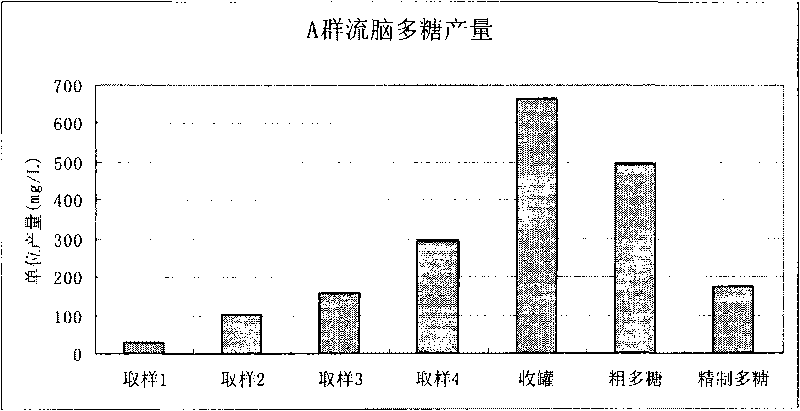
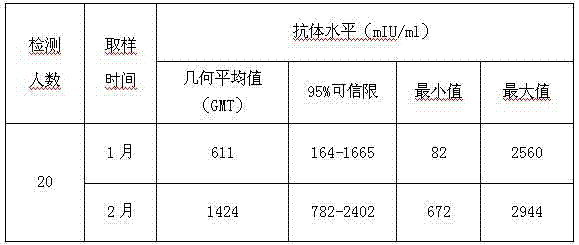
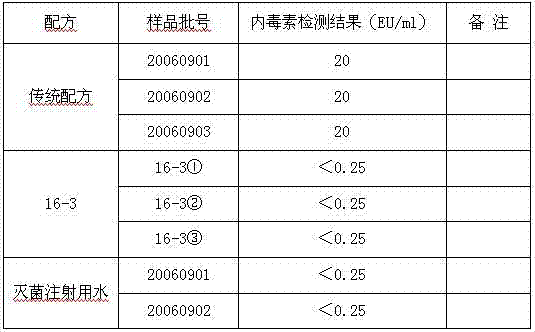
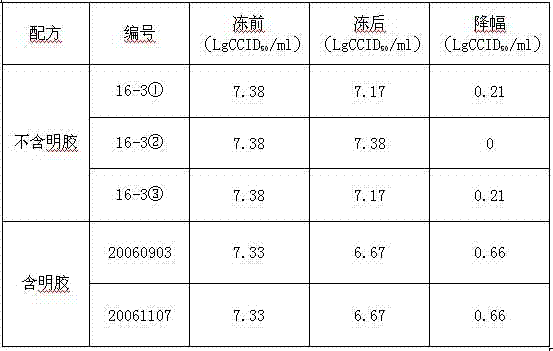
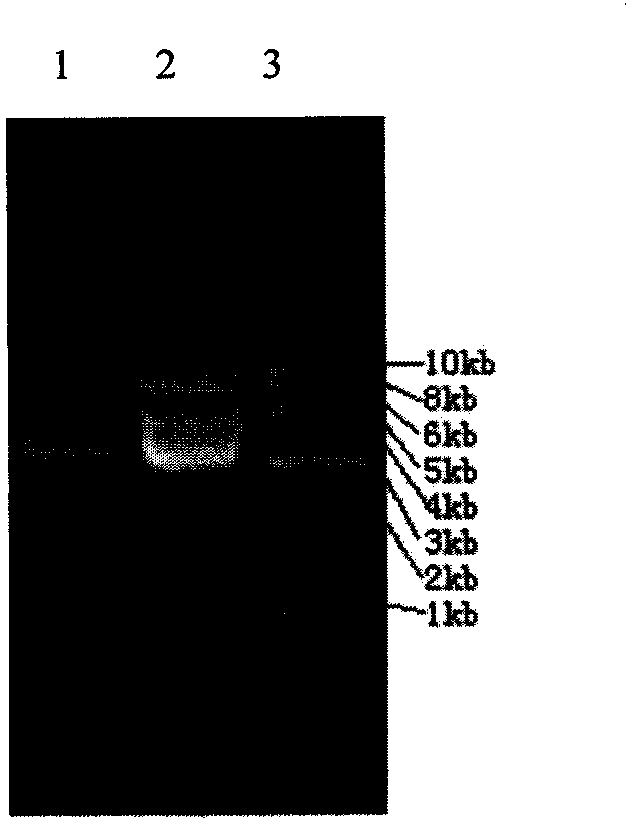
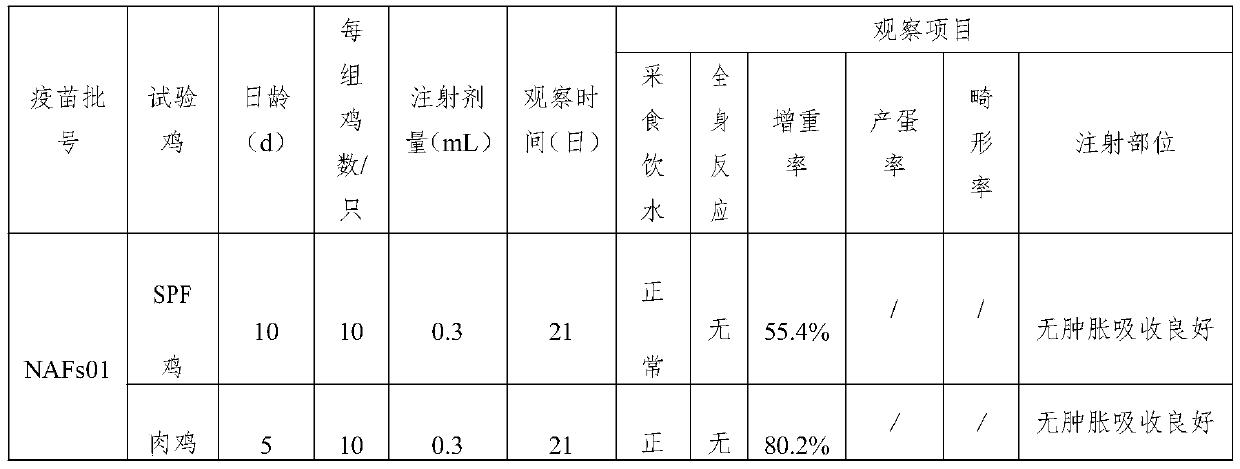
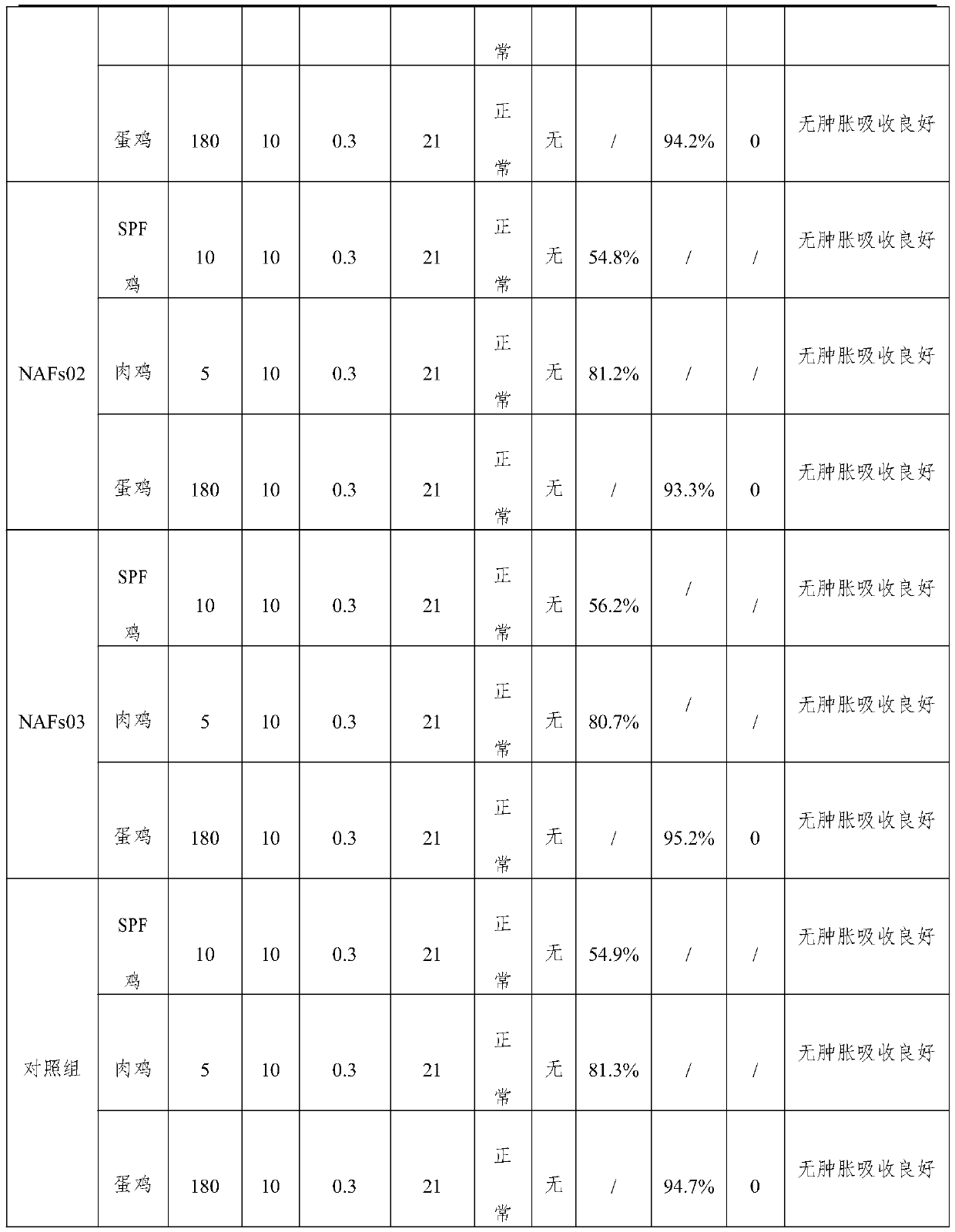
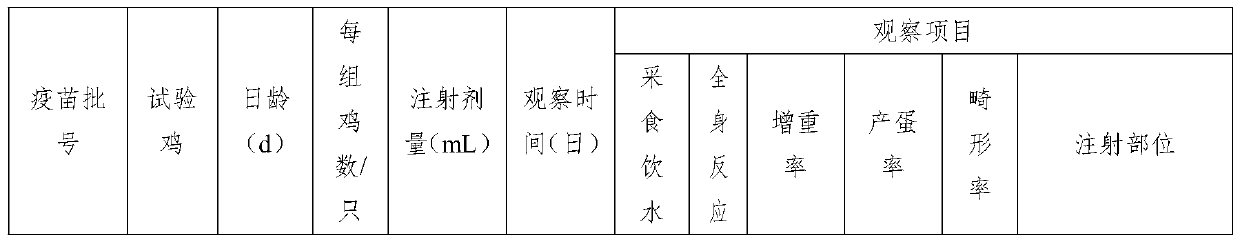
Patents
Literature
81results about How to "Low endotoxin content" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
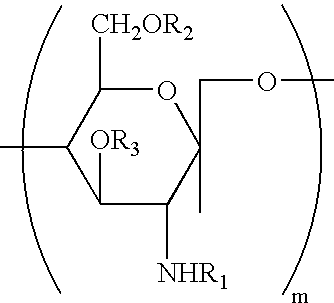
Water-soluble chitosan having low endotoxin concentration and methods for making and using the same
InactiveUS20050080245A1Low endotoxin contentOrganic active ingredientsSugar derivativesWater solubleWater soluble chitosan
Owner:ADJUVANT PHARMA
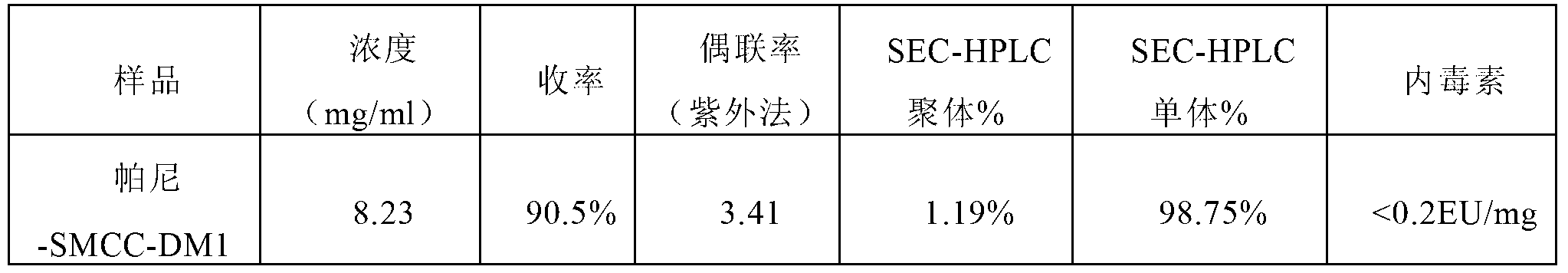
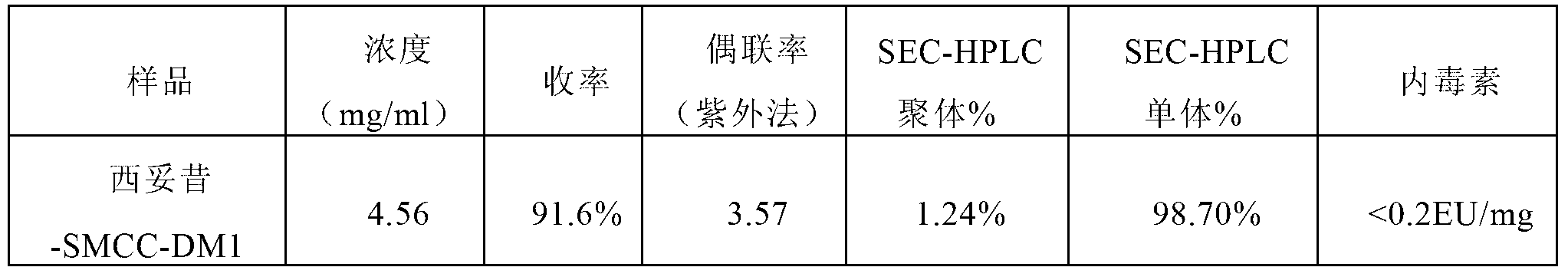
Method for preparing antibody-maytansine alkaloid medicine conjugate
ActiveCN103254311AReduce hidden dangersImprove efficiencyImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsAnion-exchange chromatographyAlkaloid
The invention relates to a method for preparing antibody-maytansine alkaloid medicine conjugate. The method comprises the following steps of replacing the antibody into a reaction buffer solution; dissolving a dual-function bridging agent-maytansine alkaloid with an organic solvent so as to prepare the mother liquor of maytansine alkaloid medicine; mixing the replaced antibody with the mother liquor of maytansine alkaloid medicine for coupled reaction for 1-4 hours at 20-30 DEG C; and carrying out anion exchange chromatography on the reaction liquid, conducting Sephadex TMG25 desalination chromatographic column purification on collected flow liquid, and collecting a first peak sample as the prepared antibody-maytansine alkaloid medicine conjugate. The prepared antibody-maytansine alkaloid medicine conjugate is proper in coupling rate, high in purity and low in endotoxin content.
Owner:QILU PHARMA CO LTD
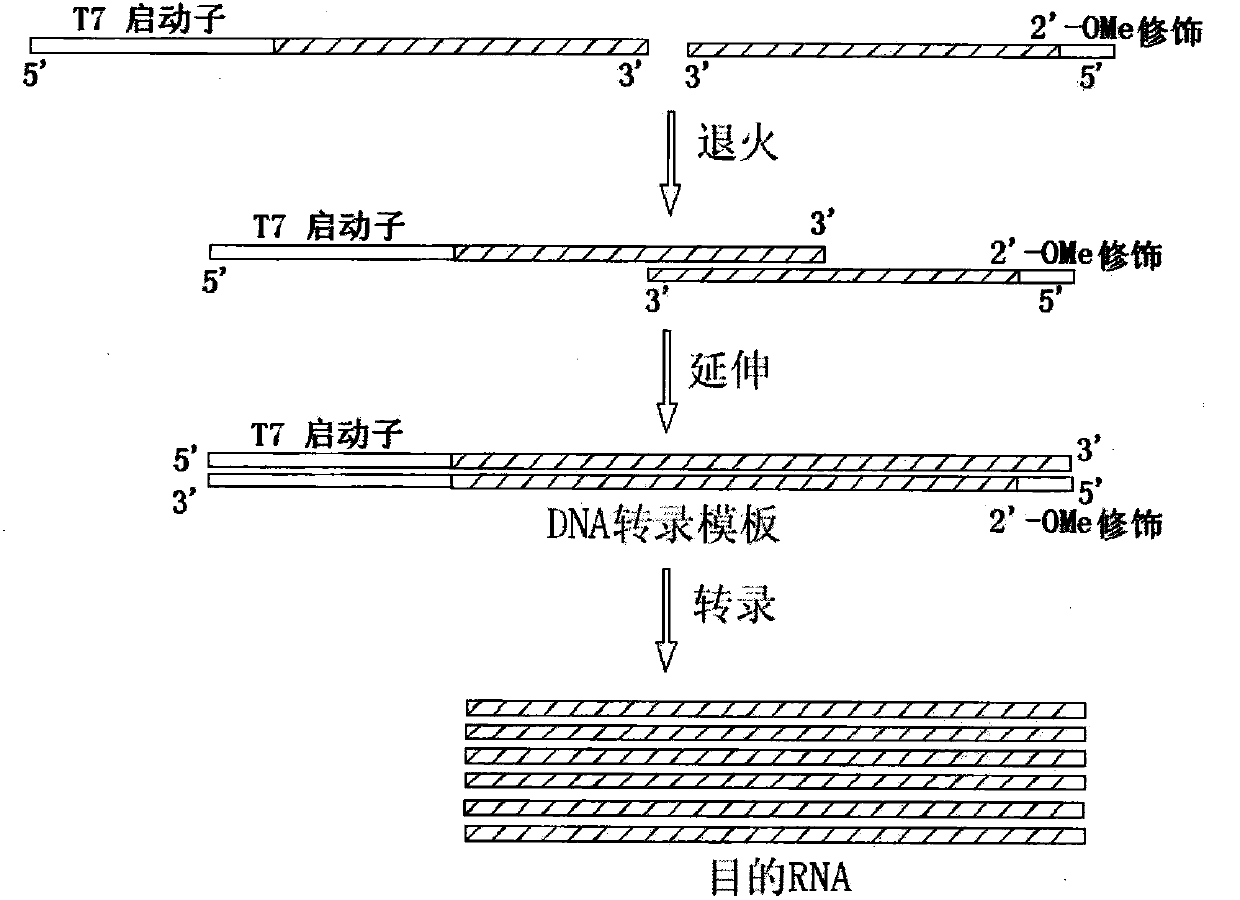
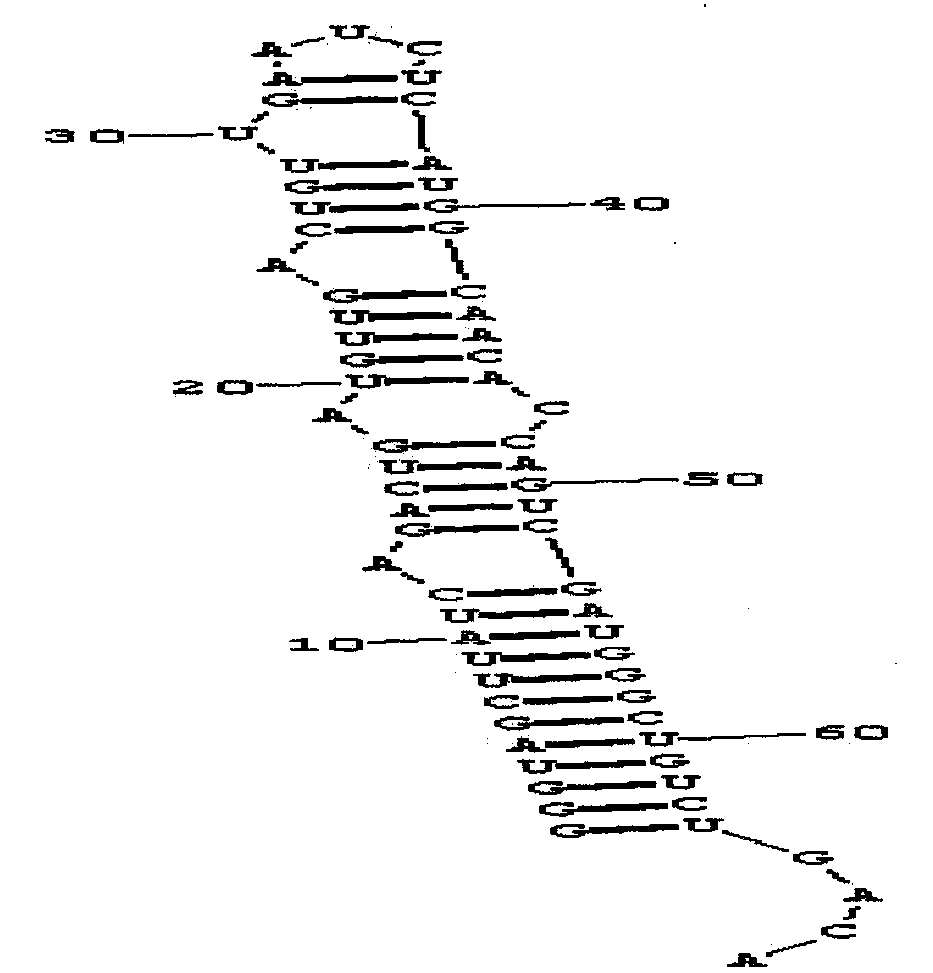
New production process for large-scale synthesis of long-chain RNA drugs
InactiveCN103993002AImprove accuracyEasy to operateGenetic material ingredientsGene therapyTemplate designProtein secondary structure
The invention provides a new production process for large-scale synthesis of long-chain RNA drugs. The process is characterized by specifically including the steps of: DNA transcription template design; template preparation and purification; in-vitro transcription; and product purification, purity detection and the like. The production process provided by the invention is especially suitable for synthesis of long-chain RNA drugs with a stable secondary structure. With the characteristics of simple operation and low cost, the process provided by the invention meets the requirements of large-scale production, and the produced long-chain RNA drugs can be used for cell experiments, animal experiments and other preclinical RNA drugs' functional study.
Owner:BIOMICS BIOTECH
Process for removing endotoxin in bacteria polysaccharide by using macroporous resin
ActiveCN1970780AGood removal effectImprove hydrophobicityUltrafiltrationMicroorganism based processesBacteroidesUltrafiltration
The invention discloses a removing method of endotoxin in the bacterial polysaccharide through large-hole resin in the biological technical domain, which comprises the following steps: fermenting bacteria through biological reactor; making rough sugar; extracting rough sugar through cooled phenol to remove protein; hyperfiltering through composite buffer of calcium ionic chelant-surface activator; removing endotoxin adsorbed by large-hole resin; obtaining bacterial polysaccharide with low endotoxin content.
Owner:YUXI WALVAX BIOTECH CO LTD
Method for purifying capsular polysaccharide
ActiveCN101724085AAvoid damageProtection securityAntibacterial agentsOrganic active ingredientsAlcoholChloroform
The invention relates to a treatment method for purifying capsular polysaccharide, comprising the following steps of: (a), adding a lower alcohol with low final concentration into a cell capsular polysaccharide precipitate, and dissolving the capsular polysaccharide to form a mixed liquid; (b), carrying out solid-liquid separation on the mixed liquid and collecting a supernate; (c), adding a lower alcohol with high final concentration into the obtained supernate to form a rough precipitate containing the cell capsular polysaccharide; (d) adding the lower alcohol with the low final concentration into the rough precipitate so that the polysaccharide in the rough precipitate is in a dissolving state; (e) adding the lower alcohol with the high final concentration into the supernate so as to form the capsular polysaccharide precipitate; and (f) collecting the obtained capsular polysaccharide precipitate to obtain a purified cell capsular polysaccharide. The method conveniently, controllably and effectively improves recycling rates of the capsular polysaccharide and improves the security without using toxic reagents of phenol or chloroform.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
Freeze-dried live attenuated hepatitis A vaccine not containing gelatin or human albumin protective agent and preparation method for freeze-dried live attenuated hepatitis A vaccine
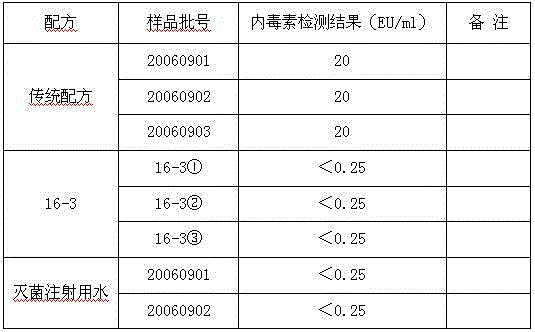
The invention belongs to the field of biological products, in particular to a freeze-dried live attenuated hepatitis A vaccine protective agent not containing gelatin or human albumin and used for preventing hepatitis A, and a preparation method for the freeze-dried live attenuated hepatitis A vaccine protective agent. The protective agent comprises trehalose, dextran 40, L-cysteine, arginine, glutamic acid, glycine, magnesium chloride, magnesium sulfate, sorbierite, mannitol and tris(hydroxymethyl)aminomethane. The protective agent with the formula is mixed with a hepatitis A vaccine stock solution to form a semi-finished product, the semi-finished product is packaged and freeze-dried, and virus infectious titers of the vaccine before and after freeze drying and the thermal stability after freeze drying are detected; after the formula is compared with the conventional production formula containing the gelatin, results show that the protective agent has good protective effect, the descent of the virus infectious titers of the vaccine in the freeze drying process is obviously decreased, and the endotoxin content in a finished product is obviously reduced (less than 0.25EU / ml); and after the freeze-dried vaccine with the formula is inoculated into a human body, results indicate that the vaccine has good immune effect and high safety.
Owner:ZHEJIANG PUKANG BIOTECH
Soybean bioactive peptide additive used for cell medium
ActiveCN104593317ALow endotoxin contentSimple and efficient operationArtificial cell constructsVertebrate cellsCell culture mediaSerum free
The invention provides a soybean bioactive peptide additive used for cell medium, mass percentage of oligopeptide with molecular weight being less than 1000 Dalton in the soybean bioactive peptide additive accounts for more than 90% of total protein, the oligopeptide at least comprises one or more selected from YE, ERQ, QDPQ, EGGAH, PQGAR and AAGGSN. The soybean bioactive peptide additive used for cell medium enables complex formulation with a plurality of basic mediums, and can be used for serum-free culture on the a plurality of animal cells, cell medium cost is greatly reduced, pollution problem due to animal source component is reduced, cell proliferation is promoted, cell vitality is increased, and cell products expression is enhanced.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD
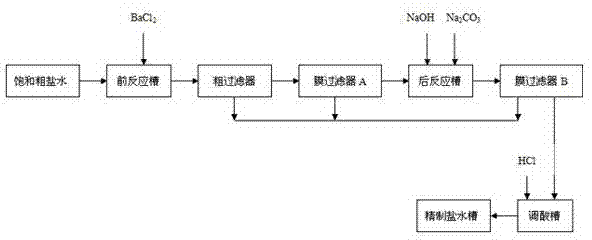
Membrane refining process of medicinal sodium chloride
ActiveCN103482658ALow endotoxin contentIncrease productivityAlkali metal halide purificationSulfate radicalsBarium dichloride
The invention discloses a membrane refining process for medicinal sodium chloride. The membrane refining process comprises the following steps: leading brine or crude salt water saturated by melting salt into a front reaction tank, adding barium chloride into the front reaction tank, filtering through a large-mesh filter after reaction, and removing sulfate radical through a first membrane filter; leading the sulfate radical-removed clear liquid into a rear reaction tank, adding sodium hydroxide and sodium carbonate into the rear reaction tank, and leading the solution into a second membrane filter after complete reaction so as to remove calcium and magnesium; leading the calcium and magnesium-removed clear liquid to an acid regulating tank, and adding hydrochloric acid to adjust the pH, so as to obtain refined salt water; producing the medicinal sodium chloride by an evaporation method, wherein the obtained medicinal sodium chloride has the advantages that the concentration of SS is less than or equal to 1mg / L, the total concentration of Ca<2+> and Mg<2+> is less than or equal to 1 mg / L, and the concentration of SO4<2-> is less than or equal to 20 mg / L. According to the membrane refining process, a two-stage membrane filtration method is adopted, no flocculant is added, the process is continuous, the operation is simple, the treatment capacity is large, the pollution resistance is high, and the quality of the medicinal salt is obviously improved after evaporation and crystallization.
Owner:JIANGSU JIUWU HITECH
A wheat bioactive peptide additive used for a cell culture medium
ActiveCN104593319AHigh in PolypeptideLow endotoxin contentVertebrate cellsArtificial cell constructsSerum igeSerum free
A wheat bioactive peptide additive used for a cell culture medium is provided. The mass of oligopeptides having molecular weights lower than 1000 Dalton is not less than 90% of the total protein mass in the additive, and the oligopeptides at least comprise one or more of VN, TFN, QVSQ, HGAQ, GGDNP and GTCGAG. The additive can be blended with various base culture media and used for serum-free culture of a plurality of animal cells. The additive largely reduces the cost of the cell culture medium, reduces pollutions and other problems caused by animal-source components, promotes cell proliferation, improves cell viability and enhances expression of cell products.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD
Method for producing endotoxin-free nucleic acids and the use thereof
InactiveUS6855816B1Reduce content of endotoxinAvoid disadvantagesSugar derivativesFermentationOligonucleotideBiology
Method for isolating and purifying nucleic acids and / or oligonucleotides from a biological sample, the use of the isolated or purified nucleic acid and / or oligonucleotide for transfecting cells and also for the production of an agent for the treatment of genetic disorders, a composition suitable for the isolation or purification method and also the use of potassium acetate and a silica gel-like support material for isolating endotoxin-free nucleic acids and / or oligonucleotides or nucleic acids and / or oligonucleotides with reduced endotoxin content.
Owner:XANTOS BIOMEDICINE
Man's serum albumin with man's parathormone (1-34) fusion protein and its application
InactiveCN1597965AEasy to purifyEasy to usePeptide/protein ingredientsSerum albuminHalf-lifePlant cell
The invention discloses a fusion protein of human sperm albumin and human parathyroid hormone (1-34), and DNA sequence coding it as well as bacteria, yeast, animal cells and plant cells that carry the DNA sequence. It contains a first region with at least 85% sequence isogeny with human sperm albumin and a second region with at least 85% sequence isogeny with hPTH (1-34), and can substitute, delete or add several aminophenol residues on the premise of not changing its own property; there is a peptide linkage between the two regions; it not only retains the functional actions of hPTH (1-34) to activate receptor and stimulate bon reconstruction but also remarkably prolongs in vivo half life, and it is a medicinal protein that can cure osteoporosis.
Owner:浙江优诺金生物工程有限公司
Method for removing endotoxin in protein
InactiveCN105001299AEfficient removalEasy to operatePeptide preparation methodsUltrafiltrationFiltration
The invention provides a method for removing endotoxin in protein. The method adopts a sequential combination of chromatography, ultrafiltration and common filtration, and accords with a protein purification process flow. With the method, protein purification is realized while endotoxin is effectively removed, and the properties of protein is not influenced. The method is especially suitable to be used in treating various genetically engineered bacteria expressed intracellular and extracellular proteins. With optimized designs of the conditions of filtering mode, filtering pressure, membrane pore size and the like, operation steps and operation time are simplified, such that production cost is reduced, and processing capacity is improved. With the method provided by the invention, the endotoxin content can be reduced to an animal clinical application standard range, and the properties of the product is not influenced while the endotoxin is removed. The method is suitable for large-scale productions and applications.
Owner:TIANJIN RINGPU BIO TECH
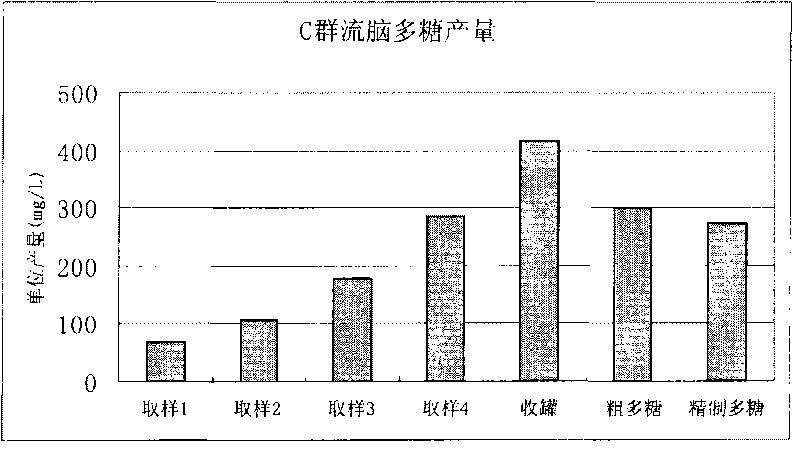
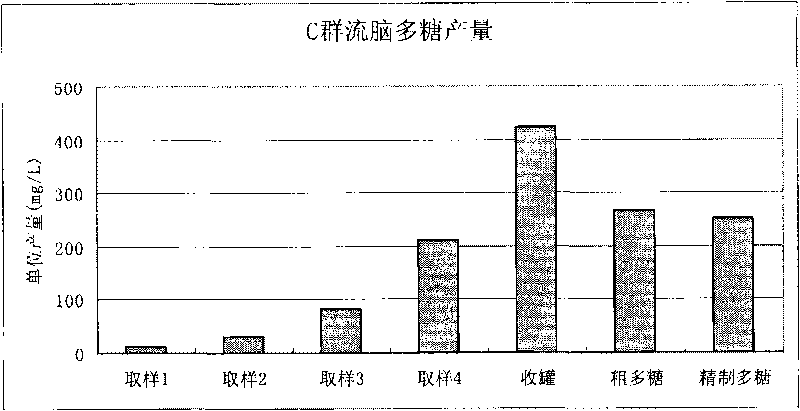
Meningitis vaccine and preparation method thereof
ActiveCN103721249ALow endotoxin contentImprove securityAntibacterial agentsBacterial antigen ingredientsLactoseMeningitis vaccines
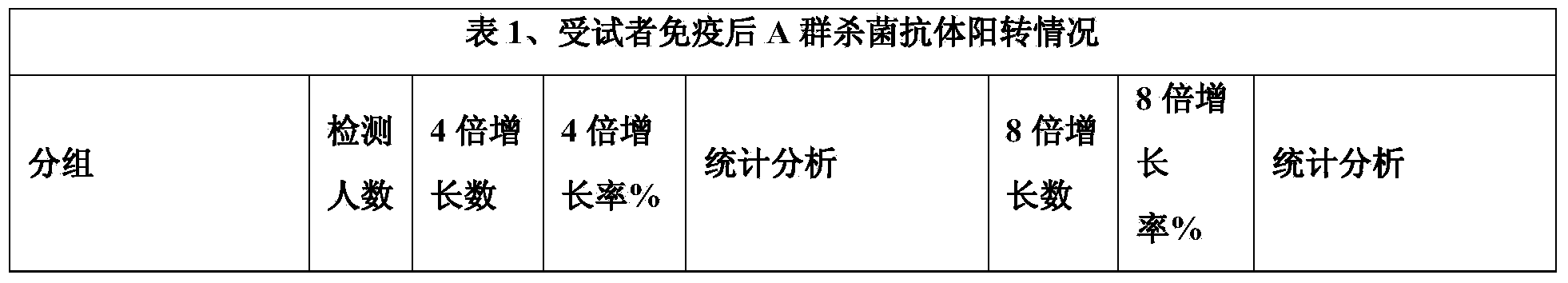
The invention discloses a meningitis vaccine and a preparation method thereof. The meningitis vaccine consists of polysaccharide obtained by fermenting A Neisseria meningitidis, polysaccharide obtained by fermenting C Neisseria meningitidis, polysaccharide obtained by fermenting Y Neisseria meningitidis, polysaccharide obtained by fermenting W135 Neisseria meningitidis, lactose and water. Experiments prove that the endotoxin content of the polysaccharide prepared by the method disclosed by the invention is very low and is 1 EU / mu g; the endotoxin content of the vaccine prepared by the polysaccharide is very low and is 1 EU / mu g. The vaccine disclosed by the invention is safe and effective; adverse reactions are smaller after the vaccine is inoculated; the vaccine can be accepted by receivers easily and is favorable to the popularization of national immunization policies.
Owner:华兰生物疫苗股份有限公司
AFP recombinant protein, and in vitro high-efficiency recombination expression method
ActiveCN103755811ANo pollution in the processHigh purityDigestive systemPharmaceutical non-active ingredientsEnzyme digestionPollution
Owner:SHANGHAI CLAISON BIOTECH
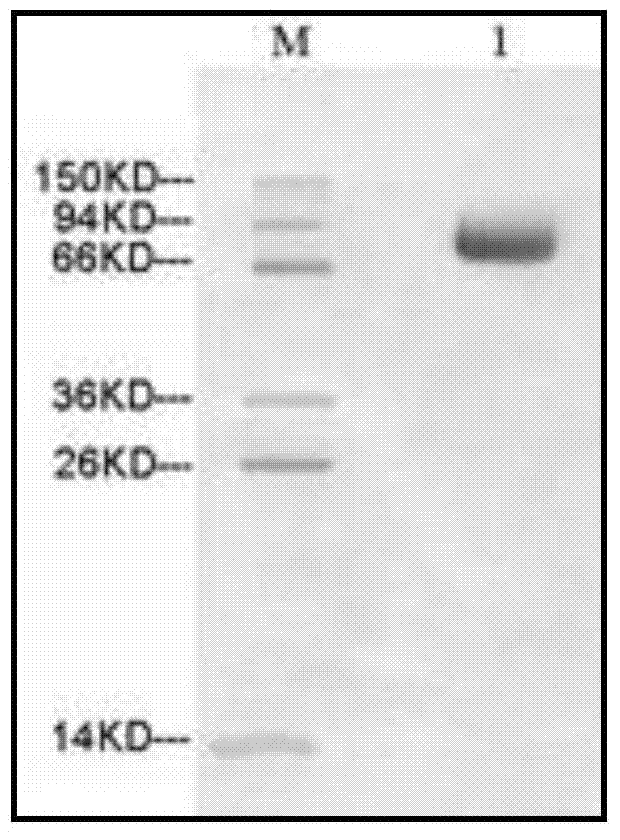
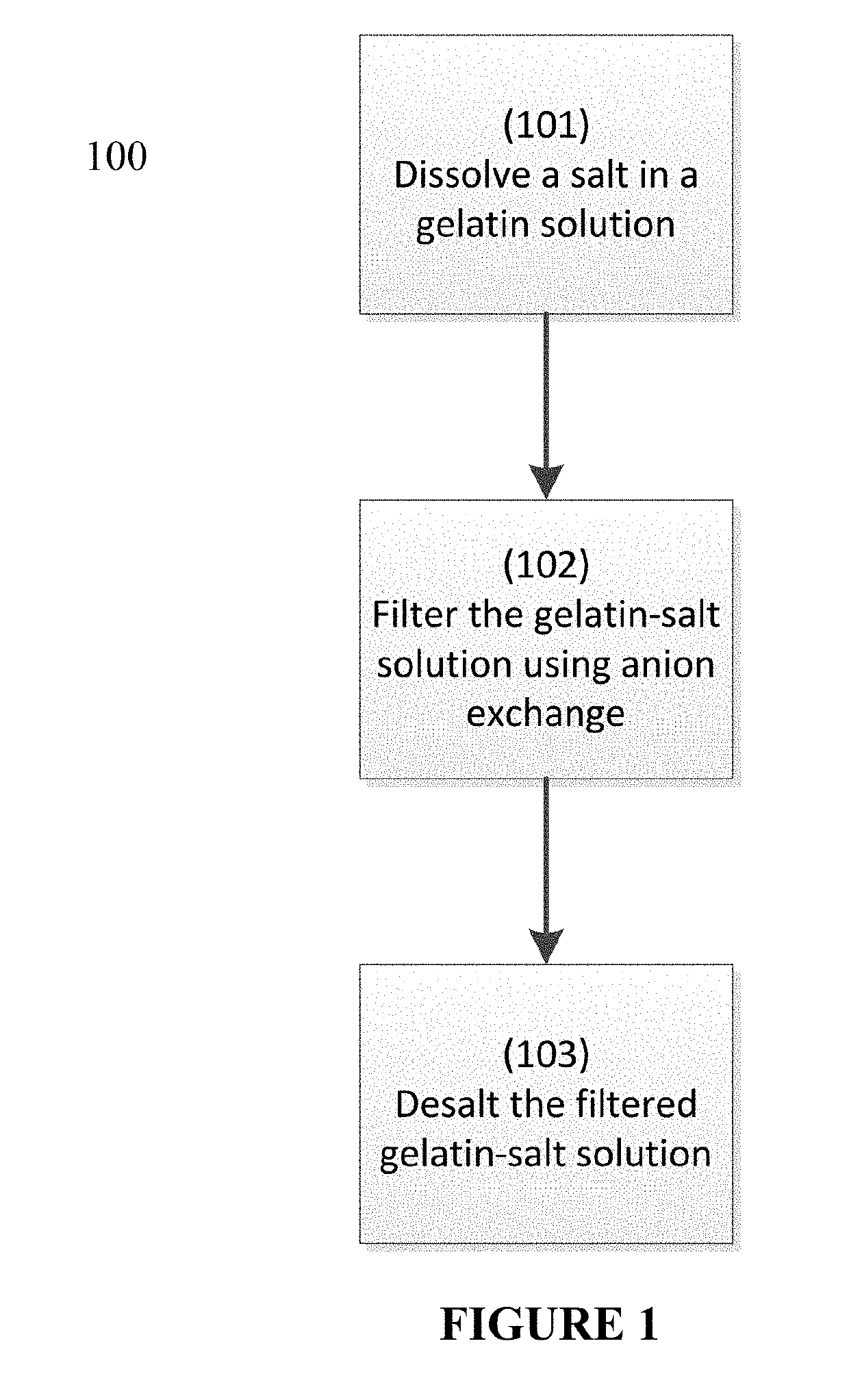
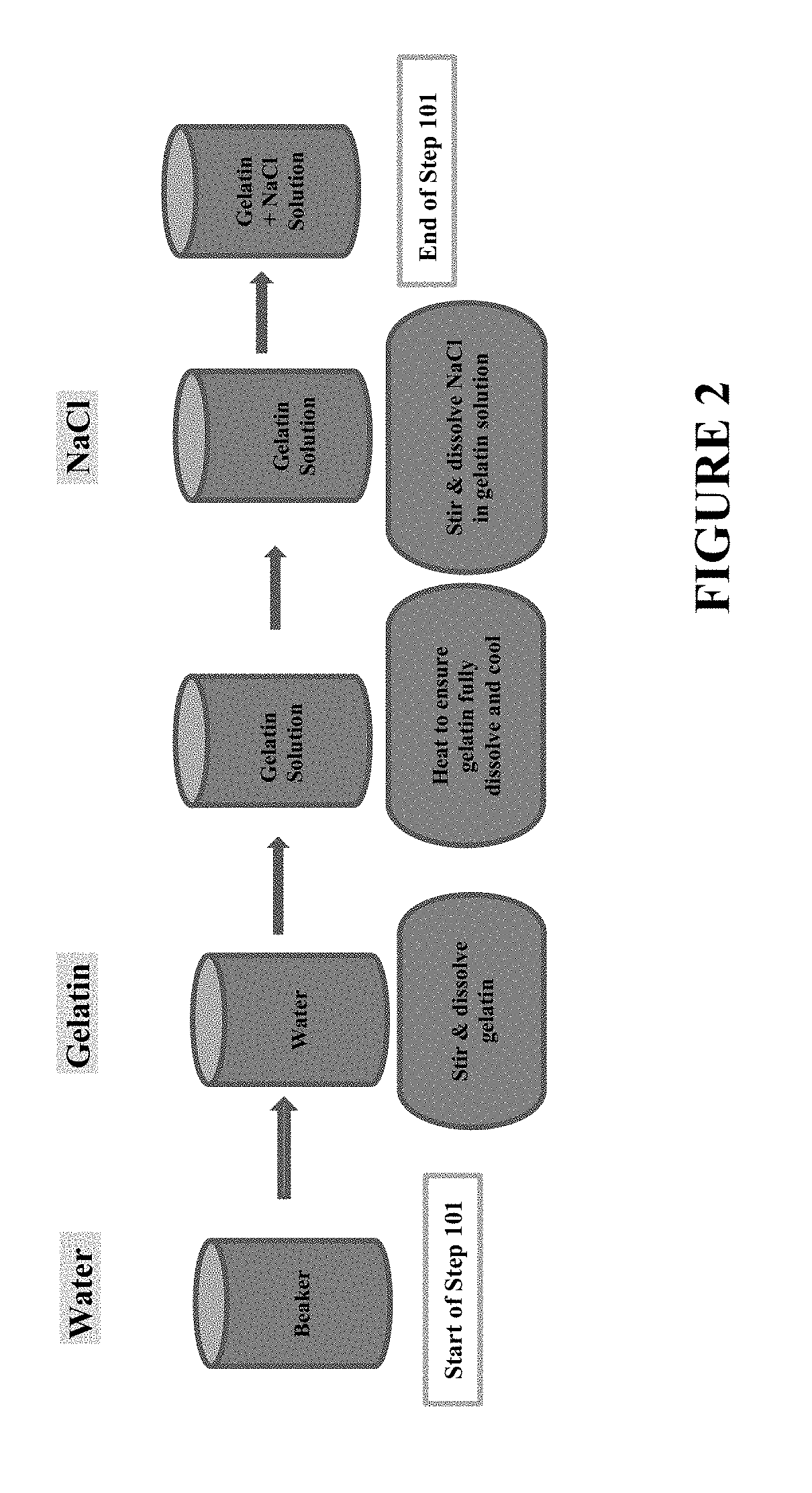
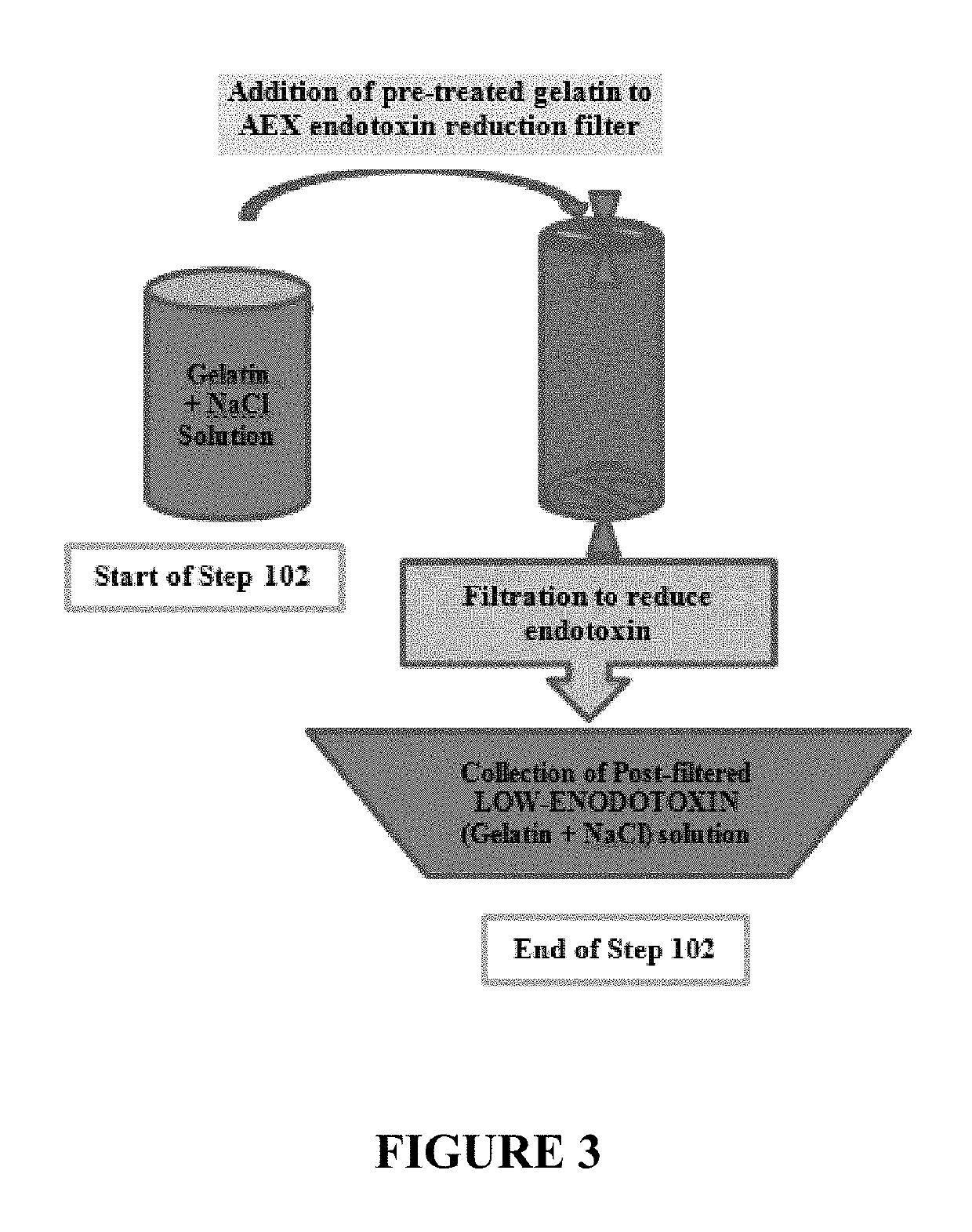
Process to reduce endotoxin in gelatin
ActiveUS20190276707A1Enhance immune responseLow endotoxin contentIon-exchange process apparatusPowder deliveryIon exchangeToxin
The present disclosure is directed to processes for reducing the endotoxin level in gelatin and the resulting gelatin with low endotoxin content. The process includes dissolving a salt in a gelatin solution and filtering the gelatin-salt solution using anion exchange to reduce the endotoxin level. After reducing the endotoxin level of the gelatin-salt solution, the low endotoxin gelatin-salt solution is desalted to remove the salt, thereby producing a low endotoxin gelatin solution.
Owner:CATALENT U K SWINDON ZYDIS LTD
Recombinant pig alpha-interferon as well as preparation method and application thereof
InactiveCN108179151AHigh potencyLow endotoxin contentBacteriaPeptide/protein ingredientsEscherichia coliInclusion bodies
The invention provides a recombinant pig alpha-interferon as well as a preparation method and application thereof and belongs to the field of genetic engineering. The recombinant pig alpha-interferonhas high antiviral activity and can be effectively applied to prevention and control of viral infectious diseases of livestock and poultry. According to the technical scheme, the preparation method comprises the following steps: optimizing a pig alpha-interferon gene, optimizing an RNA (Ribonucleic Acid) secondary structure and eliminating certain common incision enzyme sites; then cloning to a pET-23b(+) carrier to transform escherichia coli and carrying out induced expression; after carrying out isolation denaturation and purification and renaturation on an inclusion body, obtaining the recombinant pig alpha-interferon. The recombinant pig alpha-interferon provided by the invention has very high valence and the antiviral activity can reach 10<10>U / mg; the recombinant sheep tau-interferoncan be effectively applied to prevention and treatment of viral diseases of the livestock and poultry.
Owner:QINGDAO VLAND BIOTECH INC +1
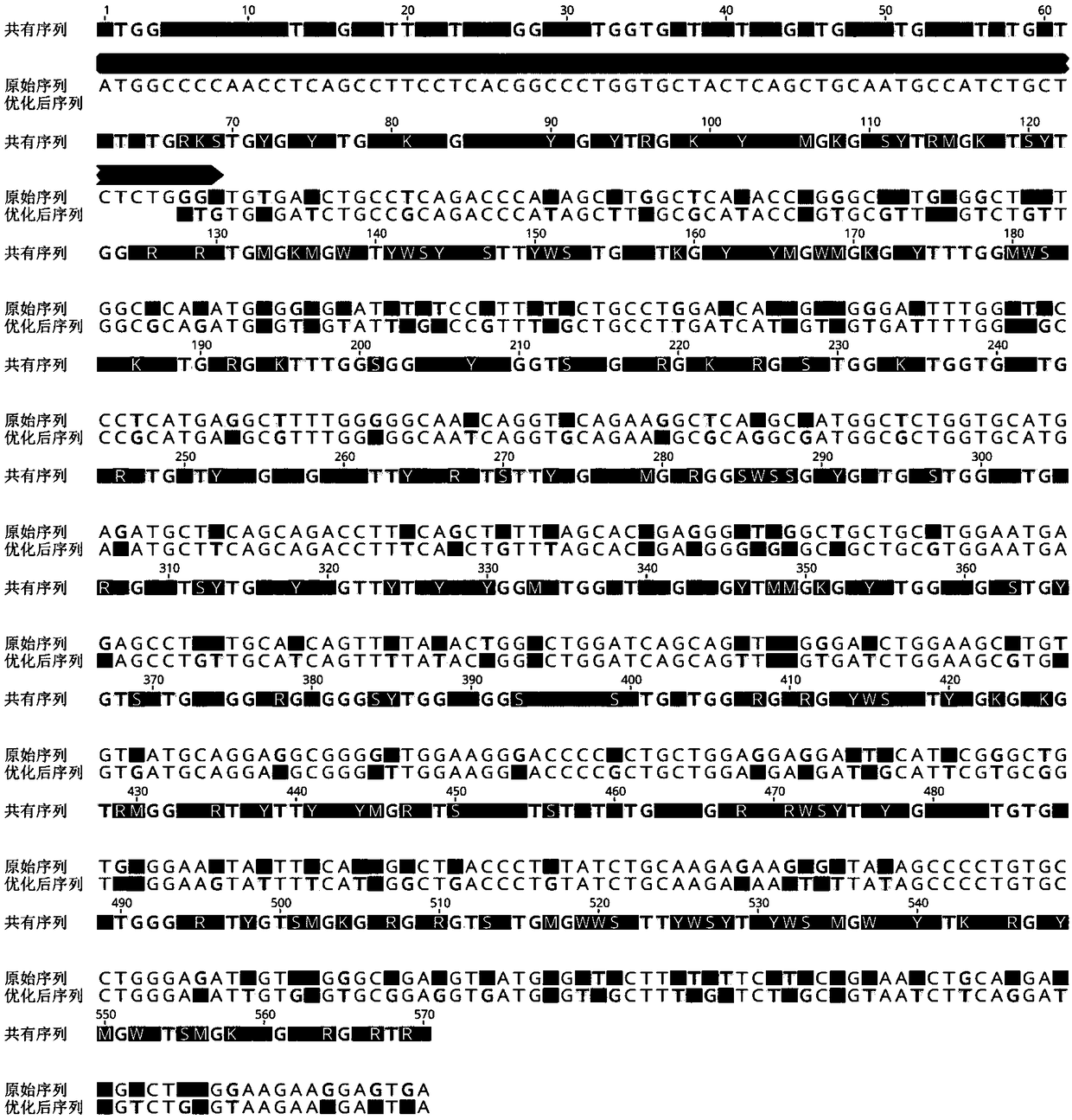
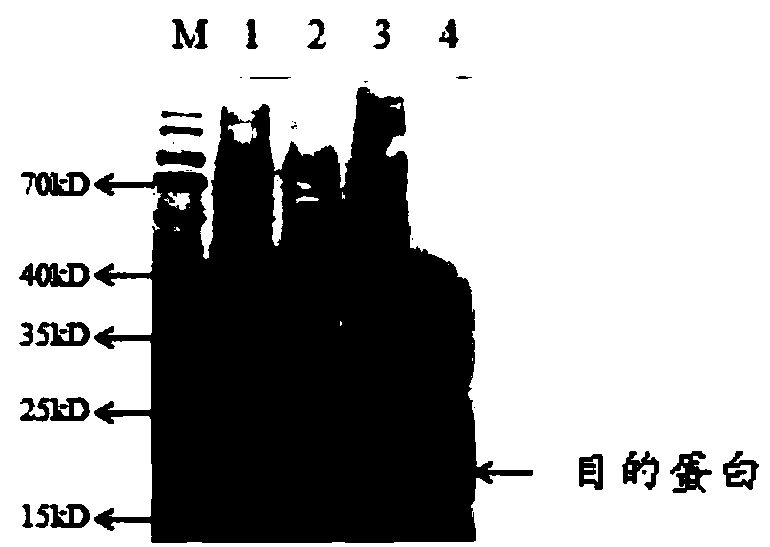
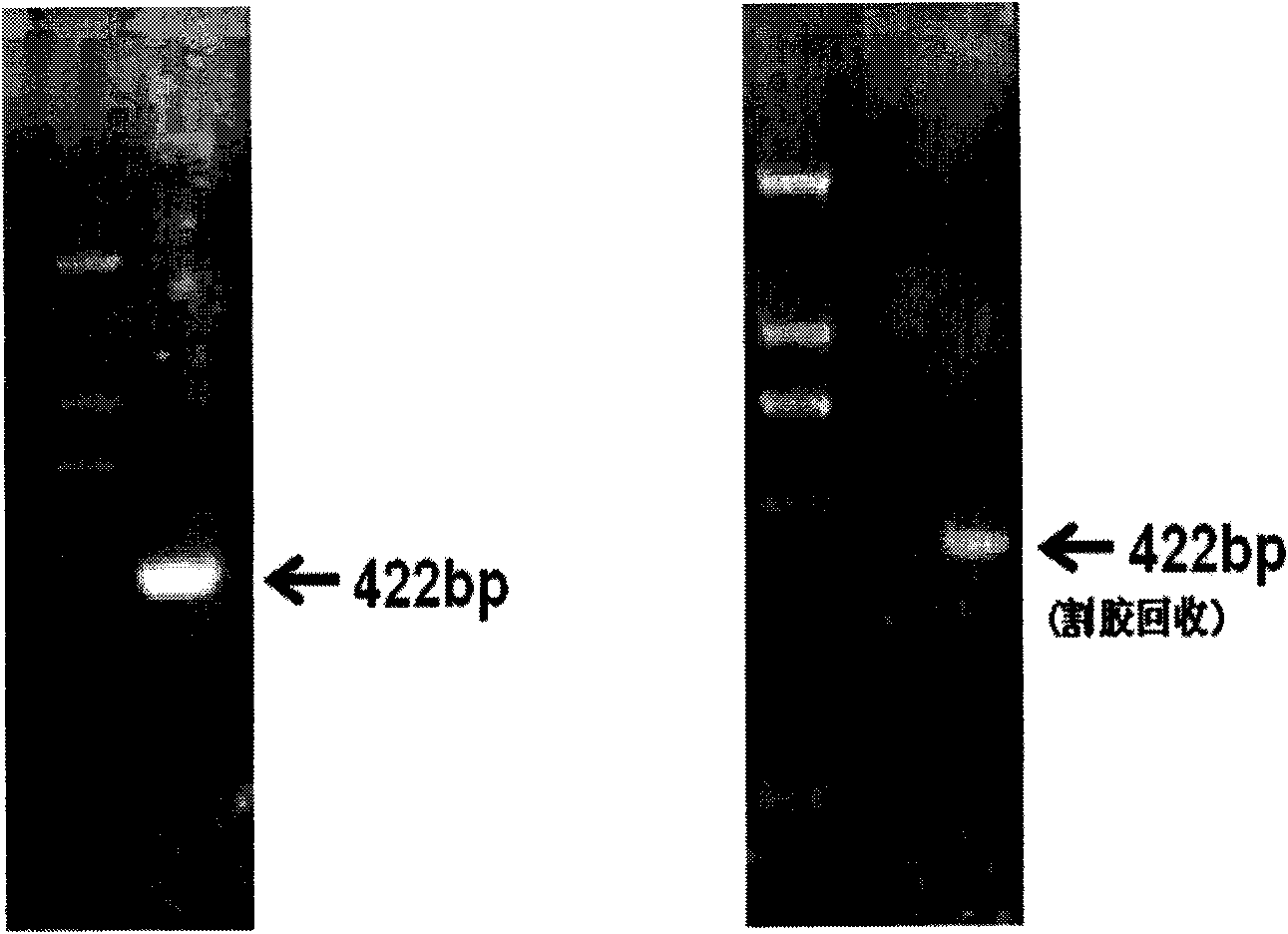
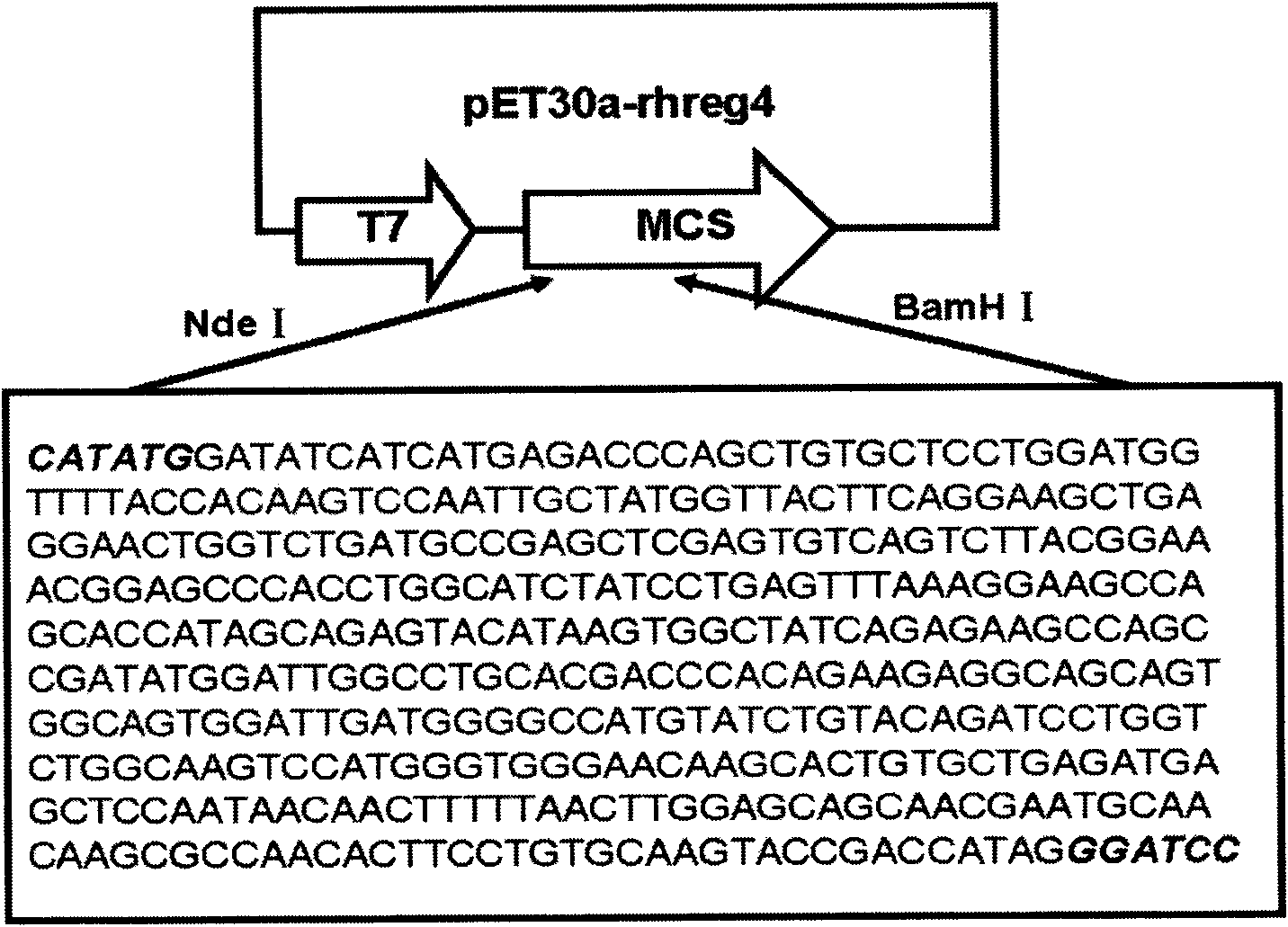
Human recombination Reg4 protein and coding gene thereof as well as preparation method thereof
InactiveCN101648995AHigh activityQuality improvementMicroorganism based processesPeptide preparation methodsCancer cellA-DNA
The invention discloses a human recombination Reg4 protein and a coding gene thereof as well as a preparation method thereof, belonging to the technical field of gene engineering. The invention relates to a human recombination Reg4 protein which is the protein of (a) or (b) as follows: (a) a protein shown as an amino acid sequence, such as SEQ ID NO: 1; and (b) a protein which is formed by substituting, deleting one or more amino acids from or adding one or more amino acids to the amino acid sequence in (a), has the added value promoting activity of a cancer cell and is derived from the (a). The invention also relates to a DNA sequence for coding the human recombination Reg4 protein. The invention also provides a method for preparing the human recombination Reg4 protein, which comprises the following steps: constructing a recombination expression vector containing DNA for coding the human recombination Reg4 protein; preparing a transformant containing the recombination expression vector obtained in the step (1); culturing the transformant and expressing the protein; purifying the protein and obtaining the human recombination Reg4 protein. The human recombination Reg4 protein is ina way of directly having activity and has simple preparation method, low cost, protein product purity more than 98 percent, low endotoxin content and high activity.
Owner:SHANGHAI JIAO TONG UNIV
Method for isolating monocytes in bovine peripheral blood
InactiveCN102329774AStable separationReduce unfavorable factors for adherent separationBlood/immune system cellsIsolation effectAnticoagulant
The invention provides a method for optimally isolating monocytes in bovine peripheral blood. ACD-A is taken as an anticoagulant, the method comprises the optimization of centrifugal conditions, the optimization of cell cleaning conditions, the optimization of wall-sticking conditions and the like, and the success rate of the isolation is greatly improved. In the method, a whole set of complete system which comprises the sampling of bovine blood, the isolation of the monocytes in the bovine peripheral blood and the final transformation of the monocytes into macrophages is established for the first time, and the counting and dyeing are not required in the process, so that the time is saved and an isolation effect is improved. The survival rate of the monocytes isolated by the method is high; and the method is high in experimental repeatability and strong in operability, and provides powerful technical support for simply, conveniently and efficiently isolating the monocytes in the bovine peripheral blood.
Owner:CHINA AGRI UNIV
Purifying method for bacterial capsular polysaccharide
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
A corn bioactive peptide additive used for a cell culture medium
ActiveCN104593318AHigh in PolypeptideLow endotoxin contentVertebrate cellsArtificial cell constructsBiotechnologySerum ige
The present invention provides a maize active peptide additive used for a cell culture medium, an oligopeptide of molecular weight less than 1000 daltons accounting for a mass percentage of ≥ 90% of the total protein in said maize active peptide additive, and said oligopeptide at least comprising one or more of AP, SAP, PAL, VNAP, PSSQ, and TQPGPQ. The maize active peptide additive of the present invention can be compounded with various basal media and can be used for various animal cell serum-free cultures.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD
Method for extracting albumin and globulin from bovine serum and method for utilizing ox blood
ActiveCN106749626AHigh purityIncrease profitSerum albuminPeptide preparation methodsBovine serum albuminBiologic Products
The invention discloses a method for extracting albumin and globulin from bovine serum and a method for utilizing ox blood and relates to the technical field of protein extraction. According to the method for extracting albumin and globulin from bovine serum provided by the invention, the principle of plasma protein depositing in saline solution in a certain saturability is utilized, (NH4)2SO4 fractional precipitation is adopted and the bovine serum albumin is further purified. The bovine serum albumin is high in purity, is low in endotoxin content and meets the requirement of Chinese Biological Product Regulation. A saturated (NH4)2SO4 fractional repeated salting out method is utilized to extract the bovine serum albumin; the high molecular weight plasma protein is extremely removed; and the purity of the extracted bovine serum albumin is high. The method for extracting albumin and globulin from bovine serum provided by the invention is simple in process; no special device is required; the method is suitable for expanded production; and the method for utilizing ox blood provided by the invention can increase the use ratio of the ox blood.
Owner:长睿生物技术(成都)有限公司
Reagent and method for extracting plasmids
The invention provides a reagent and a method for extracting plasmids. The reagent for extracting plasmids comprises a bacteria lysis solution, an endotoxin removing grain solution, an endotoxin removing solution, a plasmid combining solution, a cleaning solution, an eluant and the like. According to the reagent and the method for extracting the plasmids provided by the invention, the operation steps are simple, the time for extracting is short, the endotoxin content in the extracted plasmids can be effectively reduced, the content of endotoxin in the extracted plasmids can be reduced to be lower than 0.1EU / mu g to satisfy the clinical criteria and the obtained plasmids have high DNA (Deoxyribonucleic Acid) yield and good purity.
Owner:HANGZHOU KMB BIOTECH
Triple inactivated vaccine and preparation method thereof
InactiveCN110680914AHexon is high in proteinImproving immunogenicitySsRNA viruses negative-senseViral antigen ingredientsImmunogenicityTGE VACCINE
The invention provides a triple vaccine. Antigens of the triple inactivated vaccine include an inactivated chicken newcastle disease La Sota virus strain, an inactivated H9 subtype avian influenza QF01 strain and an inactivated I-group 8b type fowl adenovirus Hexon protein. The invention further provides a preparation method of the triple inactivated vaccine. The preparation method comprises the following steps of enabling the I-group 8b type fowl adenovirus Hexon protein to be inactivated through pyrrole, and performing mixing and emulsifying on the inactivated I-group 8b type fowl adenovirusHexon protein, an inactivated newcastle disease virus concentrated solution and an inactivated avian influenza virus concentrated solution to obtain the triple inactivated vaccine. The I-group 8b type fowl adenovirus Hexon protein inactivated by the triple inactivated vaccine is high in content, high in immunogenicity and low in formaldehyde content and endotoxin content, has better protection effects on current epidemic I-group 8b type fowl adenovirus, is good in safety properties and true in immune effects, can resist infection of clinical chicken groups on newcastle disease virusese, avianinfluenza and I-group 8b type fowl adenovirus, and is long in persistent period, the immunity persistent period can reach 5 months under the condition that 0.3mL of the vaccine is only used once, andat least 70% of the protection ratio can be provided for the chicken groups.
Owner:洛阳职业技术学院 +2
Process for preparing plasmid in large scale with high yield
InactiveCN1373214AFairly pureIncrease productionBacteriaSugar derivativesEscherichia coliProteinase K
Owner:MILITARY VETERINARY INST MILITARY SUPPIES PLA
Quadruple inactivated vaccine and preparation method thereof
InactiveCN111494617ANo injection stressImproving immunogenicitySsRNA viruses negative-senseViral antigen ingredientsDiseaseAvian adenovirus
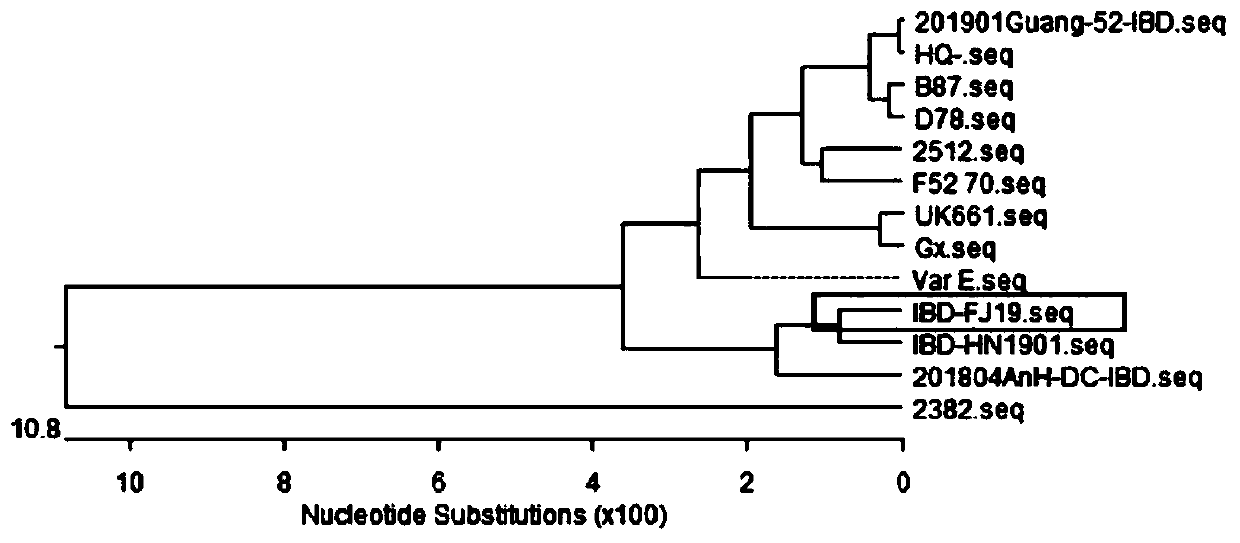
The invention provides a method for preparing a quadruple inactivated vaccine by adopting VP2 protein of a bursal disease virus variant FJ19 (with the preservation number of CGMCC NO: 19381), and a corresponding vaccine. Antigens of the vaccine are a newcastle disease La Sota virus strain, an avian influenza TL18 strain (with the preservation number of CGMCC NO: 19382), a VP2 protein of a bursal disease virus variant FJ19 and an I group 8b type avian adenovirus Hexon protein. The quadruple inactivated vaccine disclosed by the invention is high in VP2 protein and Hexon protein content, high inimmunogenicity and low in formaldehyde and endotoxin content, has a relatively good protection effect on the currently popular variant bursal disease virus FJ19 and the group I 8b type avian adenovirus, is good in safety performance and definite in immune effect, can resist infection of Newcastle disease virus, avian influenza virus, variant bursal disease virus and group I 8b type avian adenovirus to clinical chicken flocks at the same time, is small in immune dose and long in duration, can reach the immune duration of 5 months under the condition of only 0.3 mL once, and at least provides aprotection rate of 70% for the chicken flocks.
Owner:扬州优邦生物药品有限公司
Freeze-dried live attenuated hepatitis A vaccine not containing gelatin or human albumin protective agent and preparation method for freeze-dried live attenuated hepatitis A vaccine
The invention belongs to the field of biological products, in particular to a freeze-dried live attenuated hepatitis A vaccine protective agent not containing gelatin or human albumin and used for preventing hepatitis A, and a preparation method for the freeze-dried live attenuated hepatitis A vaccine protective agent. The protective agent comprises trehalose, dextran 40, L-cysteine, arginine, glutamic acid, glycine, magnesium chloride, magnesium sulfate, sorbierite, mannitol and tris(hydroxymethyl)aminomethane. The protective agent with the formula is mixed with a hepatitis A vaccine stock solution to form a semi-finished product, the semi-finished product is packaged and freeze-dried, and virus infectious titers of the vaccine before and after freeze drying and the thermal stability after freeze drying are detected; after the formula is compared with the conventional production formula containing the gelatin, results show that the protective agent has good protective effect, the descent of the virus infectious titers of the vaccine in the freeze drying process is obviously decreased, and the endotoxin content in a finished product is obviously reduced (less than 0.25EU / ml); and after the freeze-dried vaccine with the formula is inoculated into a human body, results indicate that the vaccine has good immune effect and high safety.
Owner:ZHEJIANG PUKANG BIOTECH
Preparation of an endotoxin-specific adsorption membrane and its application in the field of assisted reproduction
ActiveCN108579698BObvious anti-endotoxin effectReduce endotoxinIon-exchange process apparatusSemi-permeable membranesEndotoxin removalHexamethylenediamine
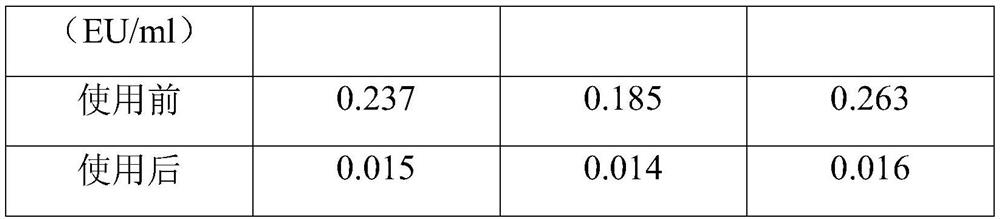
The invention belongs to the field of assisted reproduction, and in particular relates to a specific adsorption membrane for endotoxin. The endotoxin-specific adsorption membrane of the present invention is specifically as follows: a PVDF hollow fiber membrane is used as a carrier, polymyxin B is used as an adsorbent, wherein polymyxin B is fixed on the carrier in a coupled manner, and the PVDF hollow fiber membrane is HEC is coupled, and a hexamethylenediamine spacer is grafted, and the polymyxin B is coupled to the hexamethylenediamine spacer. It can be used to remove endotoxins in various buffer solutions, culture solutions, operating solutions, etc. involved in the field of assisted reproduction, and effectively reduce the content of endotoxins to an ideal range without affecting the content of active ingredients. The present invention also provides an operation method when the above-mentioned membrane is used in sperm micromanipulation fluid to remove endotoxin. The endotoxin-specific adsorption membrane of the present invention is used for removing endotoxin from the culture solution in the field of assisted reproduction, which is helpful for assisted reproduction to improve the embryo development rate, and the embryo has better morphology.
Owner:成都艾伟孚生物科技有限公司
Purification of nucleic acid from a sample containing nucleic acid and endotoxin
ActiveUS10457931B2Simple and economical processReduce investmentMicrobiological testing/measurementDNA preparationOrganic solventEndotoxin removal
Disclosed is a process for purifying nucleic acids, especially plasmid DNA, from a nucleic acid-containing, biologic sample, consisting of (a) preparation of a fluid sample containing nucleic acid and endotoxin each in dissolved form; (b) precipitation at least of nucleic acid and endotoxin from the fluid sample; (c) washing of the components of the fluid sample precipitated out in step b) in order to at least partially remove the endotoxin with at least one washing solution, which contains at least one amine compound with at least two carbon atoms and with a molar mass of ≤500 g / mol and; at least one organic solvent different from the aforementioned amine compound and has a pH-value (20° C.) in the range from pH 3.0 to pH 8.5, and; (d) dissolution of the remaining nucleic acid from the washed, precipitated constituents from step c) using a dissolving buffer and collection of the dissolved nucleic acids in a separate receptacle. The process is suitable for the effective and economical removal of endotoxins.
Owner:AXAGARIUS
Fermentation process and application of mannan peptide
ActiveCN104404114AComposition distribution is stableNarrow distribution widthPeptide/protein ingredientsMicroorganism based processesActivated carbonMicroorganism
The invention belongs to the field of a microbial fermentation technology (C12P) and a medicinal peptide (C07K), and particularly provides a fermentation preparation method of mannan peptide. According to the fermentation preparation method, vaccination, concentration and activated carbon treatment of hemolytic streptococcus and the like are improved, so that the endotoxin content of the prepared mannan peptide is significantly reduced and the safety of medication is improved. The invention further provides the mannan peptide as well as preparations and application thereof and the like.
Owner:SINOPHARM A THINK PHARMA +1
Simple method for extracting low-endotoxin bowman acinetobacter capsular polysaccharide
InactiveCN107488237AEfficient removalLow endotoxin contentAntibacterial agentsBacterial antigen ingredientsToxinPolysaccharide
The invention relates to a simple method for extracting low-endotoxin bowman acinetobacter capsular polysaccharide, which belongs to the technical field of biomaterial preparation. According to the invention, the method employs a lysate and a ultrasonic method for co-cracking the bowman acinetobacter, uses a CTAB solution for primary removal of endotoxins such as lipopolysaccharide, ethanol with concentration being 25% and ethanol with concentration being 80% are respectively used for depositing the obtained bowman acinetobacter capsular polysaccharide, the impurities such as lipopolysaccharide and nucleic acid can be removed, and the low-endotoxin bowman acinetobacter capsular polysaccharide can be finally obtained. The method has the advantages of simpleness, rapidity and low cost, the endotoxin is low, and the method can be used for in-vivo tests and researches.
Owner:JIANGSU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com