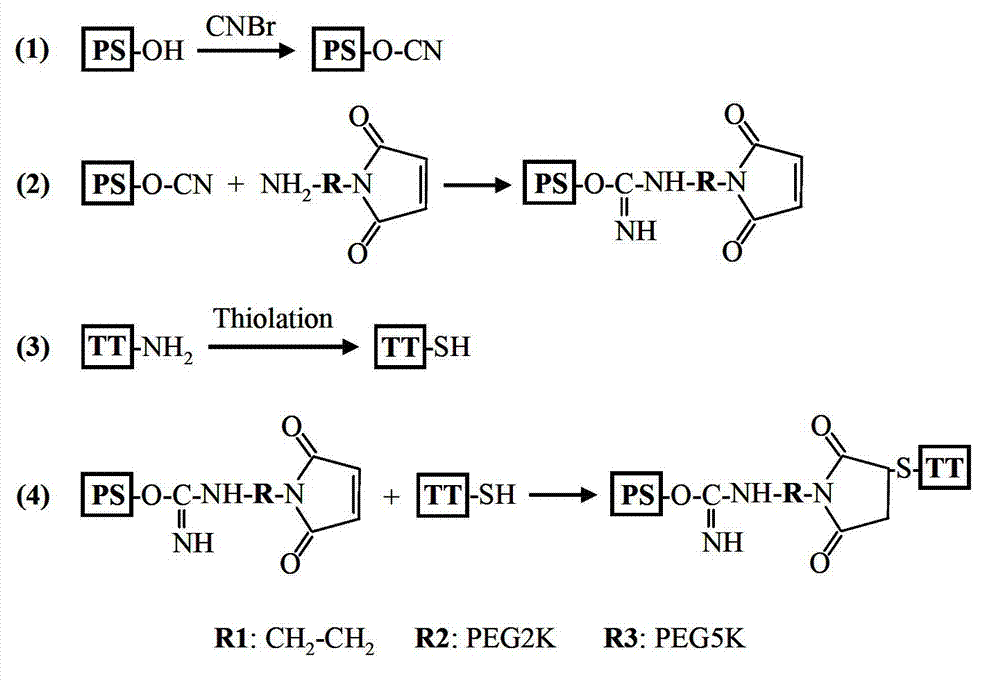
Meningococcal polysaccharide conjugate vaccine treating heterobifunctional reagent as conjugation bridge, and its preparation method
A combination vaccine and meningitis technology, applied in the field of biomedicine, can solve the problems of reducing polysaccharide-protein binding efficiency, polysaccharide and carrier protein self-crosslinking, limiting the development of polysaccharide-conjugated vaccines, etc., to improve immunogenicity and reduce space Shielding effect, benefit to quality control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of polysaccharide conjugate vaccine
[0036] Preparation of polysaccharide conjugate vaccine without PEG (PS-TT), polysaccharide conjugate vaccine with PEG2K as bridge (PS-P2K-TT), and polysaccharide conjugate vaccine with PEG5K as bridge (PS-P5K-TT) react as figure 1 shown. Dissolve 10 mg of group Y meningococcal capsular polysaccharide in 2 ml of normal saline, add 20 μL of cyanogen bromide solution (w / v 50%) for activation, and react at room temperature for 1 hour. During activation, the pH of the solution was maintained at 10.5 with 0.5M NaOH. After the activation, the pH value of the solution was adjusted to 8.5 with 0.5M hydrochloric acid to terminate the reaction. Subsequently, 10 mg N-2-aminoethyl-maleimide, 60 mg amine-PEG2K-maleimide (PEG molecular weight 2 kDa) and 75 mg amine-PEG5K-maleimide (PEG molecular weight 5 kDa) were added separately ). React overnight at room temperature. After the reaction, use a filter membrane with a ...
Embodiment 2
[0038] Example 2: Characterization of three polysaccharide conjugate vaccines
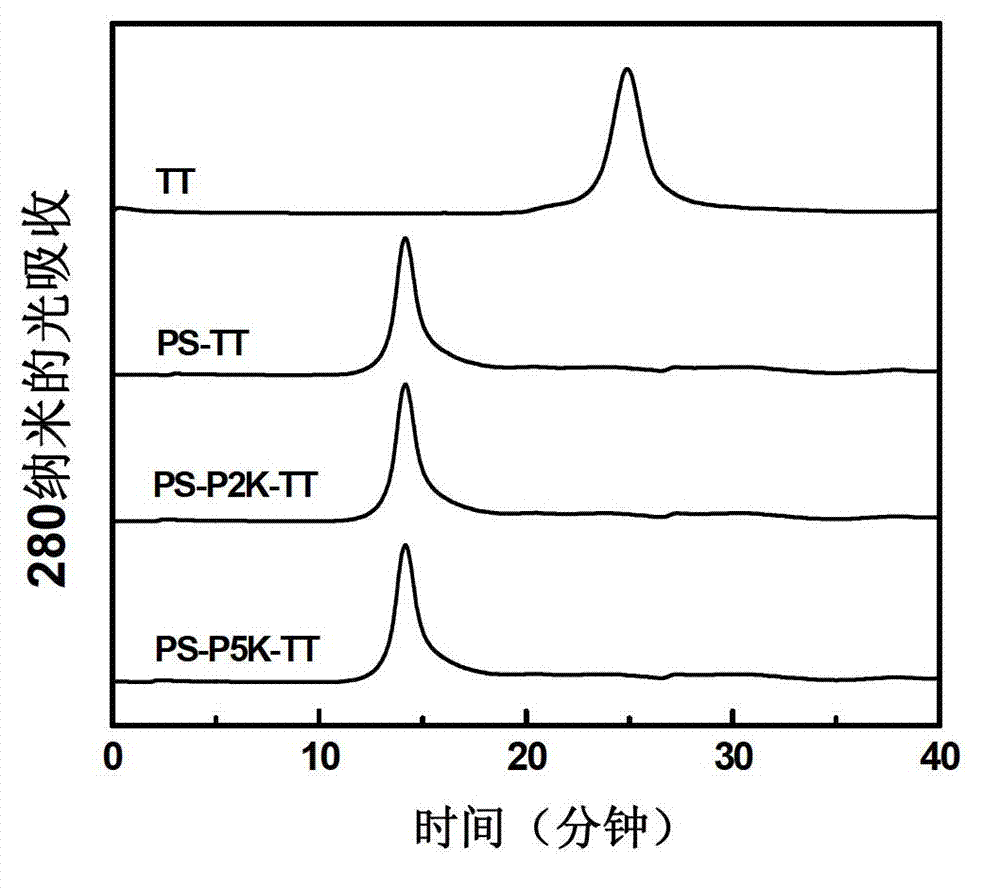
[0039] The polysaccharide-protein combination product involved in Example 1 was separated and purified with a Superdex200 gel filtration column (2.6cm×60cm), the eluent was 20mM phosphate buffer (pH7.4), and the flow rate was 3ml / min . Elution peaks corresponding to PS-TT, PS-P2K-TT and PS-P5K-TT were collected separately.
[0040] The purified product was identified by Superdex200 gel filtration column (1.0cm×30cm), the eluent was 20mM phosphate buffer (pH7.4), and the flow rate was 0.5ml / min. Such as figure 2 As shown, compared with the carrier protein, the peak time of PS-TT, PS-P2K-TT and PS-P5K-TT was significantly earlier. This indicates that the molecular weight of the carrier protein increases significantly after binding to the pneumonia capsular polysaccharide. Since the three combined products all elute in the outer water volume of the gel filtration column, the peak eluting times of...
Embodiment 3
[0042] Example 3: Determination of the immunogenicity of polysaccharide-conjugated vaccines
[0043] Select 32 5-week-old female Blab / C mice weighing 15-22 g. They were randomly divided into 4 groups, namely capsular polysaccharide group, PS-TT group, PS-P2K-TT group and PS-P5K-TT group, with 8 mice in each group. Intraperitoneal injection, each injection containing 5 micrograms of polysaccharides, once a week, a total of 3 injections. After 21 days, blood was collected from the orbit. Anti-capsular polysaccharide IgG, IgG1 and IgG2a in mouse plasma were detected by ELISA.
[0044] Such as Figure 4 As shown, the capsular polysaccharide group produced weak IgG and IgG1 titers against the capsular polysaccharide, while IgG2a was not detected. The antibody titer increased significantly after the capsular polysaccharide was coupled with the carrier protein. Compared with the capsular polysaccharide group, the anti-capsular polysaccharide IgG and IgG1 antibody titers in the P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com