Synthetic method of specific average degree of substitution sulfobutyl ether-β-cyclodextrin
A technology of sulfobutyl ether and cyclodextrin, which is applied in the field of synthesis of sulfobutyl ether-β-cyclodextrin, can solve the problems of harsh reaction conditions, low yield, environmental pollution, etc., and achieve mild reaction conditions, Increased reaction yield and easy post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
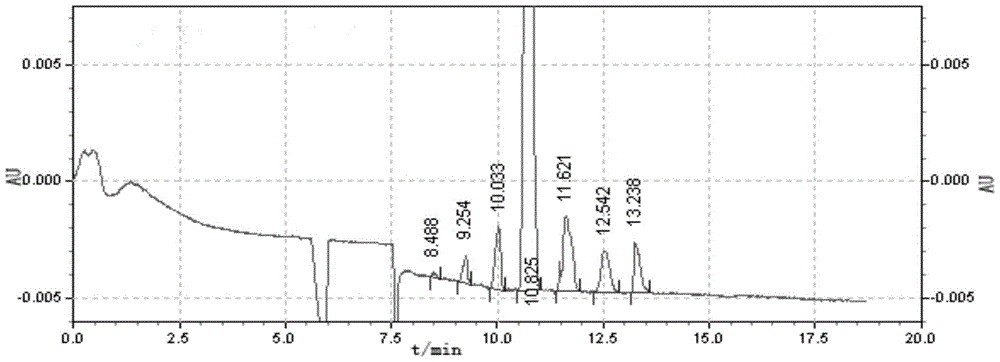
Embodiment 1
[0028] The synthetic method of the sulfobutyl ether-beta-cyclodextrin that specific average degree of substitution is 1 comprises the steps:
[0029] (1) In a 100mL three-necked flask, under stirring conditions, dissolve 10g of β-cyclodextrin in 3.8mL of 15%wt NaOH aqueous solution, NaOH is 1.6 times the molar weight of β-cyclodextrin, and control the reaction temperature at 65°C , stir for 30min;
[0030] (2) Add 1.8mL of 1,4-butanesultone dropwise to a 100mL three-neck flask, the amount of 1,4-butanesultone is twice the molar amount of β-cyclodextrin, and reflux at 65°C for ether chemical reaction, when the pH drops to 8.8, adjust the pH of the solution by the concentration of 15%wt NaOH aqueous solution installed in the sample injector, keep the pH value of the reaction system between 8.80-9.70, when the pH value of the reaction system is at 8.80-9.70 There will be no change between them, all the remaining NaOH aqueous solution contained in the sample injector is added, an...
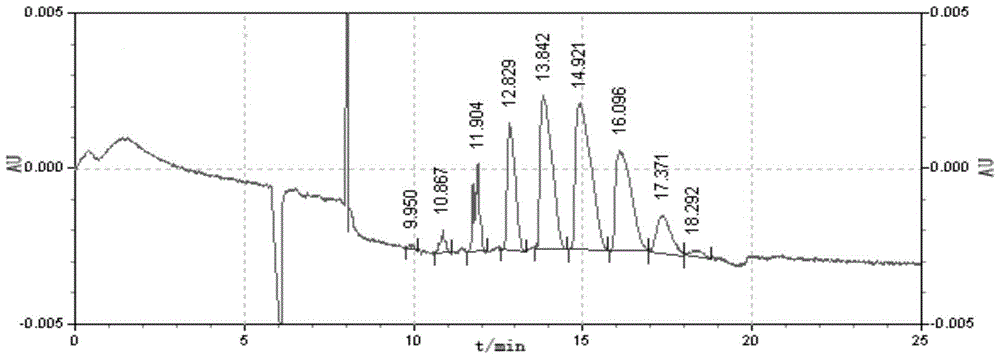
Embodiment 2
[0033] The specific average degree of substitution is a synthetic method of sulfobutyl ether-β-cyclodextrin of 3.4-4.2, comprising the following steps:
[0034] (1) In a 100mL three-necked flask, under stirring conditions, dissolve 10g of β-cyclodextrin in 11.5mL of 12.5%wt NaOH aqueous solution, the amount of NaOH added is 4 times the molar weight of β-cyclodextrin, and the reaction temperature is controlled 75°C, stirring time 30min;
[0035] (2) Add 4.5mL 1,4-butanesultone dropwise to a 100mL three-neck flask, the amount of 1,4-butanesultone added is 5 times the molar amount of β-cyclodextrin, and reflux at 75°C Etherification reaction, when the pH drops to 8.8, adjust the pH of the solution by the concentration of 12.5%wt NaOH aqueous solution installed in the sample injector, keep the pH value of the reaction system between 8.80-9.70, when the pH value of the reaction system is between 8.80- No change occurs between 9.70, all the remaining NaOH aqueous solution contained...
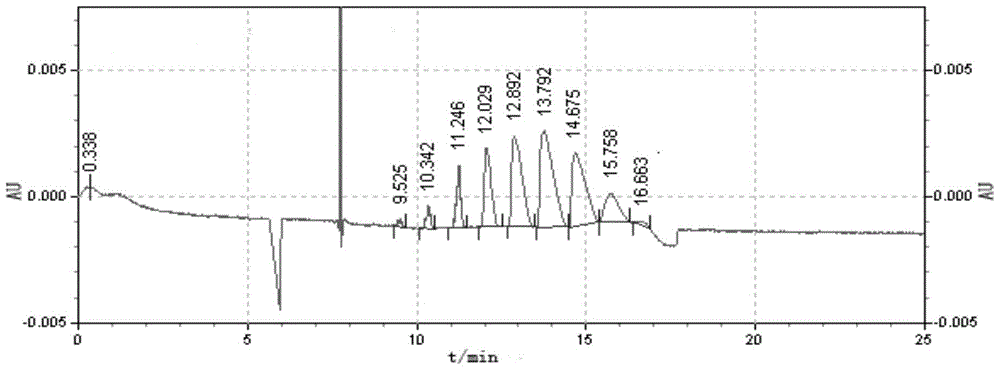
Embodiment 3
[0038] A method for synthesizing sulfobutyl ether-β-cyclodextrin with a specific average degree of substitution of 6.2-6.9, comprising the steps of:
[0039] (1) In a 100mL three-necked flask, under stirring conditions, dissolve 10g of β-cyclodextrin in 18.3mL of 12.5%wt NaOH aqueous solution, the amount of NaOH added is 6.5 times the molar weight of β-cyclodextrin, and the reaction temperature is controlled at 70 ℃, stirring time 30min;
[0040] (2) Add 7.2mL of 1,4-butanesultone dropwise to a 100mL three-neck flask, the amount of 1,4-butanesultone added is 8 times the molar amount of β-cyclodextrin, and reflux at 70°C Etherification reaction, when the pH drops to 8.8, adjust the pH of the solution by the concentration of 12.5%wt NaOH aqueous solution installed in the pH automatic balancer, keep the pH value of the reaction system between 8.80-9.70, when the pH value of the reaction system is at 8.80 No change between -9.70, all the remaining NaOH aqueous solution installed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com