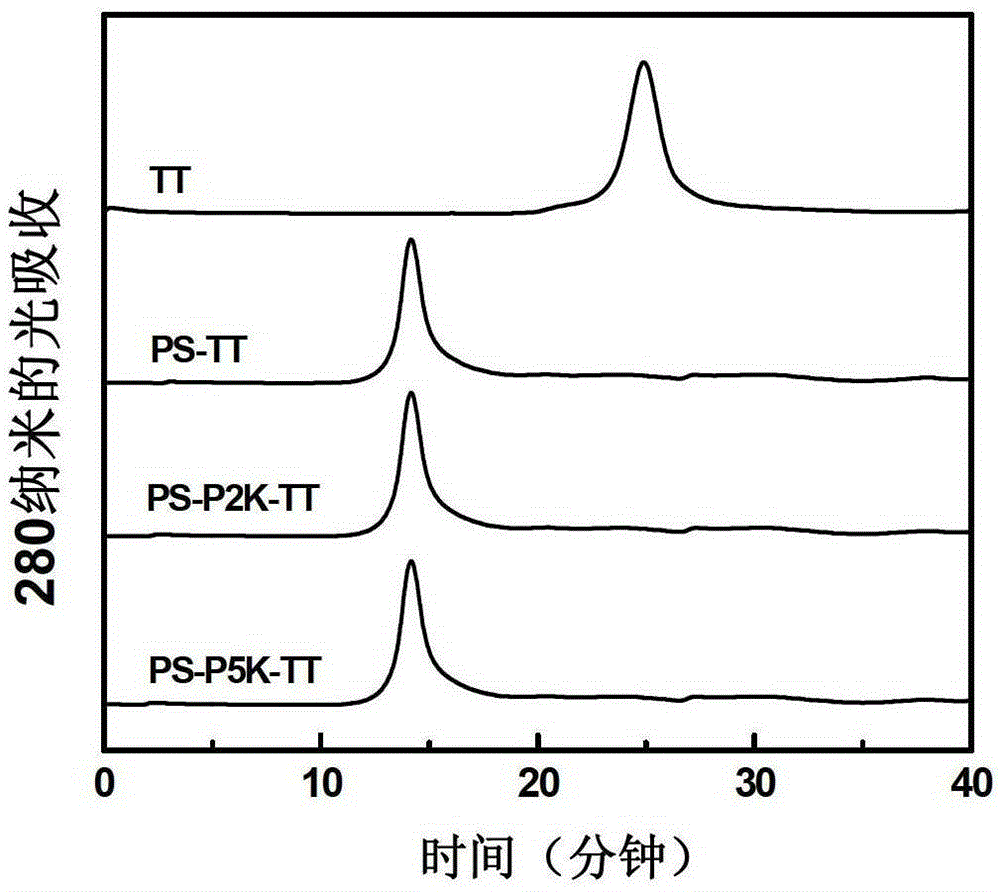
The invention provides a preparation method of a novel polysaccharide conjugate vaccine treating a PEG heterobifunctional reagent as a conjugation bridge. A meningococcal polysaccharide conjugate vaccine is exploited through the method. The method has the following advantages: 1, the respective self-crosslinking of polysaccharides and proteins in traditional methods is avoided, the yield is improved, and the quality control is benefited; and 2, a long PEG chain can increase the distance between the polysaccharides and the proteins, reduce the mutual space shield effect between the polysaccharides and the proteins, and improve the immunogenicity of the polysaccharide conjugate vaccine.
Owner:华兰生物疫苗股份有限公司 +1
Bacterial polysaccharide protein conjugate vaccine using hepatitis B surface antigen as carrier protein and preparation method of bacterial polysaccharide protein conjugate vaccine
InactiveCN104383532AAddressing Immunization IssuesPlay a role in preventionAntibacterial agentsAntiviralsAntigenConjugate vaccine
The invention discloses a bacterial polysaccharide protein conjugate vaccine using a hepatitis B surface antigen as carrier protein and a preparation method of the bacterial polysaccharide protein conjugate vaccine. According to the vaccine, protein is the hepatitis B surface antigen, and a bacterial polysaccharide is selected from any one or more of a haemophilus influenza type b polysaccharide, group A, group C, group Y and group W135 meningococcal polysaccharides, a salmonella typhi type Vi polysaccharide, a group B streptococcus type Ia polysaccharide and the like, pneumococcus serotype type 1, 2 and the like, and salmonella paratyphi type A or salmonella paratyphi type B. Animal experiments show that the antibody positive conversion rates of the bacterial polysaccharide and the hepatitis B surface antigen in the vaccine are both more than 85%, so that the vaccine is relatively high in antibody positive conversion rate; carrier protein plays a role in transforming the bacterial polysaccharide from a T-cell-independent antigen into a T-cell-dependent antigen, and also can be used for preventing diseases caused by hepatitis B virus; and by adopting the bacterial polysaccharide protein conjugate vaccine disclosed by the invention, the problem of performing immunization inoculation on infants and young children under 2 years old can be solved, the function of one injection with multiple immune effects also can be achieved, and the use crowd and coverage rate of the vaccine can be expanded.
Owner:云南沃森生物技术股份有限公司
Meningitis polysaccharide conjugate vaccine and preparing method thereof
InactiveCN103690944AImproving immunogenicityRelieve painAntibacterial agentsCarrier-bound antigen/hapten ingredientsConjugate vaccineEpitope
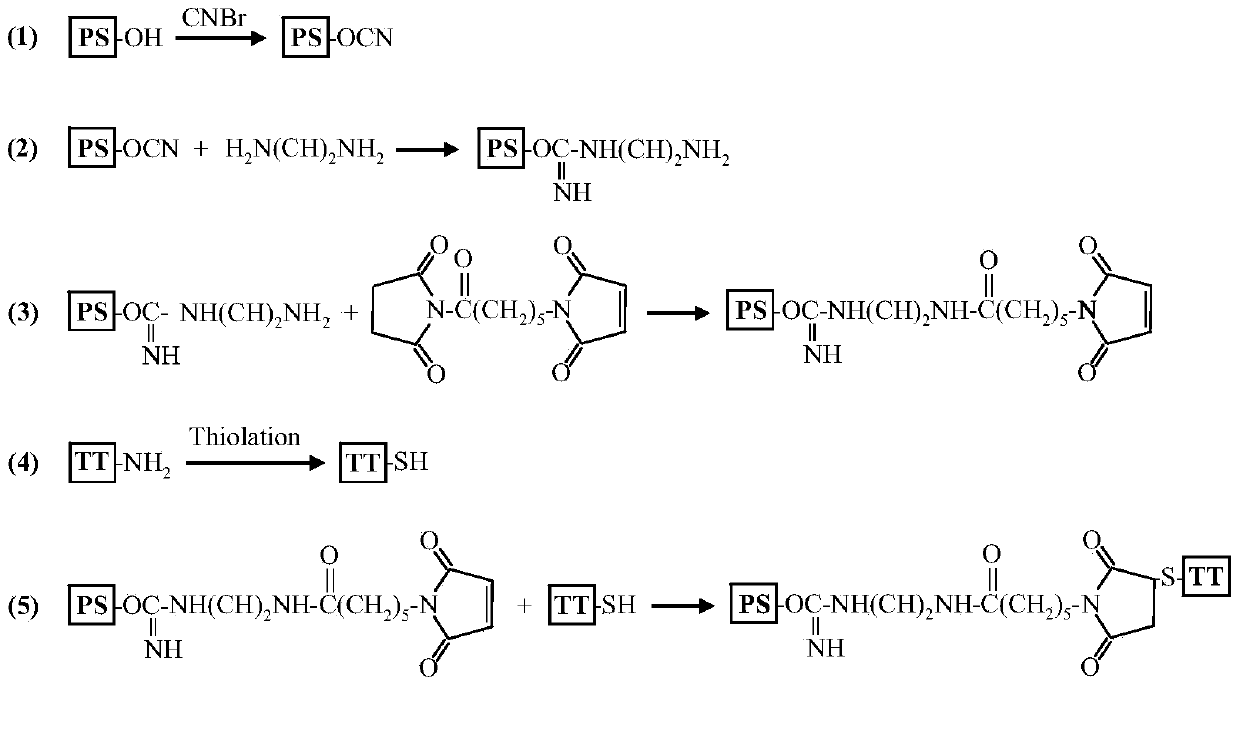
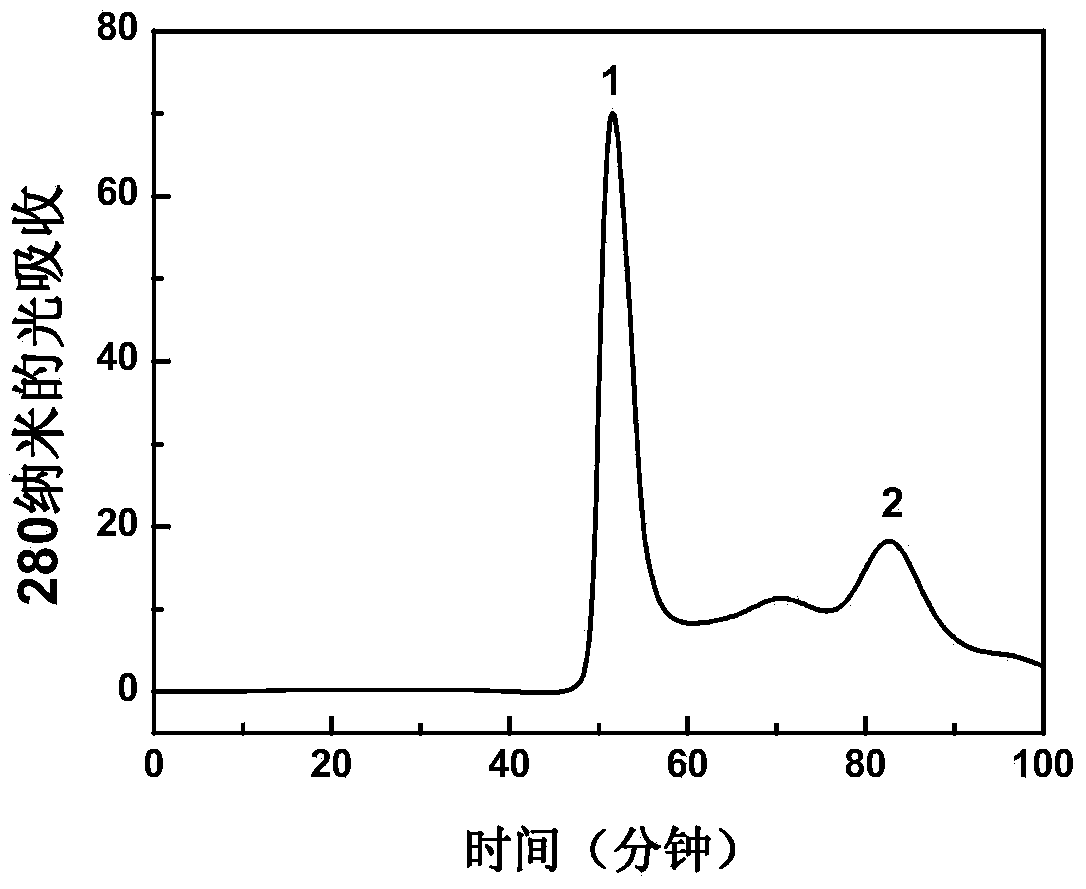
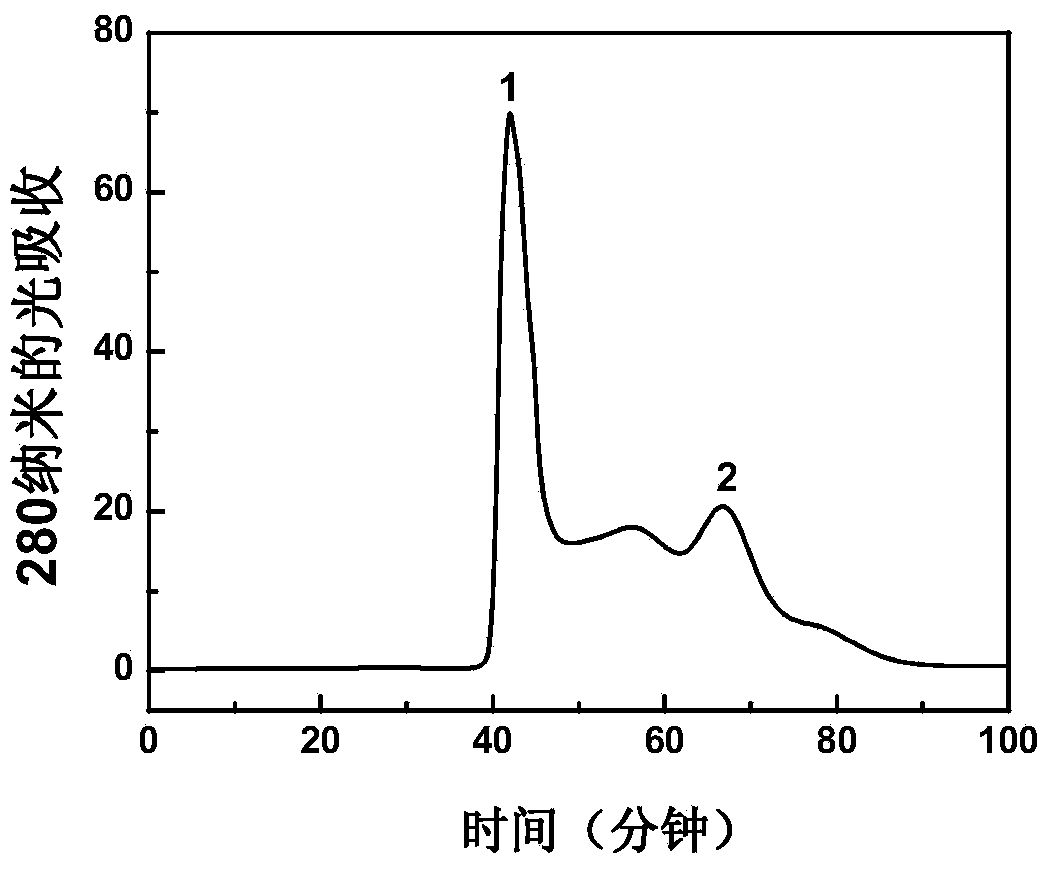
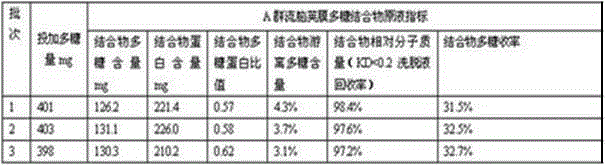
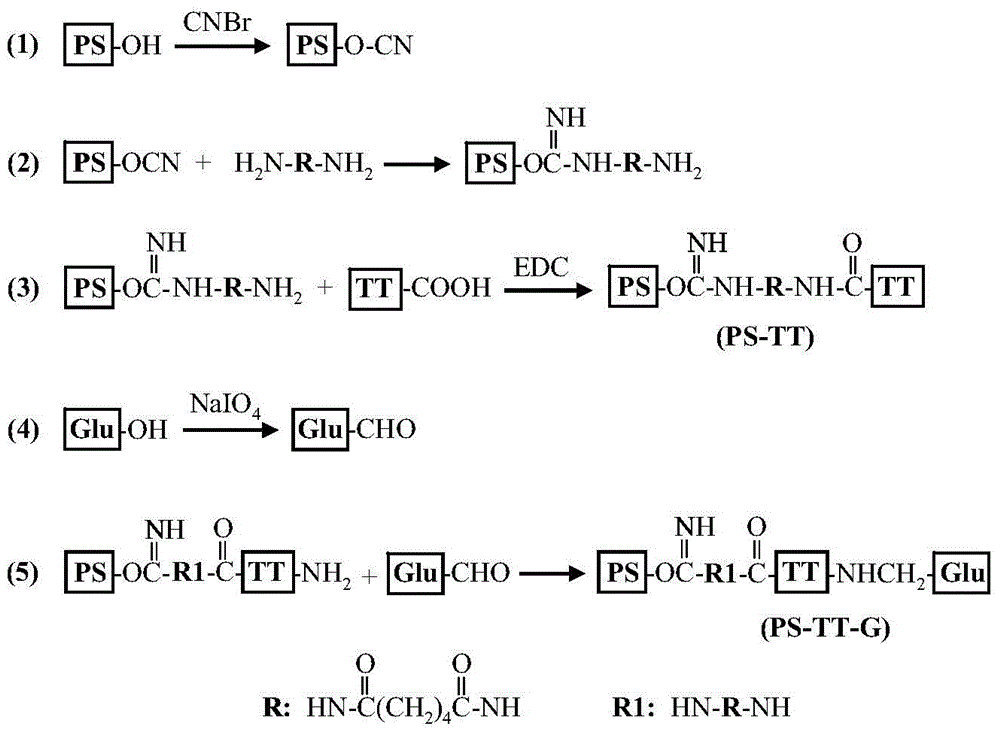
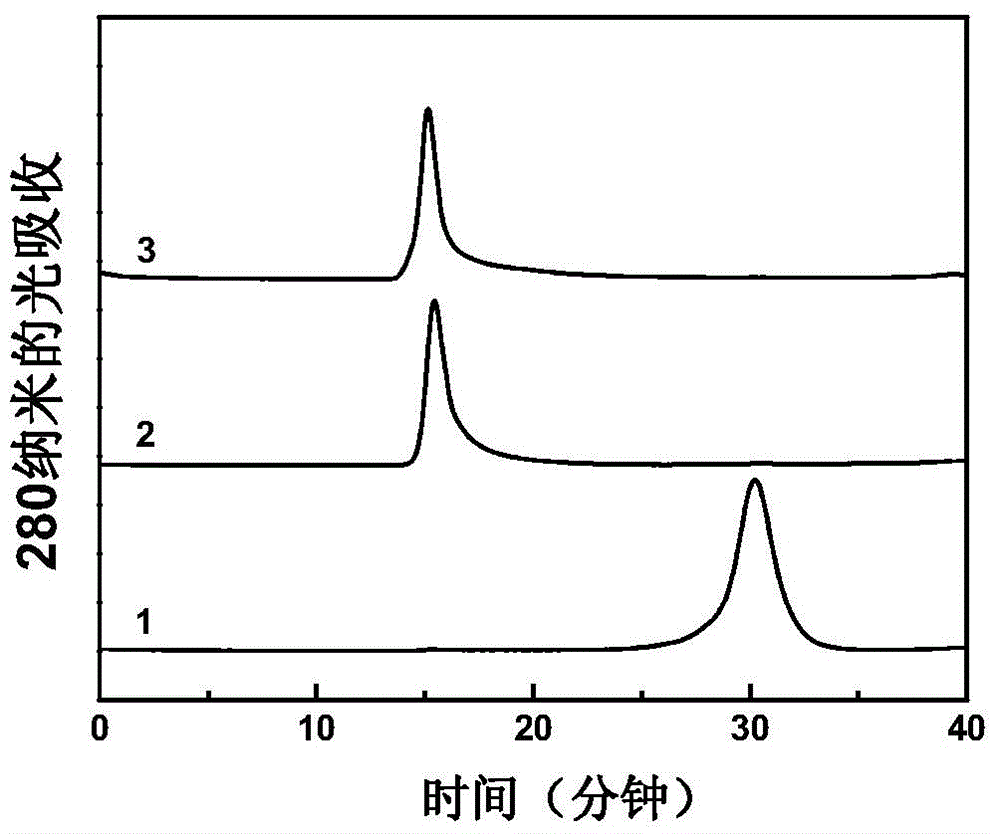
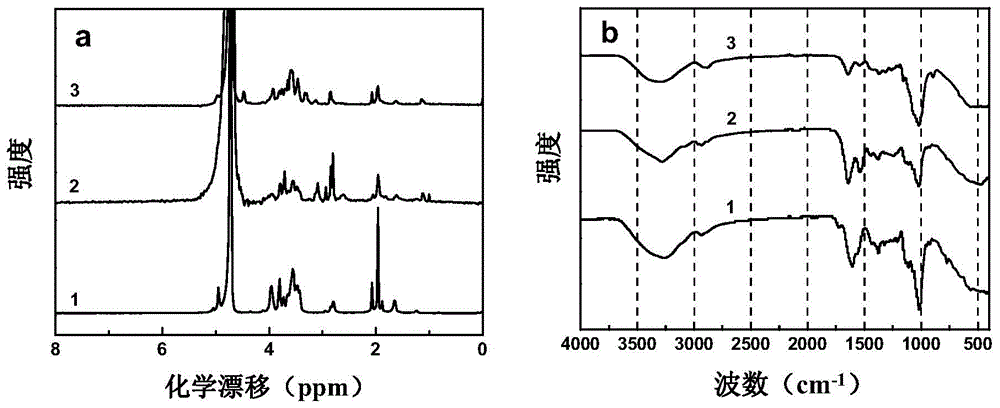
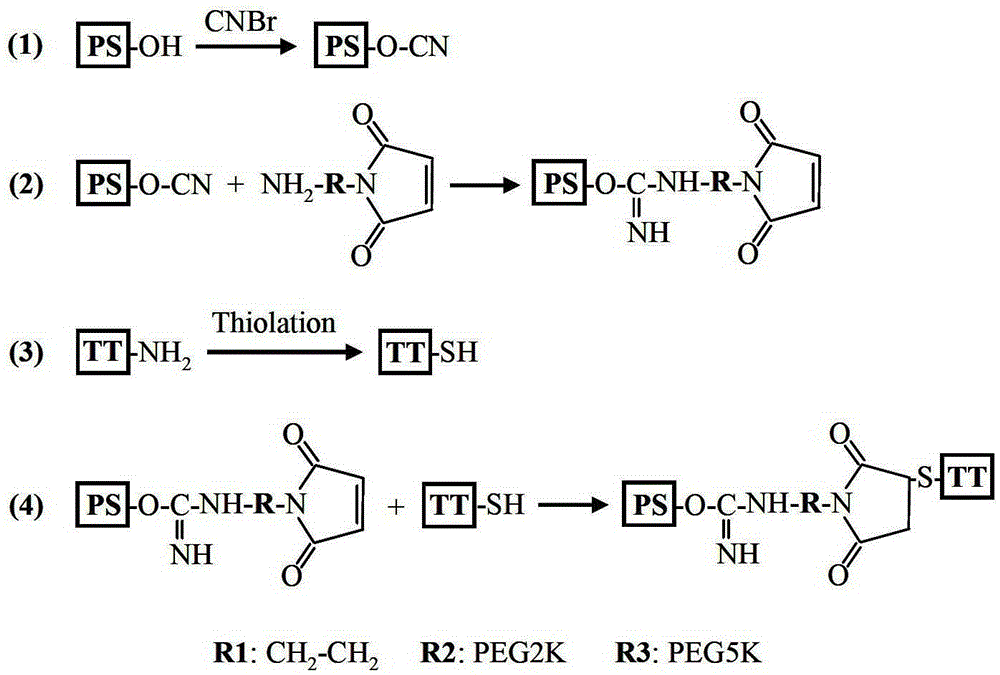
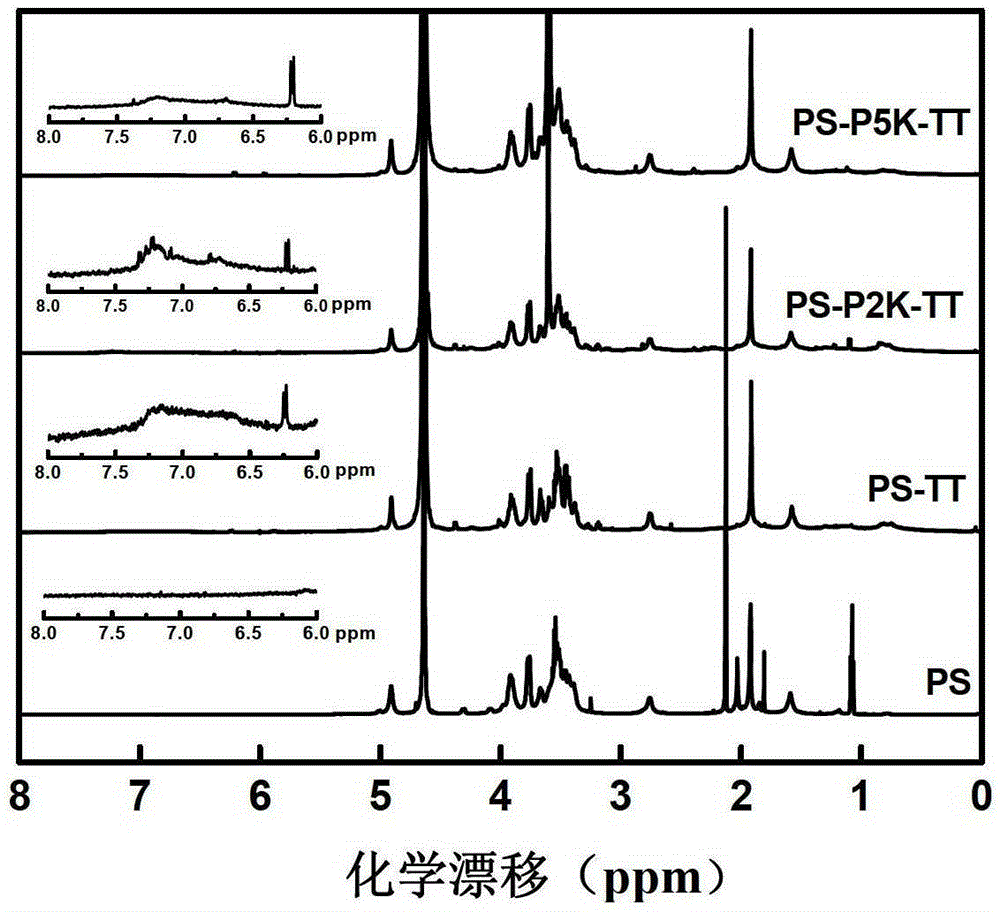
The invention describes a method for preparing a meningitis polysaccharide conjugate vaccine. The meningitis polysaccharide conjugate vaccine is developed based on the method. The method comprises the following steps: (1) activating meningococcus polysaccharides by cyanogen bromide, then deriving the meningococcus polysaccharides which are activated by the cyanogen bromide by ethanediamine, finally deriving the polysaccharides by a reagent which is of a structure of succinimidyl ester-R-maleimide; (2), sulfhydrylating carrier proteins; (3) combining the derived meningococcus polysaccharides with the sulfhydrylated carrier proteins. As conjugation bridges between the meningococcus polysaccharides and the carrier proteins are very long, the spatial shielding effect of the carrier proteins on the antigenic epitopes of the polysaccharides is reduced, and the original immunization property of the polysaccharide conjugate vaccine is improved.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for preparing group A meningococcal capsular polysaccharide conjugate vaccine
InactiveCN105056228AAvoid steric hindranceMolecular chain lengthAntibacterial agentsCarrier-bound antigen/hapten ingredientsTetanus toxoidsCarrier protein
The invention relates to a method for preparing group A meningococcal capsular polysaccharide conjugate vaccine. The method includes the following steps that group A meningococcal capsular polysaccharide is diluted, activated and coupled into a group A meningococcal polysaccharide-sebacic dihydrazide derivative together with sebacic dihydrazide; then the group A meningococcal polysaccharide-sebacic dihydrazide derivative and a tetanus toxoid solution are subjected to a catalytic reaction through carbodiimide, so that group A meningococcal capsular polysaccharide and carrier protein conjugate is obtained. According to the method, the sebacic dihydrazide is used as a coupling agent in the preparation process of the group A meningococcal conjugate vaccine; compared with adipic dihydrazide, the sebacic dihydrazide has two more methylenes on carbon chains, molecular chains are longer, the steric hindrance of biomacromolecules can be better resisted, and the conjugation yield is increased.
Owner:CHENGDU OLYMVAX BIOPHARM
Beta-glucan modified meningitis polysaccharide conjugate vaccine and preparation method thereof
ActiveCN104548090AImproving immunogenicityReduce the number of vaccinationsAntibacterial agentsCarrier-bound antigen/hapten ingredientsCyanogen bromideMeningococcal meningitis
The invention relates to a beta-glucan modified meningitis polysaccharide conjugate vaccine and a preparation method thereof. The preparation method of the beta-glucan modified meningitis polysaccharide conjugate vaccine comprises the following steps: (1) activating meningococcus polysaccharide by cyanogen bromide, and then deriving by adopting adipic dihydrazide; (2) combining a derived meningococcus polysaccharide derivative with carrier protein; (3) activating beta-glucan; and (4) modifying polysaccharide-protein conjugate by the activated beta-glucan. By virtue of the steps, a novel and efficient meningitis polysaccharide conjugate vaccine can be prepared and can be used for preventing infection caused by epidemic cerebrospinal meningitis Neisseria gonorrhoeae.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Immunogenic composition
ActiveUS9198977B2Antibacterial agentsBacterial antigen ingredientsSalmonella serotype typhiCarrier protein
The invention provides immunogenic polysaccharide protein conjugates comprising capsular polysaccharides from N. Meningitidis serogroup X and methods for preparation thereof. The present invention relates to N. meningitidis X saccharide-carrier protein conjugates prepared by a conjugation reaction. Accordingly, the instant invention relates to multivalent meningococcal polysaccharide protein conjugate composition comprising capsular saccharide from serogroups X and at least one capsular saccharide from A, C, W135 and Y wherein, i) polysaccharides A C W135 X are sized mechanically whereas polysaccharide Y is sized chemically, ii) all saccharide are conjugated to carrier protein via a linker with a cyanylation conjugation chemistry iii) all saccharide to protein ratios in final conjugates are between 0.2-0.6 and iv) at least two different carrier proteins selected from the group consisting of TT, DT and CRM197 are utilized.
Owner:SERUM INST OF INDIA PTE LTD
Process for refining group C/Y/W135 meningococcal polysaccharides
ActiveCN102911285AProtein content controlLow in proteinAntibacterial agentsAntibody medical ingredientsMeningococcal carriageMENINGOCOCCAL POLYSACCHARIDE
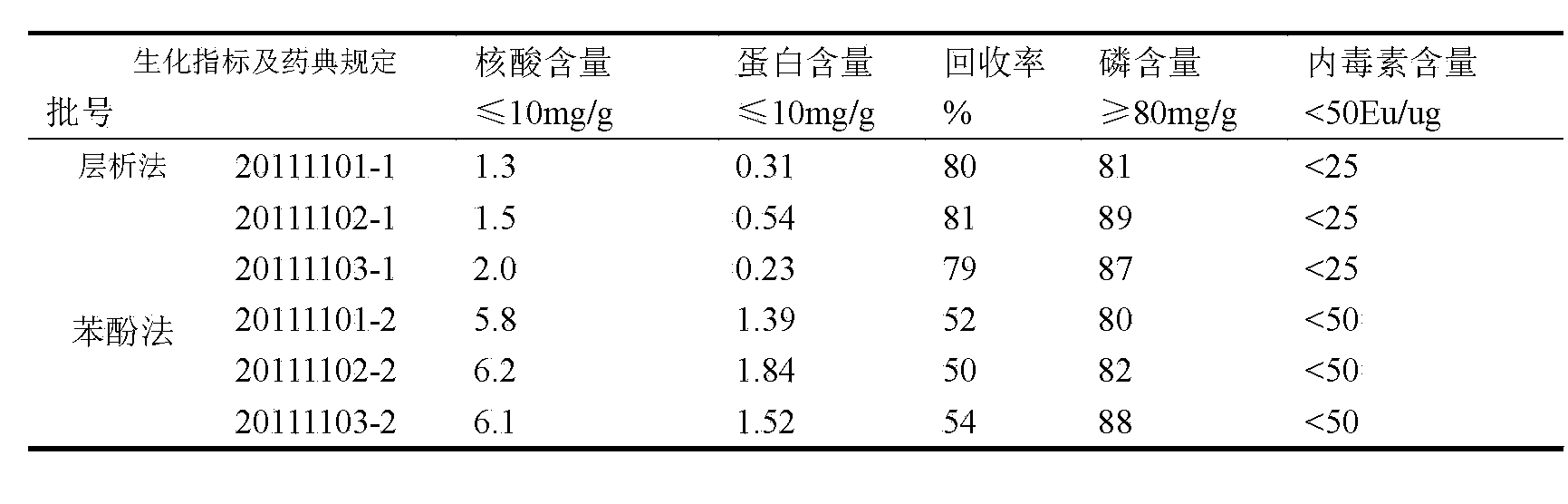
The invention discloses a process for refining group C / Y / W135 meningococcal polysaccharides. The process includes dissolving one of a coarse group C meningococcal polysaccharide, a coarse group Y meningococcal polysaccharide and a coarse group W135 meningococcal polysaccharide, displacing the polysaccharide into an equilibration buffer solution, feeding a collected polysaccharide to an anion exchange column, using an eluant to elute absorption substances from the anion exchange column, piecewise collecting absorption peaks with wavelength smaller than 206 according to different elution peaks and using a desalting method to remove micromolecule materials. According to the process for refining the group C / Y / W135 meningococcal polysaccharides, protein contents can be effectively controlled, so that the polysaccharide protein content is far lower than the polysaccharide protein content in a cold phenol method, the recovery rate can reach around 80%, and the process is applicable to mass production with 100g of coarse polysaccharide yield per batch.
Owner:罗益(无锡)生物制药有限公司
Multivalent meningococcus preparation box, vaccine preparation and preparation method thereof
ActiveCN104958759AConvenient and quick content determinationFreeze-drying process optimizationAntibacterial agentsPowder deliveryFreeze-dryingMeningococcal carriage
The invention relates to a multivalent meningococcus product which comprises a freeze-dried component and a liquid component. The freeze-dried component and the liquid component each comprise one or two or three kinds of A, C, Y and W135 group meningococcal polysaccharides, and the same meningococcal polysaccharides exist in the freeze-dried component and the liquid component at different times. In the clinic using process, the freeze-dried component and the liquid component are mixed to obtain the multivalent meningococcus preparation. The multivalent meningococcus product can stably keep the polysaccharide antigen activity, easily detect the effective antigen components in the preparation and control the preparation quality conveniently; in addition, the preparation technology is easy, and the multivalent meningococcus product capable of being stably preserved and high in antigen activity can be obtained by optimizing the polysaccharide antigen freeze-drying technology and the dissolution conditions.
Owner:CANSINO BIOLOGICS INC
Method for determining content of polysaccharide of each group of meningococcus polysaccharide conjugate vaccine finished products
ActiveCN102809655AEliminate the effects ofAccurate measurementBiological testingCarrier proteinMENINGOCOCCAL POLYSACCHARIDE
The invention relates to a method for determining the content of the polysaccharide of each group of meningococcus polysaccharide conjugate vaccine finished products and belongs to the technical field of biology. The method comprises the following steps of: treating by using protease K during preparation of a detection product sample, wherein the total size of an enzymolysis reaction system is 420 mu l; and by using the total size as reference, adding the protease K, a protease K buffer solution which occupies one tenth of the total size and an ultra-filtration concentrated solution which occupies one sixth of the total size, uniformly mixing the protease K, the protease K buffer solution and the ultra-filtration concentrated solution, and then incubating, wherein the addition amount of the protease K is two to eight times of the content of proteins in the enzymolysis reaction system. By adoption of the method, influence of each group of conjugate carrier proteins on immunoelectrophoresis is eliminated, an appropriate detection system for detecting the polysaccharide of each of more than four groups of meningococcus polysaccharide conjugate vaccine finished products is established, and the content of the polysaccharide of each of more than four groups of meningococcus polysaccharide conjugate vaccine finished products can be accurately and quickly determined. The method has the characteristics of high durability, high accuracy and high precision. A method for evaluating the quality of meningococcus polysaccharide conjugate vaccine is established.
Owner:YUXI WALVAX BIOTECH CO LTD
Polysaccharide combined vaccine for epidemic encephalitis and preparation method thereof
InactiveCN101428144ALow endotoxin contentRelieve painAntibacterial agentsPowder deliveryDiseaseFreeze-drying
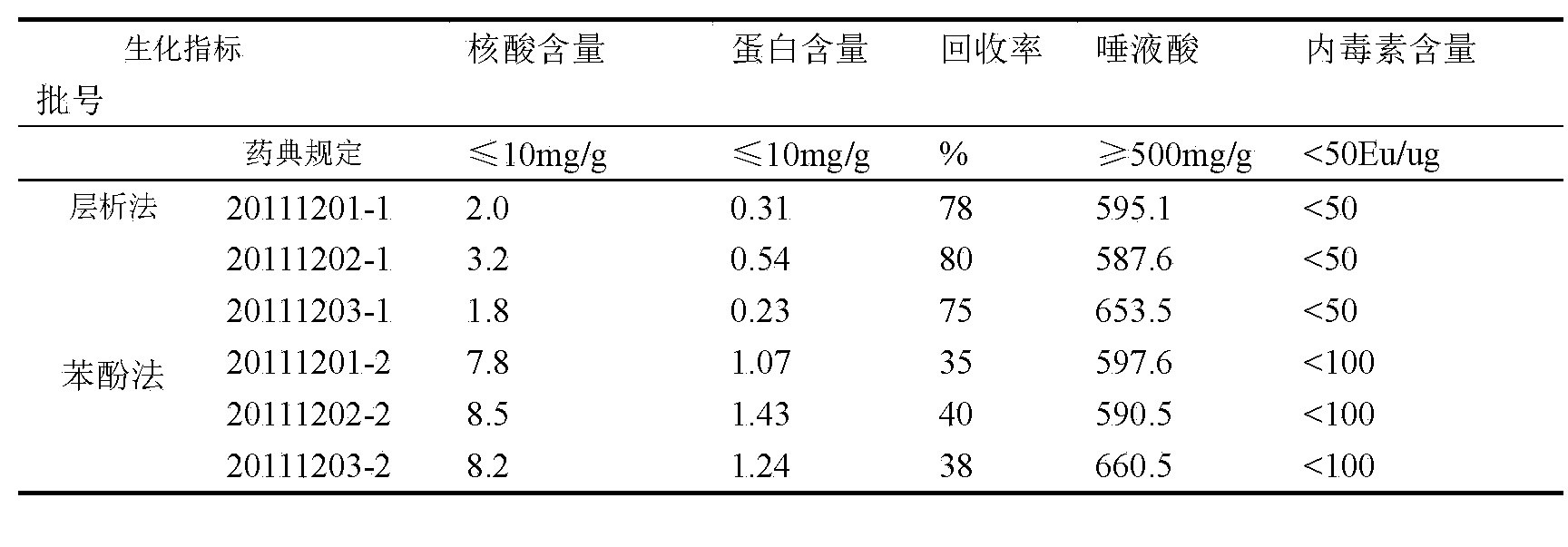
The invention provides an epidemic meningitis polysaccharide combined vaccine, and aims to prepare a freeze-dried ACYW135 meningococcal polysaccharide vaccine by the process of removing endotoxins by the ultracentrifugation method so as to ensure that the content of endotoxins present in ACYW135 group meningococcal polysaccharide stock solution is far lower than that before the centrifugation. The vaccine provided by the invention has the advantages of ensuring that only one injection is enough to prevent a patient from being infected with epidemic meningitis disease, achieving, if not greater, as much preventive effect as four injections initially, alleviating the suffering of the patient, and reducing the prevention cost.
Owner:长春长生生物科技股份有限公司
Preparation method of group A/C meningococcal polysaccharide
InactiveCN106367451AAvoid purification processReduce usageMicroorganismsMicroorganism based processesPurification methodsUltrafiltration
The invention discloses a preparation method of group A / C meningococcal polysaccharide. The preparation method comprises the following steps: (1) carrying out fermentation culture on meningococcus, and carrying out sterilization treatment, thus obtaining a sterilized culture solution; (2) preparing meningococcus capsular polysaccharide; and (3) dissolving the rough polysaccharide, carrying out chromatography elution, collecting target peaks containing the polysaccharide, carrying out desalting on the collected polysaccharide solution by adopting an ultrafiltration membrane and adopting water for injection as an ultrafiltrate, thus obtaining the desalted solution, namely, the group A / C meningococcal polysaccharide solution. According to the preparation method, firstly, sterilization treatment is carried out, so that the inactivated culture solution is obtained, then, the capsular polysaccharide is prepared, and then, the capsular polysaccharide is purified by adopting the chromatography method; the use of abundant phenol in the traditional purification method is avoided, on the basis that the purification quality is guaranteed, the destruction of phenol to the environment is effectively avoided, meanwhile, the recovery rate of polysaccharide is approximate to that of the traditional phenol extraction method, in addition, the operation is simple, and the preparation method is suitable for large-scale production.
Owner:CHENGDU OLYMVAX BIOPHARM
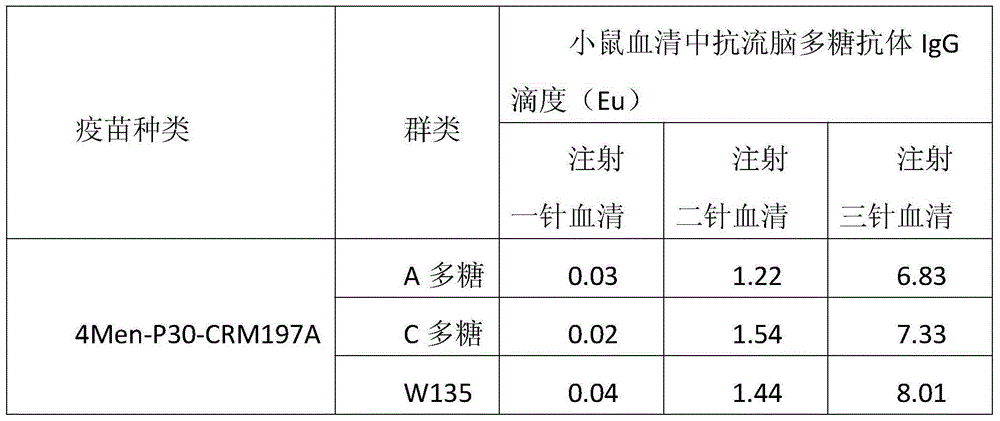
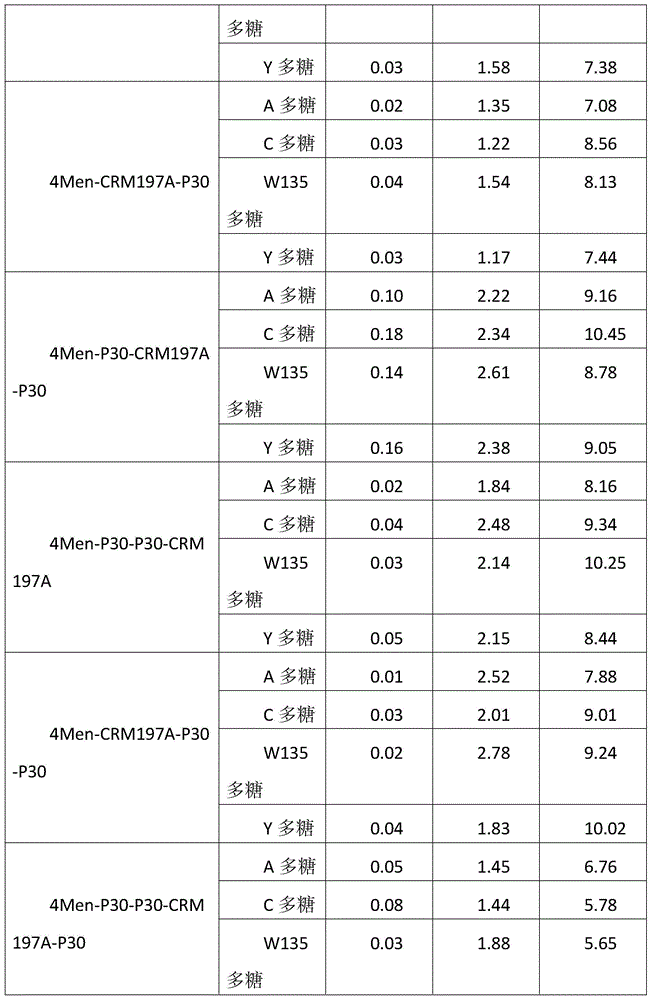
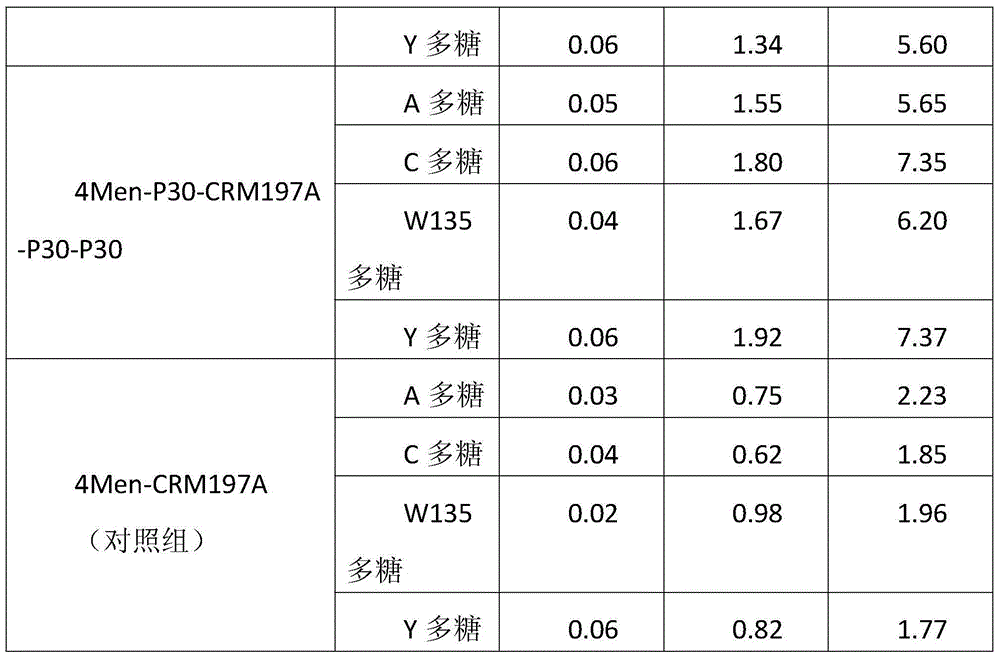
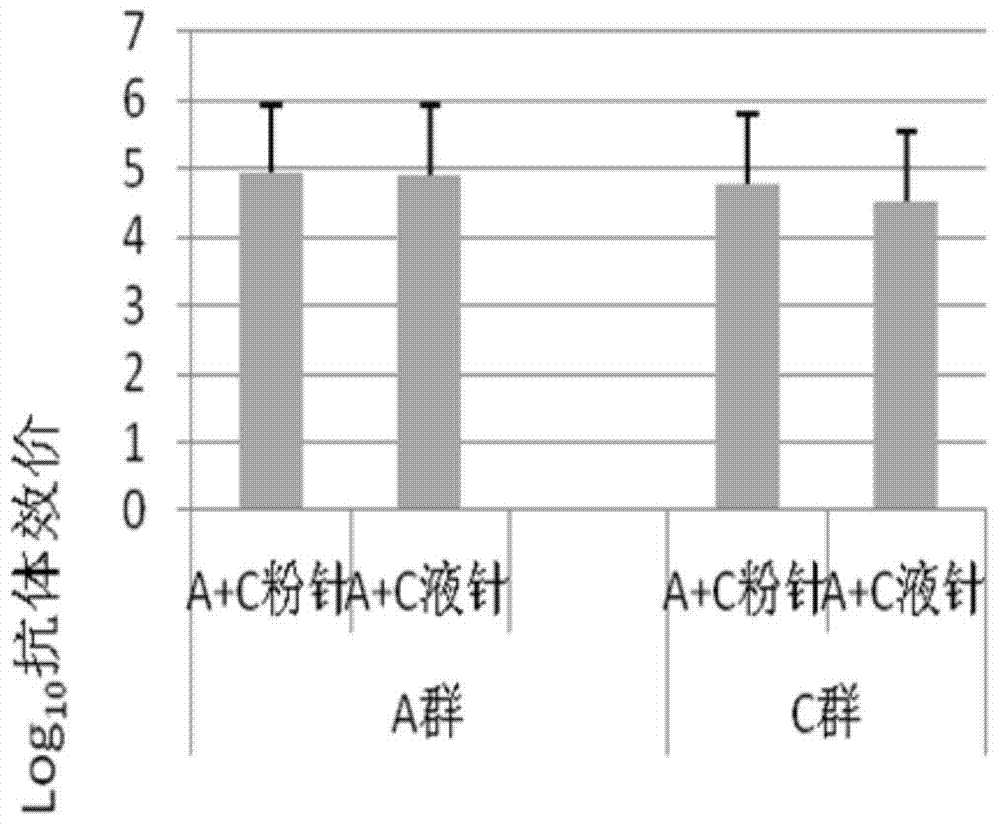
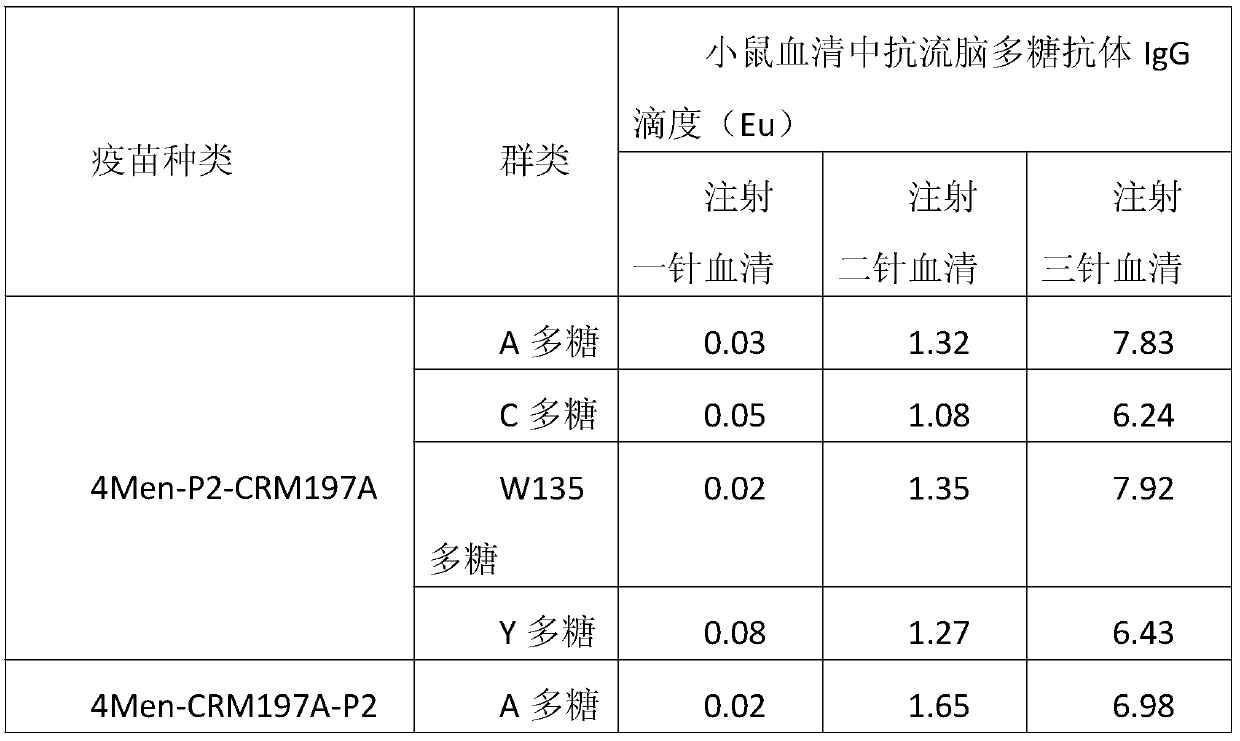
Method for enhancing 4-valent epidemic meningococcal polysaccharide protein bonder immunogenicity
ActiveCN104096226AImproving immunogenicityHigh titerAntibacterial agentsCarrier-bound antigen/hapten ingredientsSerum igeEpitope
The invention discloses a method for enhancing 4-valent epidemic meningococcal polysaccharide protein bonder immunogenicity, and the method is as follows: adding all-powerful epitope peptide (P30) into CRM197A (A chain of diphtheria toxin variant 197), using genetic recombinant Escherichia coli to produce a P30-containing protein carrier P30CRM197A of the A chain of the diphtheria toxin variant 197 (CRM197); and connecting 4 different serogroups (comprising A, C, W135 and Y) capsular polysaccharides to the P30-containing protein carrier P30CRM197A by covalent bonds to form a 4-valent epidemic meningococcal polysaccharide-P30CRM197A bonder; compared with a 4-valent epidemic meningococcal polysaccharide-CRM197A bonder obtained by a corresponding protein carrier CRM197A which does not contain the all-powerful epitope peptide (P30), the immunogenicity of the 4-valent epidemic meningococcal polysaccharide-P30CRM197A bonder obtained by the method is increased by 3-5 times than that of a contrast.
Owner:KANVAX BIOPHARM
ACY W135 group meningococcal polysaccharide type-b haemophilus influenzae conjugate vaccine
InactiveCN101590226ASuitable for reproduction and growthLow endotoxin contentAntibacterial agentsBacterial antigen ingredientsDiseaseConjugate vaccine
The invention provides an ACY W135 group meningococcal polysaccharide type-b haemophilus influenzae conjugate vaccine. A frozen-dry ACY W135 meningococcal polysaccharide vaccine is prepared by using the technology of removing endotoxin through ultracentrifugation so as to ensure that the endotoxin content of ACY W135 group meningococcal polysaccharide stock solution is greatly reduced before centrifugation. The conjugate vaccine can prevent disease infection of epidemic encephalitis for a patient by one dosage of injection, reaches and exceeds the preventing effect of the prior five dosages of injection, reduces the pain of the patient, and lowers the prevention cost.
Owner:长春长生生物科技股份有限公司
Group-A and group-C meningococcus polysaccharide-adsorption diphtheria tetanus combined vaccine and production process thereof
InactiveCN109513001ALower titerReduce morbidityAntibacterial agentsBacterial antigen ingredientsDiseaseTetanus toxoids
The invention provides a group-A and group-C meningococcus polysaccharide-adsorption diphtheria tetanus combined vaccine and a production process thereof. The combined vaccine contains an adsorption diphtheria tetanus vaccine and a group-A and group-C meningococcus polysaccharide vaccine which are respectively contained in two reagent bottles, wherein the content of diphtheria toxoid in each 1ml of the adsorption diphtheria tetanus vaccine is below 20Lf, the content of tetanus toxoid is below 3Lf, the content of aluminum hydroxide is below 3mg, and the content of sodium chloride is 7.5mg-9mg;and each human dose, namely 0.5ml of the group-A and group-C meningococcus polysaccharide vaccine contains 50 micrograms of group-A meningococcus polysaccharide, 50 micrograms of group-C meningococcuspolysaccharide vaccine and 8mg of lactose. The combined process is mainly used for preventing multiple high-incidence diseases of children of 5 and 6 years old through one injection. The invention further provides the production process of the group-A and group-C meningococcus polysaccharide-adsorption diphtheria tetanus combined vaccine.
Owner:LIAONING MAOKANGYUAN BIO TECH CO LTD +1
Method for detecting content of each group of free polysaccharide in meningococcus polysaccharide conjugate vaccine finished product
ActiveCN102809656BSimple and fast operationEasy to operateBiological testingMENINGOCOCCAL POLYSACCHARIDE CONJUGATESodium acetate
The invention relates to a method for measuring the free polysaccharide content of each group of meningococcal polysaccharide-conjugated vaccine finished products, belonging to the field of biotechnology. In this method, proteinase K treatment is carried out in the preparation of the test product, the amount of proteinase K added is 2 to 8 times the protein content in the enzymatic hydrolysis reaction system, and cold phenol treatment is used to separate the proteinase K with saturated cold phenol solution of sodium acetate. Combined polysaccharides and free polysaccharides in the conjugate are combined with immunoelectrophoresis detection technology to measure the content of free polysaccharides in each group of the finished conjugated vaccine. The method of the invention eliminates the influence of carrier proteins of each group of conjugates on electrophoretic mobility and other related factors in immunoelectrophoresis, effectively separates the bound polysaccharides and free polysaccharides in the conjugates, and establishes a method for detecting the combination of polysaccharides of more than 4 groups of meningococci. The suitable detection system for the free polysaccharides of each group of vaccine products has the characteristics of strong durability, high accuracy and precision. A method for evaluating the quality of finished vaccines with more than 4 groups of meningococcal polysaccharide conjugate vaccines has been established.
Owner:云南沃森生物技术股份有限公司
Method for enhancing 4-valent epidemic meningococcal polysaccharide protein bonder immunogenicity
The invention discloses a method for enhancing 4-valent epidemic meningococcal polysaccharide protein bonder immunogenicity, and the method is as follows: adding all-powerful epitope peptide (P2) into CRM197A (A chain of diphtheria toxin variant 197), using genetic recombinant Escherichia coli to produce a P2-containing protein carrier P2CRM197A of the A chain of the diphtheria toxin variant 197 (CRM197); and connecting 4 different serogroups (comprising A, C, W135 and Y) capsular polysaccharides to the P2-containing protein carrier P2CRM197A by covalent bonds to form a 4-valent epidemic meningococcal polysaccharide-P2CRM197A bonder; compared with a 4-valent epidemic meningococcal polysaccharide-CRM197A bonder obtained by a corresponding protein carrier CRM197A which does not contain the all-powerful epitope peptide (P2), the immunogenicity of the 4-valent epidemic meningococcal polysaccharide-P2CRM197A bonder obtained by the method is increased by 3-5 times than that of a contrast.
Owner:KANVAX BIOPHARM
Meningitis polysaccharide conjugate vaccine with heterogenic dual-functional reagent as connecting bridge and preparation method of meningitis polysaccharide conjugate vaccine
InactiveCN104815326AAvoid self-couplingHigh yieldAntibacterial agentsCarrier-bound antigen/hapten ingredientsMENINGOCOCCAL POLYSACCHARIDE CONJUGATEHeterobifunctional reagent
The invention provides a preparation method of a novel polysaccharide conjugate vaccine treating a PEG heterobifunctional reagent as a conjugation bridge. A meningococcal polysaccharide conjugate vaccine is exploited through the method. The method has the following advantages: 1, the respective self-crosslinking of polysaccharides and proteins in traditional methods is avoided, the yield is improved, and the quality control is benefited; and 2, a long PEG chain can increase the distance between the polysaccharides and the proteins, reduce the mutual space shield effect between the polysaccharides and the proteins, and improve the immunogenicity of the polysaccharide conjugate vaccine.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +2
Method for purifying A-group and C-group meningococcus polysaccharides
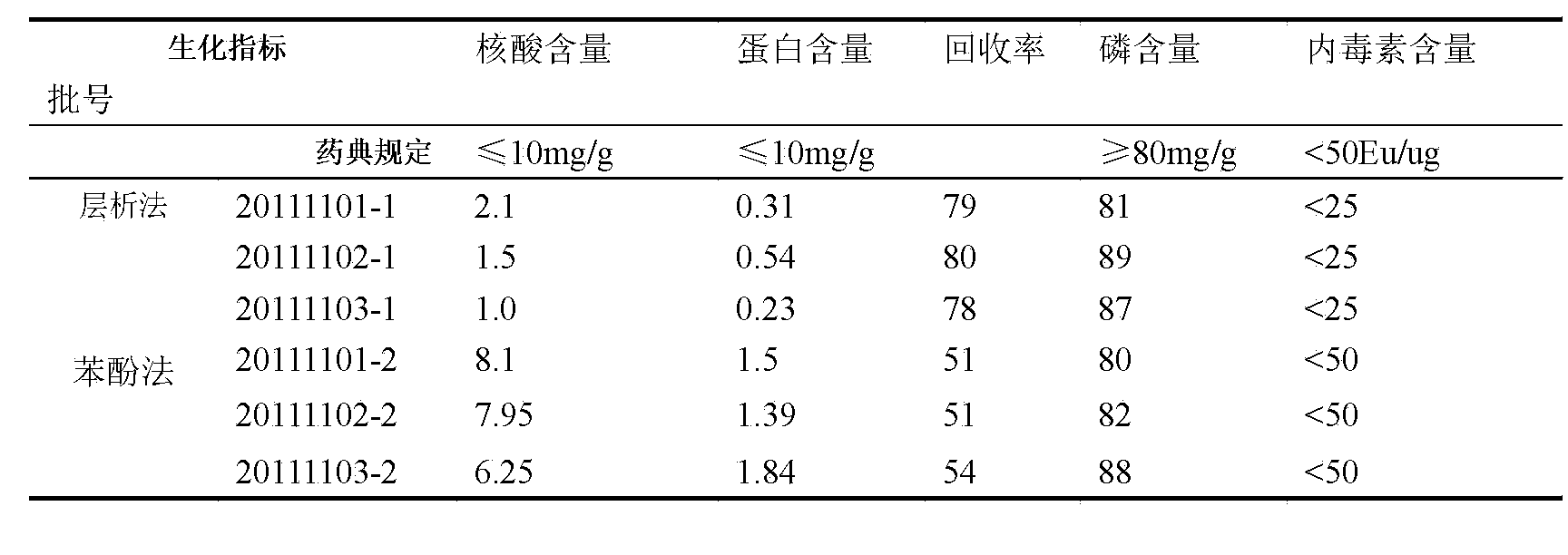
The invention discloses a method for purifying A-group and C-group meningococcus polysaccharides. The method comprises the following steps: (1) carrying out fermentation culture on meningococcus, and carrying out sterilizing treatment, so as to obtain a sterilized culture solution; (2) preparing meningococcus capsular polysaccharides; (3) carrying out chromatographic eluting on a crude prepared polysaccharide solution, collecting polysaccharide containing target peaks, carrying out desalting on the collected polysaccharide solution by using an ultrafiltration membrane pack in a manner of taking water for injection as ultrafiltrate, so as to obtain a desalted solution, i.e., an A-group and C-group meningococcus polysaccharide solution, wherein a buffer solution A is prepared from 20mM Tris-HCl and 0.5% sodium deoxycholate and has the pH of 8.0, and a buffer solution B is prepared from 20mM Tris-HCl and has the pH of 8.0. According to the method, chromatography is adopted creatively, so that the large-amount consumption of phenol in the traditional purification methods is avoided, and the environmental disruption caused by the phenol is effectively avoided on the basis of guaranteeing the quality of purifying; the polysaccharide recovery ratio is close to that of the traditional phenol extraction methods, and the operation is simple, so that the method is applicable to large-scale production.
Owner:CHENGDU OLYMVAX BIOPHARM
Multivalent meningococcal preparation kit, vaccine preparation and preparation method thereof
ActiveCN104958759BEasy to detectStable maintenance of effective antigenic activityAntibacterial agentsPowder deliveryFreeze-dryingMeningococcal carriage
Owner:CANSINO BIOLOGICS INC
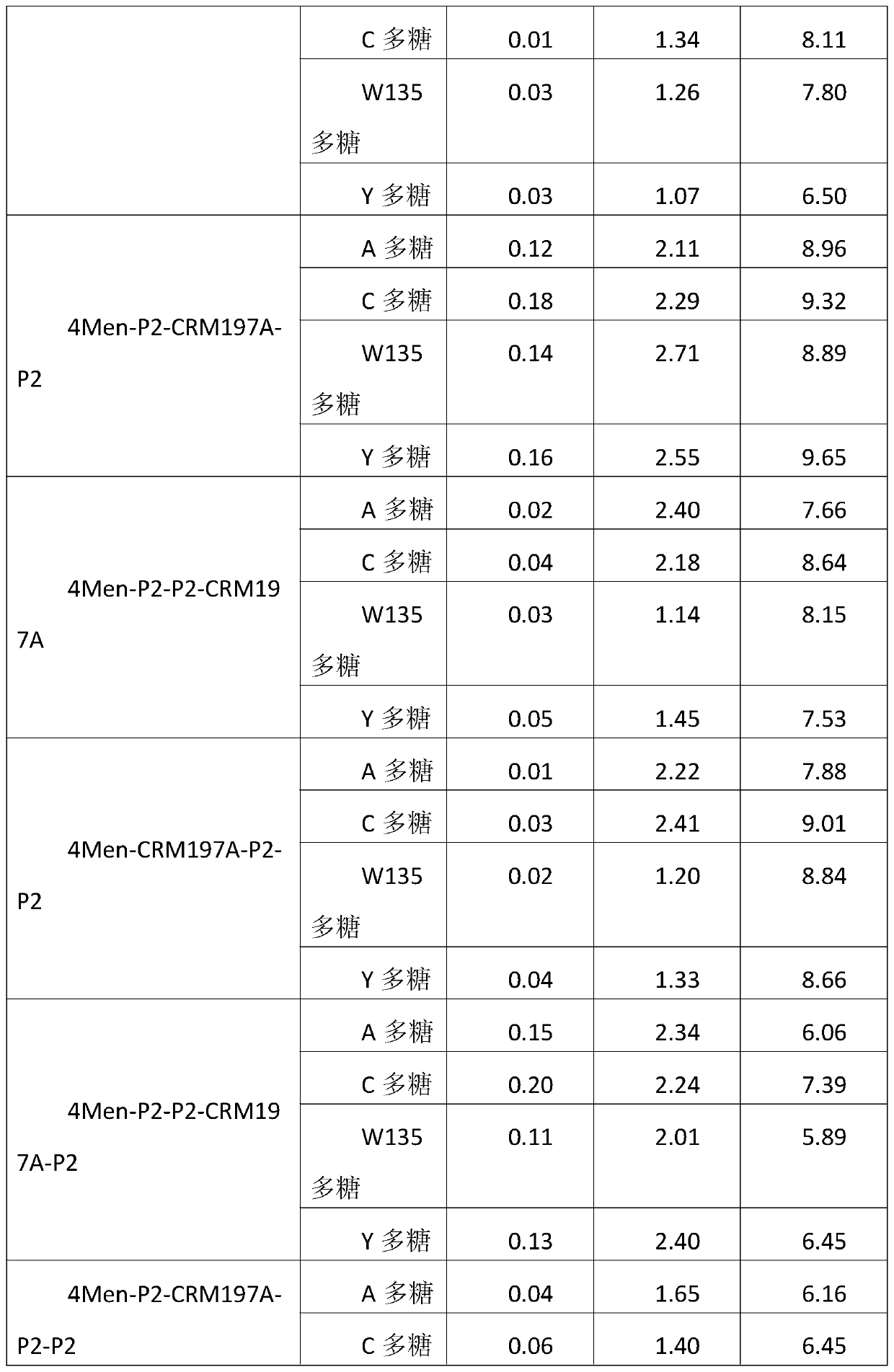
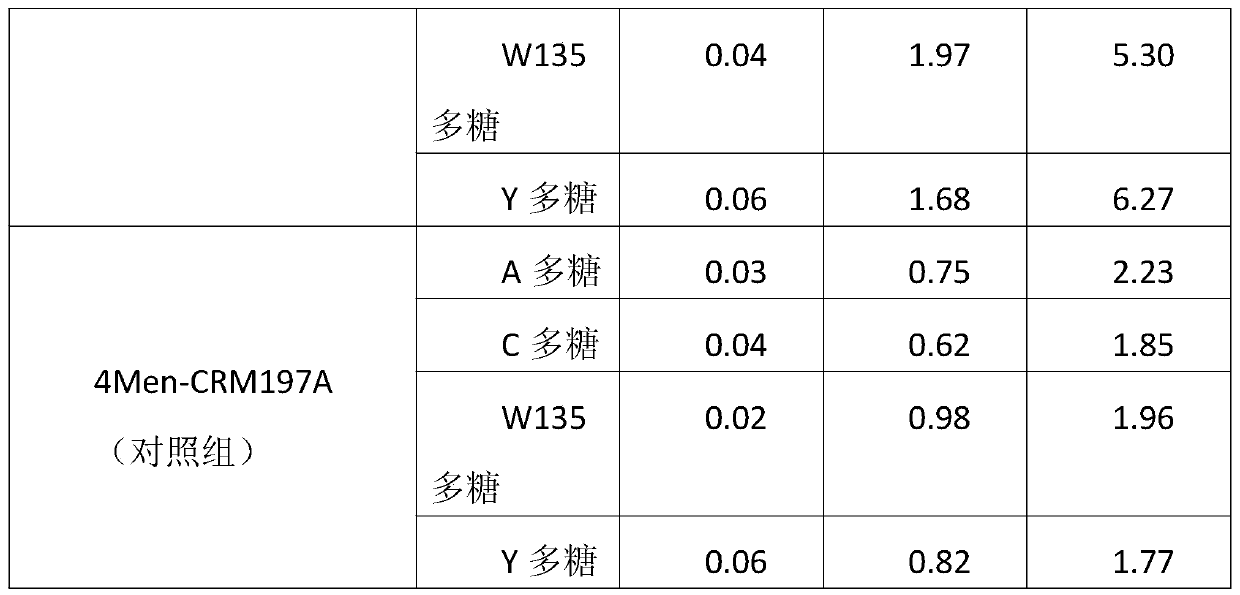
A method for enhancing the immunogenicity of a 4-valent meningococcal polysaccharide-protein conjugate
ActiveCN104096227BAntibacterial agentsCarrier-bound antigen/hapten ingredientsEscherichia coliMENINGOCOCCAL POLYSACCHARIDE
The invention discloses a method for enhancing 4-valent epidemic meningococcal polysaccharide protein bonder immunogenicity, and the method is as follows: adding all-powerful epitope peptide (P2) into CRM197A (A chain of diphtheria toxin variant 197), using genetic recombinant Escherichia coli to produce a P2-containing protein carrier P2CRM197A of the A chain of the diphtheria toxin variant 197 (CRM197); and connecting 4 different serogroups (comprising A, C, W135 and Y) capsular polysaccharides to the P2-containing protein carrier P2CRM197A by covalent bonds to form a 4-valent epidemic meningococcal polysaccharide-P2CRM197A bonder; compared with a 4-valent epidemic meningococcal polysaccharide-CRM197A bonder obtained by a corresponding protein carrier CRM197A which does not contain the all-powerful epitope peptide (P2), the immunogenicity of the 4-valent epidemic meningococcal polysaccharide-P2CRM197A bonder obtained by the method is increased by 3-5 times than that of a contrast.
Owner:KANVAX BIOPHARM
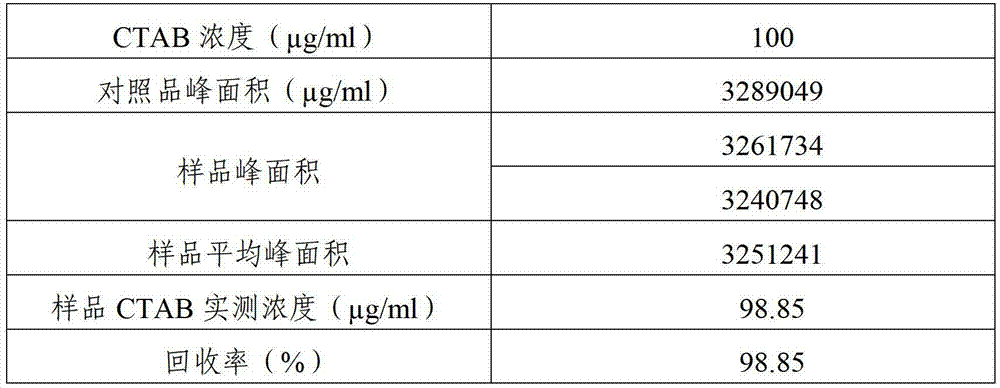
Measuring method of CTAB content in meningococcal polysaccharide
ActiveCN103245738AGood repeatabilityConvenient CTAB contentComponent separationMeningococcal carriageMENINGOCOCCAL POLYSACCHARIDE
The invention relates to a measuring method of the CTAB (Cetyltrimethyl Ammonium Bromide) content in meningococcal polysaccharide. The method comprises the following steps: taking CTAB standard solution and meningococcal polysaccharide solution samples, respectively placing the samples on a high-efficiency gel chromatographic column, and calculating the concentration of CTAB in the meningococcal polysaccharide solution sample according to a chromatogram. The measuring method of the CTAB content in the meningococcal polysaccharide, provided by the invention, is accurate, good in repeatability, quick and convenient.
Owner:BEIJING MINHAI BIOTECH
A kind of meningitis polysaccharide conjugate vaccine with heterotype bifunctional reagent as connecting bridge and preparation method thereof
ActiveCN103083652BAvoid self-couplingHigh yieldAntibacterial agentsPharmaceutical non-active ingredientsMENINGOCOCCAL POLYSACCHARIDE CONJUGATEHeterobifunctional reagent
The invention provides a preparation method of a novel polysaccharide conjugate vaccine treating a PEG heterobifunctional reagent as a conjugation bridge. A meningococcal polysaccharide conjugate vaccine is exploited through the method. The method has the following advantages: 1, the respective self-crosslinking of polysaccharides and proteins in traditional methods is avoided, the yield is improved, and the quality control is benefited; and 2, a long PEG chain can increase the distance between the polysaccharides and the proteins, reduce the mutual space shield effect between the polysaccharides and the proteins, and improve the immunogenicity of the polysaccharide conjugate vaccine.
Owner:HUALAN BIOLOGICAL VACCINE INC +1
Multivalent meningococcal conjugates and methods of making conjugates
ActiveCN104428008BAntibacterial agentsBacterial antigen ingredientsMeningococcal carriageMENINGOCOCCAL POLYSACCHARIDE
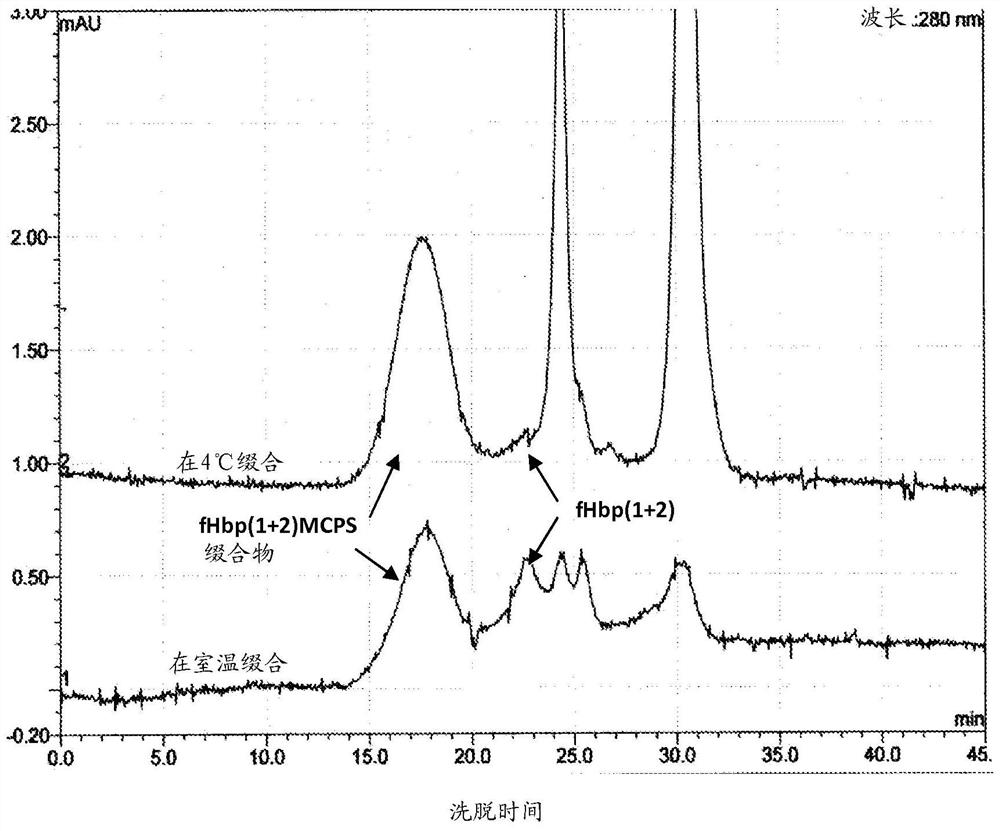
Meningococcal immunogenic conjugates that elicit immune responses against meningococcal polysaccharides (PS) from groups A, C, W-135 and Y and group B factor H binding protein (fHbp) are disclosed herein. The disclosed conjugates also exhibit bactericidal activity against meningococcal A, C, W-135, Y, B and X serogroups. Also disclosed is an improved method of preparing a conjugate, eg, an immunogenic conjugate, comprising activating the polysaccharide with a cyanating agent at about 4°C.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Process for refining group C/Y/W135 meningococcal polysaccharides
ActiveCN102911285BProtein content controlLow in proteinAntibacterial agentsAntibody medical ingredientsMeningococcal carriageMENINGOCOCCAL POLYSACCHARIDE
The invention discloses a process for refining group C / Y / W135 meningococcal polysaccharides. The process includes dissolving one of a coarse group C meningococcal polysaccharide, a coarse group Y meningococcal polysaccharide and a coarse group W135 meningococcal polysaccharide, displacing the polysaccharide into an equilibration buffer solution, feeding a collected polysaccharide to an anion exchange column, using an eluant to elute absorption substances from the anion exchange column, piecewise collecting absorption peaks with wavelength smaller than 206 according to different elution peaks and using a desalting method to remove micromolecule materials. According to the process for refining the group C / Y / W135 meningococcal polysaccharides, protein contents can be effectively controlled, so that the polysaccharide protein content is far lower than the polysaccharide protein content in a cold phenol method, the recovery rate can reach around 80%, and the process is applicable to mass production with 100g of coarse polysaccharide yield per batch.
Owner:罗益(无锡)生物制药有限公司
Multivalent Meningococcal Polysaccharide-Protein Conjugate Vaccine
InactiveUS20130177588A1Enhance antibody productionAntibacterial agentsBacterial antigen ingredientsConjugate vaccineCarrier protein
The present invention describes a combined vaccine that offers broad protection against meningococcal disease caused by the pathogenic bacteria Neisseria meningitidis. The vaccine is comprised of four distinct polysaccharide-protein conjugates that are formulated as a single dose of vaccine. Purified capsular polysaccharides from Neisseria meningitidis serogroups A, C, W-135, and Y are chemically activated and selectively attached to a carrier protein by means of a covalent chemical bond, forming polysaccharide-protein conjugates capable of eliciting long-lasting immunity to a variety of N. meningitidis strains in children as well as adults.
Owner:SANOFI PASTEUR INC
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com