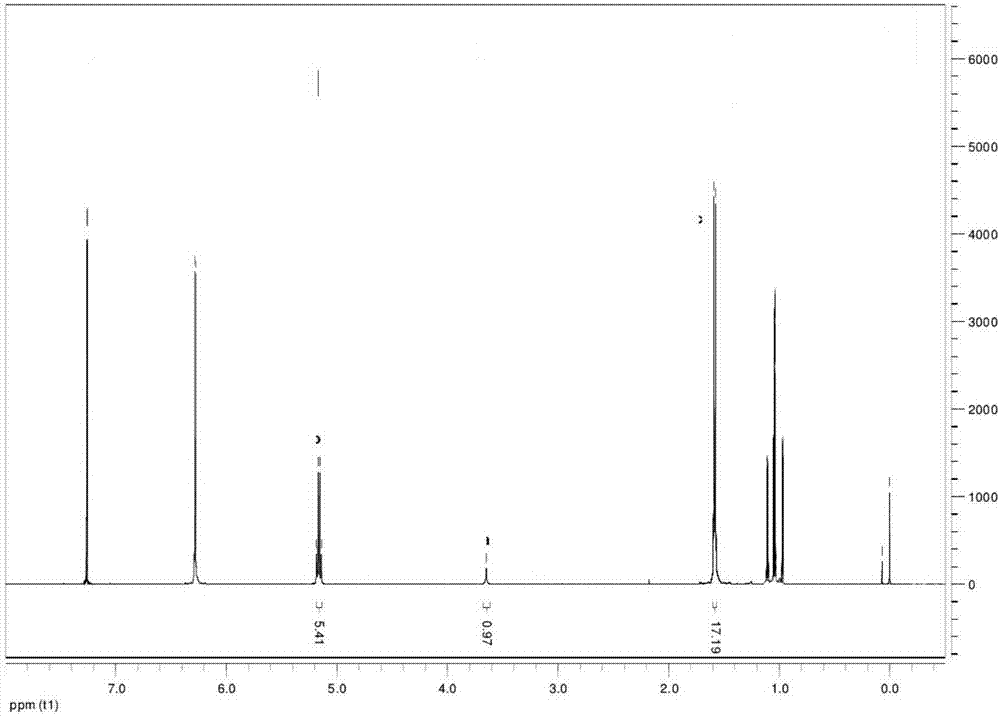
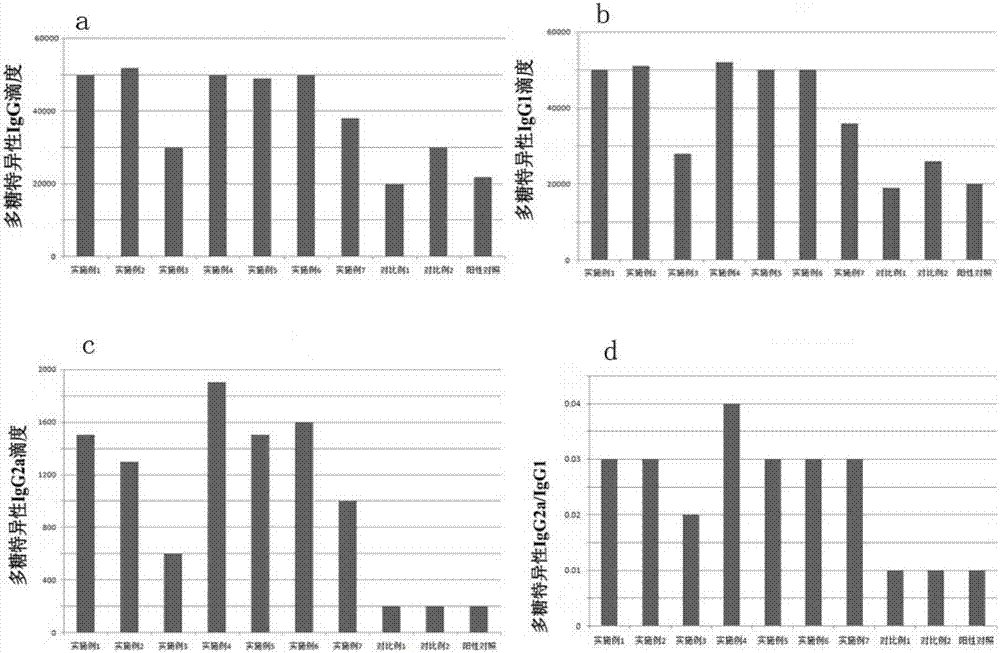
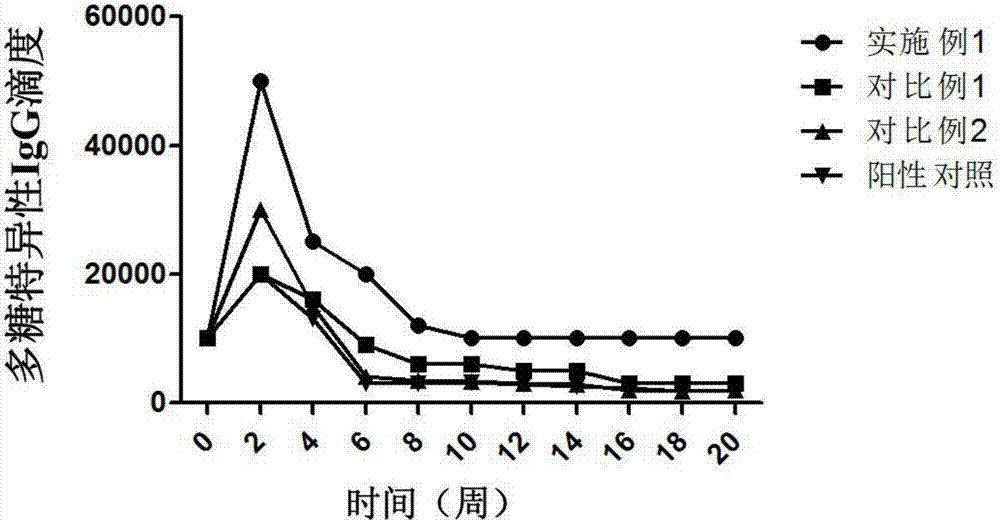
Pneumonia multivalent conjugate vaccine and preparation method thereof
A technology combining vaccines and pneumococcal polysaccharides, applied in the biological field, can solve the problems of inability to induce immune memory effects, inability to effectively activate the complement system, and low-affinity antibodies.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] 1. Preparation of magnetic nanospheres
[0100] 1. Take 2.24g FeSO 4 -7H 2 O and 3.24 g FeCl 3 -6H 2 O dissolved in 10 mL and 15 mL of dddH 2 In O, mix well after dissolving, add 100mL of dddH filtered to remove oxygen 2 O;
[0101] 2. In N 2 Stir for 5 minutes under the protection of the solution, add 50mL1mol / L NaOH solution at one time, adjust the pH of the solution to 9-10, increase the stirring speed to 200-250r / min, and continue stirring for 30min;
[0102] 3. Transfer the reaction vessel to a 65-70°C water bath and continue to 2 Stirring and aging under the protection of 30min;
[0103] 4. After the reaction, set the volume to 100mL, and observe the synthesis of magnetic particles under a microscope;
[0104] 5. Dissolve 400mgPLGA in 10mLEA solvent as the oil phase (O), add 3mL of the above magnetic particle solution as the inner water phase (W 1 ), using an ultrasonic cell breaker (120W, 60s) to carry out colostrum in an ice-water bath to prepare colos...
Embodiment 2
[0150] The difference between this example and Example 1 is that the carrier protein of the pneumonia multivalent conjugate vaccine is tetanus toxoid carrier protein and PspA.
[0151] The compositional analysis results of the prepared pneumonia multivalent conjugate vaccine are consistent with the results obtained in Example 1 within the theoretical error range.
Embodiment 3
[0153] The difference between this example and Example 1 is that the carrier protein of the pneumonia multivalent conjugate vaccine is a rotavirus carrier protein and a tetanus toxoid carrier protein.
[0154] The compositional analysis results of the prepared pneumonia multivalent conjugate vaccine are consistent with the results obtained in Example 1 within the theoretical error range.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com