Fermentation process and application of mannan peptide
A mannan peptide and fermentation product technology, applied in the field of microbial fermentation technology and medicinal peptides, can solve the problems of increasing endotoxin content, unable to eliminate bacterial residues, and not being popularized and used.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
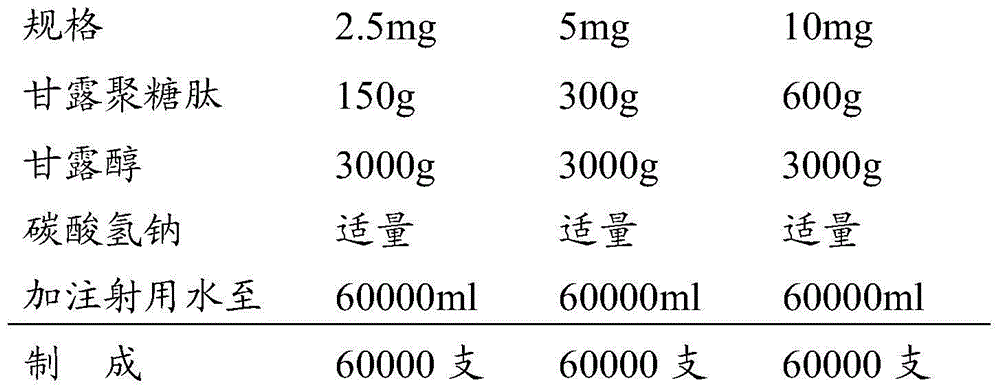
Examples
preparation example Construction
[0042] Preparation of mannan peptides optimized by the prior art of comparative examples
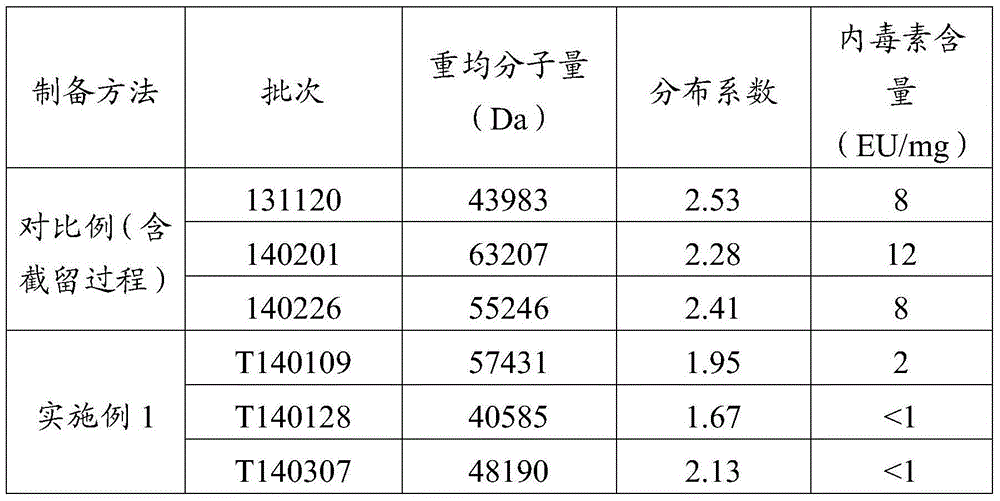
[0043] Basically refer to Example 1 (which includes the identification process of Example 2) described in Chinese Patent 03117578 to prepare mannan peptide by fermentation, except that the use of activated carbon is carried out with reference to the optimal conditions (80°C for 30 minutes) recorded in Chinese Patent 200510020106, and obtain Mannan peptides of the prior art (comparative). The molecular weight distribution of different batches of products produced by this method is unstable, and the weight average molecular weight appears in the range of 20,000 to 200,000, and the distribution width is very wide. Further steps such as membrane filtration to increase the cut-off of a specific molecular weight can be obtained, so mannan peptides with lower weight-average molecular weights can be prepared by increasing the cut-off of membrane filtration.
Embodiment 1
[0044] Embodiment 1 Preparation of mannan peptide of the present invention
[0045] Basically refer to the example 1 described in Chinese patent 03117578, the difference is: α-hemolytic streptococcus is inserted into the fermenter under sterile conditions at a 0.07% inoculum size, and cultured at a constant temperature of 37±1°C for 70 hours , during which sterile air is introduced so that the tank pressure is between 0.005-0.01MPa; then, the bacteria are inactivated, the fermentation liquid in the fermenter is released, and passed into a scraper type thin film concentrator (available from Shanghai Zhongchen Digital Technology Co., Ltd. Equipment Co., Ltd.) concentrated until the specific gravity of the concentrated solution reaches 1.16;
[0046] Then, add 90% (V / V) ethanol to the concentrated solution until the ethanol concentration reaches 78% (V / V), stir while adding, then let stand at room temperature (25°C) for 12 hours, centrifuge to collect the precipitate, and obtain ...
Embodiment 2
[0055] Example 2 Endotoxin determination of the present invention and comparative mannan peptides
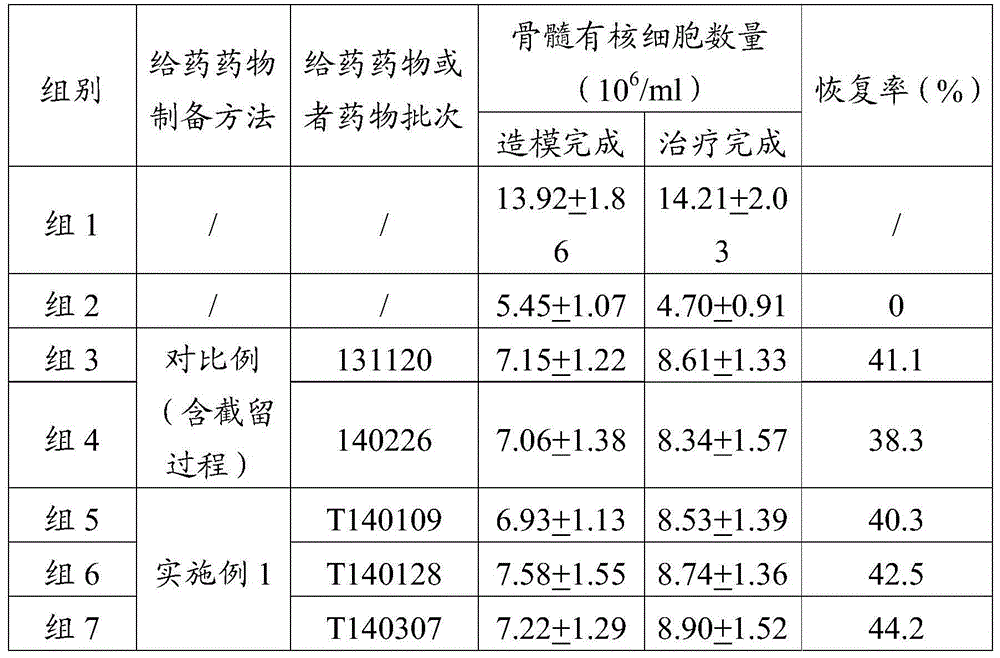
[0056] The endotoxin of the mannan peptide prepared according to the method of Comparative Example and Example 1 was measured, and the results are shown in Table 2, indicating that the amount of endotoxin contained in every mg of the mannan peptide prepared by the method of the present invention was The amount is significantly smaller than that of the prior art, which is almost an order of magnitude difference, which improves the safety of medication, facilitates popularization, and can easily make the endotoxin index of each batch of products reach the standard and can be used for intravenous injection, reducing product waste or low Use of added value.
[0057] Table 2 Physicochemical properties and endotoxin content of mannan peptides prepared by different methods
[0058]
PUM
| Property | Measurement | Unit |
|---|---|---|
| distribution coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com