Method for preparing group A meningococcal capsular polysaccharide conjugate vaccine
A technology of meningococcal and capsular polysaccharides, applied in the field of biomedicine, can solve the problem of low yield of polysaccharides, achieve the effect of increasing the yield of binding and overcoming steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
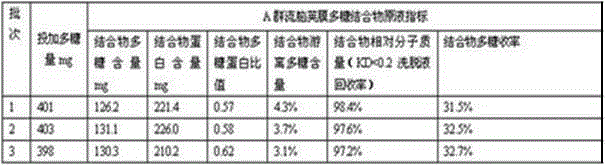
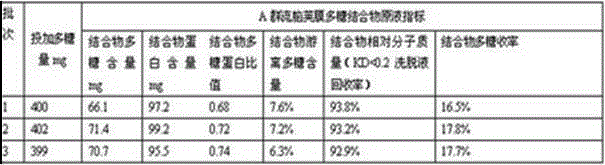
[0022] A preparation method of group A meningococcal capsular polysaccharide conjugate vaccine, comprising the following steps:
[0023] B1: Dilute group A meningococcal capsular polysaccharide with water for injection to a solution of 8 mg / ml, keep the temperature at 20°C, and adjust the pH to 10.50;
[0024] B2: adding cyanogen bromide equal to the mass of the polysaccharide described in B1 for activation treatment, maintaining pH 10.5 for activation for 20 minutes, and adjusting the pH to 8.5;
[0025] B3: adding sebacic acid dihydrazide solution of equal volume and concentration to the polysaccharide solution described in B1 for coupling reaction, maintaining pH 8.5 for 15 minutes;
[0026] B4: Dilute the reactant obtained in B3 multiple times - concentrate by ultrafiltration, remove residual cyanogen bromide, and obtain group A meningococcal polysaccharide-sebacic acid dihydrazide derivative;
[0027] B5: Add a tetanus toxoid protein solution equal in quality to the poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com