Meningitis vaccine and preparation method thereof
A technology for vaccines and meningitis, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, bacterial antigen components, etc., and can solve problems such as fever reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1, prepare meningitis vaccine I
[0063] 1. Separately ferment Neisseria meningitidis group A, Neisseria meningitidis group C, Neisseria meningitidis group Y and Neisseria meningitidis group W135
[0064] (1) Production strain preparation
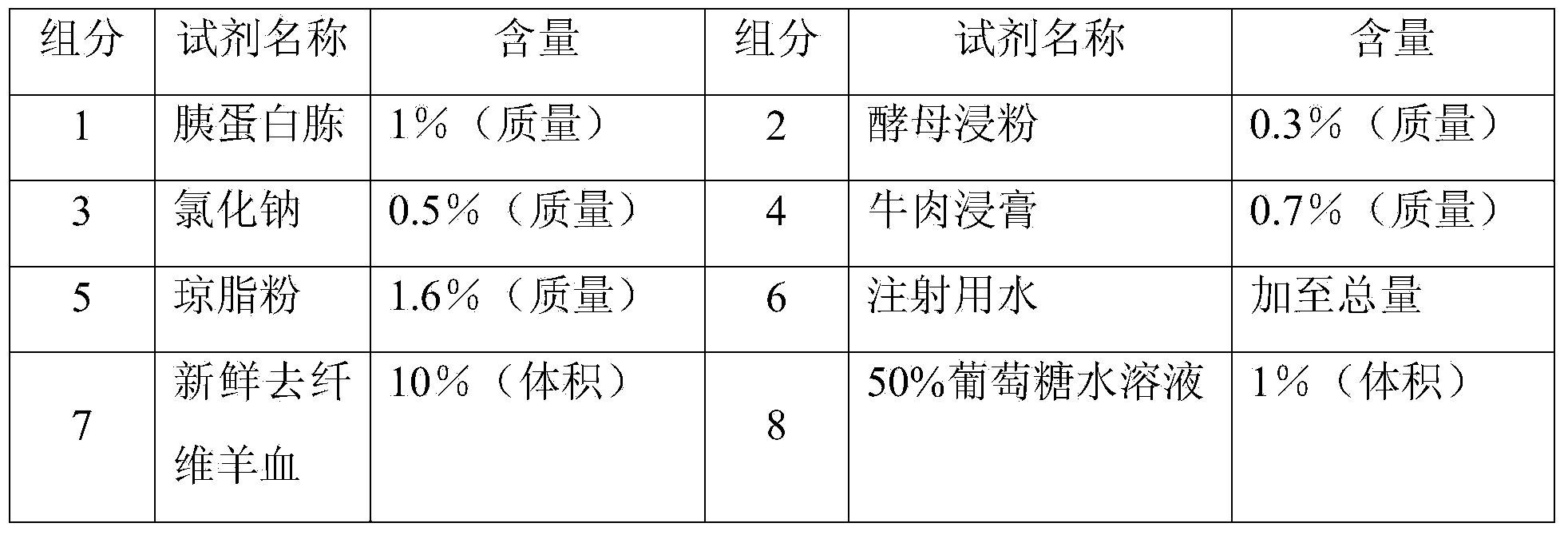
[0065] The first generation: open the batch of working seed strains, inoculate on 10% sheep blood ordinary agar medium, and culture at 37°C and 8% carbon dioxide environment for 18 hours.
[0066] Second-generation and third-generation culture: Inoculate the first-generation strains into semi-comprehensive meningitis medium and incubate at 37°C for 12 hours; inoculate the second-generation strains into semi-comprehensive meningitis medium and culture at 37°C for 12 hours .
[0067] (2) The fourth generation (strain tank) culture
[0068] The third-generation strains were divided into 5×10 8 The concentration of / ml was inoculated in the strain jar that the ECM semi-comprehensive liquid medium was housed, and cultivated...
Embodiment 2
[0105] Embodiment 2, preparation meningitis vaccine II
[0106] 1. Separately ferment Neisseria meningitidis group A, Neisseria meningitidis group C, Neisseria meningitidis group Y and Neisseria meningitidis group W135
[0107] (1) Production strain preparation
[0108] The first generation: open the batch of working seed strains, inoculate on 10% sheep blood ordinary agar medium, and culture at 37°C and 8% carbon dioxide environment for 18 hours.
[0109] Second-generation and third-generation culture: Inoculate the first-generation strains into semi-comprehensive meningitis medium and incubate at 37°C for 12 hours; inoculate the second-generation strains into semi-comprehensive meningitis medium and culture at 37°C for 12 hours .
[0110] (2) The fourth generation (strain tank) culture
[0111] The third-generation strains were divided into 5×10 8 The concentration of / ml was inoculated in a strain tank equipped with ECM semi-comprehensive liquid medium, and cultivated f...
Embodiment 3
[0148] Embodiment 3, preparation meningitis vaccine III
[0149] 1. Separately ferment Neisseria meningitidis group A, Neisseria meningitidis group C, Neisseria meningitidis group Y and Neisseria meningitidis group W135
[0150] (1) Production strain preparation
[0151] The first generation: open the batch of working seed strains, inoculate on 10% sheep blood ordinary agar medium, and culture at 37°C and 8% carbon dioxide environment for 18 hours.
[0152] Second-generation and third-generation culture: Inoculate the first-generation strains into semi-comprehensive meningitis medium and incubate at 37°C for 12 hours; inoculate the second-generation strains into semi-comprehensive meningitis medium and culture at 37°C for 12 hours .
[0153] (2) The fourth generation (strain tank) culture
[0154] The third-generation strains were divided into 5×10 8 The concentration of / ml was inoculated in the strain jar that the ECM semi-comprehensive liquid culture medium was housed, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com