A-group C-group Neisseria meningitidis polysaccharide conjugate vaccine activating process
A meningococcal and conjugated vaccine technology, applied in the direction of carrier-binding antigen/hapten components, antibacterial drugs, antibody medical components, etc., can solve problems such as adverse protection of human health and the environment, and achieve better derivative effects and extraction rates. High, easy purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
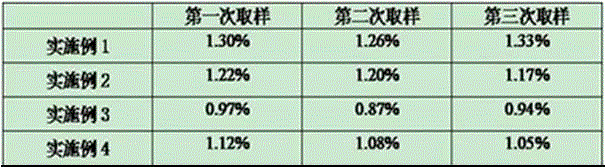
Embodiment 1
[0045] The activation process of group A group C meningococcal polysaccharide conjugate vaccine comprises the following steps:
[0046] (1) Cultivate meningococci;
[0047] (11) Preparation of purified working seed batch strains: Inoculate group A Neisseria meningitidis strains on the purified medium, incubate at 38°C for 20 hours, select robust and free strains of bacteria and inoculate again Carry out secondary culture on the purified medium, and then cultivate at 38°C for 12 hours, select robust and non-contaminated strains and add them to aseptic skim milk, mix and freeze-dry to prepare purified working seed batch strains ;
[0048] (12) Dissolve the working seed batch strains into sterile water, inoculate them on 10% sheep blood agar medium, and culture them at 35-37°C for 16-20 hours, then select the robust and impurity-free strains. Bacteria-contaminated strains; inoculate the opened purified seeds into a semi-comprehensive liquid medium for activation culture; the co...
Embodiment 2
[0072] The only difference between this example and Example 1 is that the weight ratio of polysaccharide to CDAP in step (42) in this example is 1:0.6; the weight ratio of polysaccharide to ADH in step (43) is 1:4.
Embodiment 3
[0074] The difference between this embodiment and embodiment 1 is only that the proportion and concentration of each chemical substance in this embodiment are added to the reaction, and the specific settings are as follows:
[0075] The concentration of the polysaccharide solution in step (41) is 10 mg / ml, and the pH is adjusted to 9.0 with sodium hydroxide;
[0076] The concentration of CDAP solution in step (42) is 80mg / ml, polysaccharide:CDAP=1:0.7;
[0077] The concentration of ADH solution in step (43) is 80mg / ml ADH solution, polysaccharide: ADH=1:5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com