Simple method for extracting low-endotoxin bowman acinetobacter capsular polysaccharide
A technology of Acinetobacter baumannii and capsular polysaccharide, which is applied in antibacterial drugs, pharmaceutical formulations, bacterial antigen components, etc., and can solve the problems of weak removal of bacterial endotoxin, failure to meet clinical needs, and low capsular expression , to achieve the effect of high-efficiency endotoxin content, high cost and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Acinetobacter baumannii capsular polysaccharide extraction
[0024] (1) Bacterial culture: resuscitate Acinetobacter baumannii, inoculate the bacteria on a sheep blood agar plate (BD Difco, USA), and culture at 37°C for 16 h to 24 h to form colonies visible to the naked eye. baumannii colonies, diluted to OD with sterile phosphate (PBS) buffer (pH 7.4) 600 =1. The above Acinetobacter baumannii suspension was evenly spread on 40 sheep blood agar plates, each plate was inoculated with 10 μL of bacterial solution, and cultured in a constant temperature incubator (37°C) for 16 h to 24 h until a lawn was formed.
[0025] (2) Bacteria collection: Add 30 mL of sterilized PBS buffer dropwise to each sheep blood agar plate to wash the bacteria repeatedly by blowing and blowing, and transfer all the washed bacteria liquid to 4 sterilized round-bottomed centrifuge tubes (50 mL) , centrifuged at 8 000 rpm for 10 min to collect the bacterial pellet, and discarded the...
Embodiment 2
[0029] Example 2: Detection of Capsular Polysaccharide Concentration of Acinetobacter baumannii
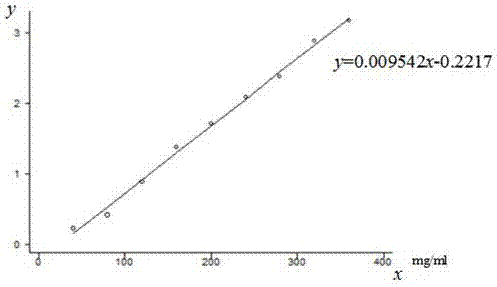
[0030] (1) Establish a standard curve: Accurately weigh 200 g of analytically pure glucose dried at 105°C to constant weight, dilute to 500 mL with three-distilled water, and absorb 0.2 mL, 0.4 mL, 0.6 mL, 0.8 mL, 1.0 mL, For 1.2 mL, 1.4 mL, 1.6 mL and 1.8 mL, add an appropriate amount of triple-distilled water to make up the volume to 2.0 mL. Using triple-distilled water as the blank control, add 1.0 mL of 6% phenol solution and 5.0 mL of concentrated sulfuric acid to each tube to fully Shake well and react at room temperature for 40 min. After the reaction, the absorbance at 490nm of each tube was detected by a 722-type spectrophotometer, and the standard curve was drawn with the concentration of glucose in each tube as the abscissa and the absorbance value as the ordinate.
[0031] (2) Test the absorbance of the sample: draw 1.0 mL of the extracted capsular polysaccharide solu...
Embodiment 3
[0035] Example 3: Capsular polysaccharide endotoxin detection
[0036] The endotoxin content of the extracted capsular polysaccharide of Acinetobacter baumannii was detected by an endotoxin detector (product of Xiamen Limulus Reagent Experimental Factory Co., Ltd.) and its supporting reagents. The detection reaction adopts the endpoint method, and the endotoxin concentration is calculated according to the absorbance at 545 nm of the reaction system. After multiple tests, the endotoxin content of the purified capsular polysaccharide is lower than 0.01 EU / mL, which is lower than the standard of no more than 0.03 EU / mL for human injection, and can be directly used for human injection, such as Further coupled reverse-phase high-performance liquid chromatography purification can improve reverse-phase high-performance liquid chromatography purification efficiency and greatly reduce costs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com