Multivalent meningococcal conjugates and methods of making conjugates
A technology of meningococcus and conjugates, applied in the field of conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
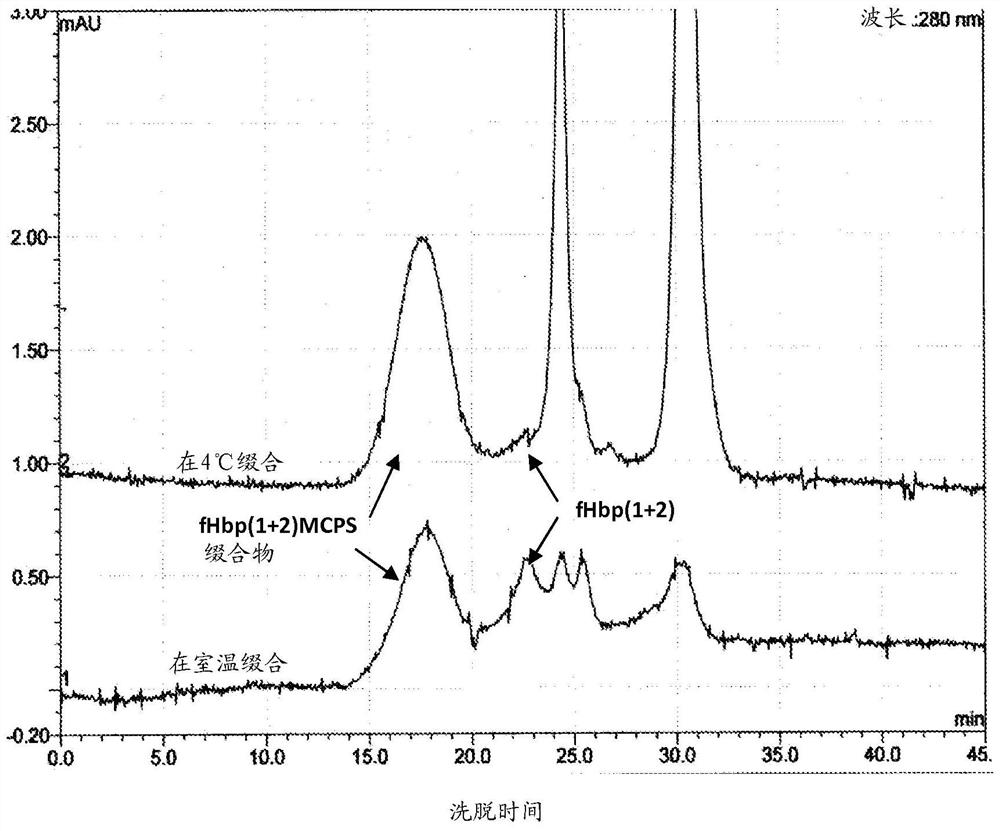
[0119] C. Preparation of Multivalent Immunogenic Conjugates
[0120] In some embodiments, the disclosed methods use multiple immunogenically distinct polysaccharides and / or multiple proteins to prepare multivalent immunogenic conjugates. For example, using the methods described above to conjugate a mixture of two or more (eg 2, 3, 4, 5 or more) immunogenically distinct polysaccharides (eg a plurality of immunogenically distinct polysaccharides) to at least one Protein (eg fHbp or NspA). In some examples, the plurality of immunogenically distinct polysaccharides includes two or more meningococcal polysaccharides (e.g., two or more meningococcal polysaccharides selected from serotypes A, B, C, W-135, and Y). cocci polysaccharide). In a specific example, the plurality of polysaccharides with different immunogenicity comprises meningococcal serogroup A polysaccharide (MAPS), meningococcal serogroup C polysaccharide (MCPS), meningococcal serogroup W-135 polysaccharide (MWPS ) an...
Embodiment 1
[0153] Preparation of conjugates
[0154] method
[0155] MAPS is produced by SynCo BioPartners, Amsterdam, The Netherlands. MCPS was from FioCruz, BioMaguinhus, Brazil. MWPS and MYPS were from Chiron (Emoryville, CA). Tetanus toxoid (TT) was obtained from Wyeth Vaccines and Serum Institute, India. 1-Cyano-4-dimethylaminopyridine tetrafluoroborate (CDAP) was purchased from Sigma-Aldrich (St. Louis, MO).
[0156] The fHbpl gene was amplified by PCR using genomic N. meningitidis H44 / 76 genomic DNA as template, forward primer AAGCTTCCTCGAGTGAGCAGTGGAGGGGGTGGTGTCGCC (SEQ ID NO: 9) and reverse primer GGCGGGGAATTCACTTATTGCTTGGCGGCAAGGCCGAT (SEQ ID NO: 10). The fHbp2 gene was amplified by PCR using genomic N. meningitidis 7608 genomic DNA as template, forward primer AAGCTTCCTCGAGTGAGCAGTGGAGGCGGCGGTGTCGCC (SEQ ID NO: 11 ) and reverse primer GGCGGGGAATTCACTTACTACTGTTTGCCGGCGATGCC (SEQ ID NO: 12). The PCR product was cloned into the XhoI-EcoRI site of the pT7-MAT-Tag-Flag-1 vector...
Embodiment 2
[0165] Immunogenicity of fHbp conjugates
[0166] method
[0167] Groups of 5 or 10 NIH-Swiss mice were immunized subcutaneously on days 0, 14 and 28 with 0.1 mL inoculum containing the conjugate prepared in Example 1 or fHbp and serum was collected on day 35 and natural PS mixture (control).
[0168] Will 1B plate (Dynatech) was coated for 2 hours with 100 μL of coating solution containing PBS (pH 7.4), mixed with methylated human serum albumin (5 μg / mL) or 1 μg / mL fHbp1 or fHbp2 Native MnA, C, W135 or Y PS (5 μg / mL), and washed with 150 μL wash buffer (PBS, pH 7.4, 0.05% 20 and 0.02% NaN 3 ) washed three times. Serum samples or reference serum (specified with 3200 units / mL anti-Mn A, C, W135 or Y PS antibodies, or 32000 units / mL anti-fHbp1 or fHbp2 antibodies) were diluted with dilution buffer (PBS, pH 7.4, 4 % newborn calf serum, 0.02% NaN 3 ) dilutions, a series of two-fold dilutions starting from 1:200. 100 μL of these diluted samples were added to each well of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com