Sesquiterpene lactone (1 beta, 10 beta-epoxy-13-alpha-dimethylamino-1,2-propanediol 3-(4)-alkene-6,12-lactone) dimethylamine hydrochloride freeze-drying preparation for injection and preparation method for sesquiterpene lactone (1 beta, 10 beta-epoxy-13-alpha-dimethylamino-1,2-propanediol 3-(4)-alkene-6,12-lactone) dimethylamine hydrochloride freeze-drying preparation
A technology of dimethylamine hydrochloride and freeze-dried preparations, applied in freeze-dried transportation, inorganic inactive ingredients, powder transportation, etc., can solve the problems of preparation stability, high product related substances, high energy consumption, etc. The effect of sublimation drying temperature, reduction of related impurities, and improvement of product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
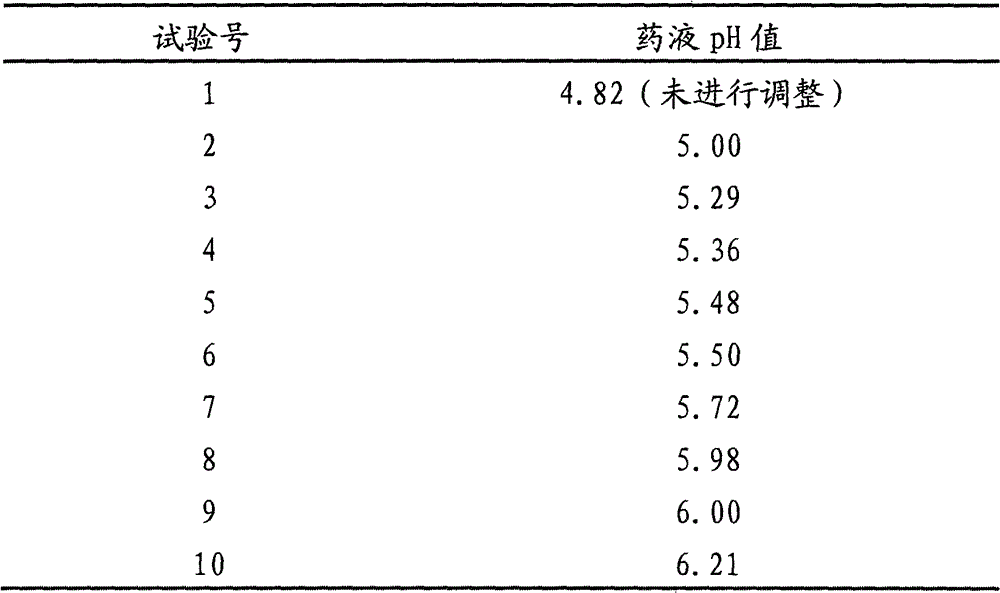
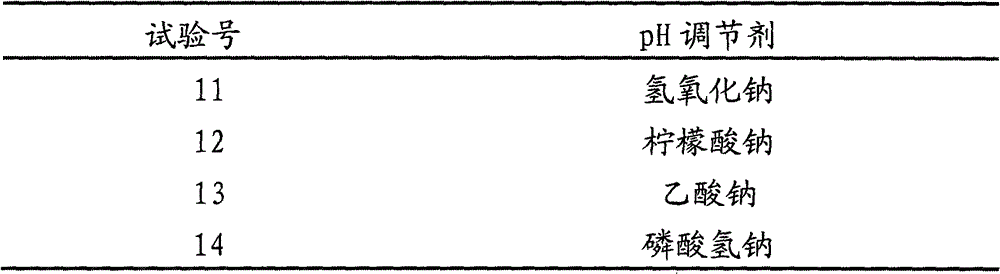
[0063] Example 1: Adjust the pH value to 5.02 with KOH, filter with a polyethersulfone microporous membrane, and dry at a final temperature of 18°C
[0064] Prescription (1000 bottles)
[0065] Agrabine Dimethylamine Hydrochloride 40g
[0066] 0.1mol / L KOH solution Appropriate amount
[0067] Water for injection 2L
[0068] Solution preparation: Weigh 90% of the prescription amount of sterile water for injection, add the prescription amount of agrabine dimethylamine hydrochloride and stir to dissolve, measure the pH value of the solution, add 0.1mol / L KOH solution, adjust the pH value of the liquid medicine at 5.02 , Diluted to 2000ml, filtered through a 0.22μm polyethersulfone microporous membrane, and then packed into type I glass bottles.
[0069] Freeze-drying: place the glass bottle containing agrabin dimethylamine hydrochloride solution in a Minifast-04 freeze-dryer for freeze-drying. The sample was frozen and cooled to -40°C, and after 4-5 hours of heat preservation...
Embodiment 2
[0071] Others: Appearance white loose powder, moisture 0.8%, residual oxygen 0.368%, clear and transparent after reconstitution. Example 2: Adjust the pH value to 5.20 with sodium acetate, filter with a polyethersulfone microporous membrane, and dry at a final temperature of 18°C
[0072] Prescription (1000 bottles)
[0073] Agrabine Dimethylamine Hydrochloride 40g
[0074] 0.1mol / L sodium acetate solution appropriate amount
[0075] Water for injection 2L
[0076] Solution preparation: take by weighing 90% of the prescription amount of sterile water for injection, add the prescription amount of agrabine dimethylamine hydrochloride and stir to dissolve, measure the pH value of the solution, add 0.1mol / L sodium acetate solution, adjust the pH value of the medicinal solution at 5.20, set the volume to 2000ml, filter through a 0.22μm polyethersulfone microporous membrane, and pack into type I glass bottles.
[0077] Freeze-drying operation is as embodiment 2
[0078] Total c...
Embodiment 3
[0079] Others: Appearance white loose powder, moisture 0.8%, residual oxygen 0.371%, clear and transparent after reconstitution. Example 3: Adjust the pH value to 5.36 with sodium citrate, filter with a polyethersulfone microporous membrane, and dry at a final temperature of 18°C
[0080] Prescription (1000 bottles)
[0081] Agrabine Dimethylamine Hydrochloride 40g
[0082] 0.1mol / L sodium citrate solution appropriate amount
[0083] Water for injection 2L
[0084] Solution preparation: Weigh 90% of the prescription amount of sterile water for injection, add the prescription amount of agrabine dimethylamine hydrochloride and stir to dissolve, measure the pH value of the solution, add 0.1mol / L sodium citrate solution to adjust the pH value of the liquid medicine At 5.36, set the volume to 2000ml, filter through a 0.22μm polyethersulfone microporous membrane, and then pack into type I glass bottles.
[0085] Freeze-drying operation is as embodiment 2
[0086] Total content ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com