Multivalent meningococcus preparation box, vaccine preparation and preparation method thereof
A meningococcal and vaccine technology, applied in antibacterial drugs, pharmaceutical formulations, bacterial antigen components, etc., can solve the problems of difficult multi-component antigen content analysis and quality control, complex vaccine production process, etc., to maintain high activity, The effect of lyophilization process optimization and solvent parameter optimization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of A, C, W135, Y group meningococcal polysaccharide or polysaccharide protein binding stock solution
[0039] After the working seeds of meningococcus groups A, C, W135, and Y are recovered, they are inoculated with fermentation medium, and after the fermentation, the bacteria are collected by centrifugation. The bacterial pellet is discarded, and the capsular polysaccharide antigen is separated and purified from the supernatant.
[0040] The purification of polysaccharides adopts ultrafiltration concentration, membrane encapsulation washing, gel separation and other steps to remove phenol, chloroform, protease and other reagents that are obviously harmful to the human body used in the traditional process.
[0041] Qualified purified polysaccharides can be diluted according to a certain ratio and then prepared to obtain a stock solution of meningococcal polysaccharides of groups A, C, W135 and Y.
[0042] The polysaccharide protein binding stock solution needs to be...
Embodiment 2
[0046] Preparation of tetravalent meningococcal vaccine (polysaccharide protein conjugate vaccine) preparation
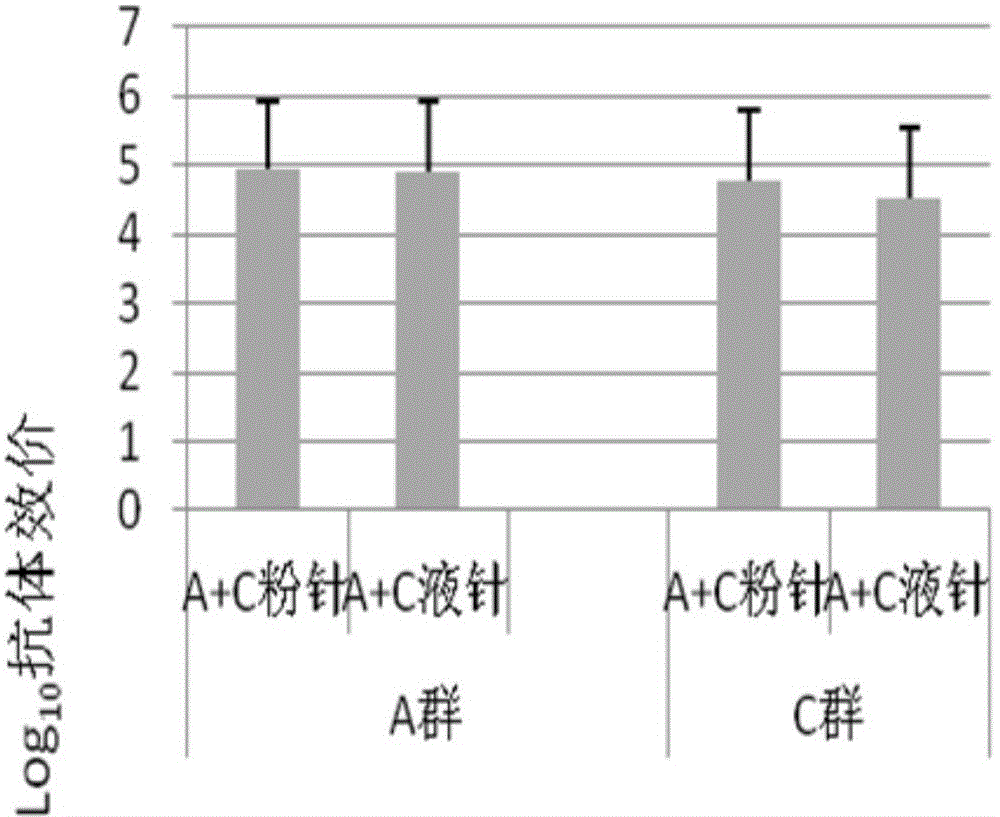
[0047] Take 10μg group A meningococcal polysaccharide-CRM197 conjugate combination stock solution, 10μg group C meningococcal polysaccharide CRM197 conjugate combination stock solution and place it in a vial, add freeze-dried protectant (20mg mannitol and 5mg sucrose), half stopper Then put it into the lyophilizer cavity, pre-freeze for 240-300 minutes at a temperature of -45℃, reduce the temperature to -35℃ and apply a vacuum, maintain a vacuum of 0.2±0.02mBar, and vacuum at a temperature of -35℃ Dry for 840 to 960 minutes, heat up to 35°C, and dry for 300 to 600 minutes to prepare freeze-dried components;
[0048] Take 10μg Y group meningococcal polysaccharide-CRM197 conjugate combined stock solution and 10μg W135 group meningococcal polysaccharide CRM197 conjugate combined stock solution into a vial, add 10mmol phosphate buffer and 0.45% physiological sodium chloride ...
Embodiment 3
[0053] Preparation of tetravalent meningococcal vaccine (polysaccharide vaccine) preparation
[0054] Take 50μg group A meningococcal polysaccharide stock solution and 50μg group C meningococcal polysaccharide stock solution in a vial, add freeze-dried protective agent (20mg mannitol and 5mg sucrose), half stoppered and put it into the lyophilizer cavity. Pre-freeze at -45℃ for 240~300 minutes, reduce the temperature to -35℃ and vacuum, keep the vacuum degree 0.2±0.02mBar, vacuum dry at -35℃ for 840~960 minutes, and heat up to 35℃, Dry for 300-600 minutes to prepare freeze-dried components;
[0055] Take 50μg Y group meningococcal polysaccharide stock solution and 50μg W135 group meningococcal polysaccharide stock solution in a vial, add a diluent consisting of a mixture of 10mmol phosphate buffer and 0.45% physiological sodium chloride solution, and prepare the dilution Liquid component of the agent;
[0056] In clinical use, the freeze-dried component and the liquid component are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com