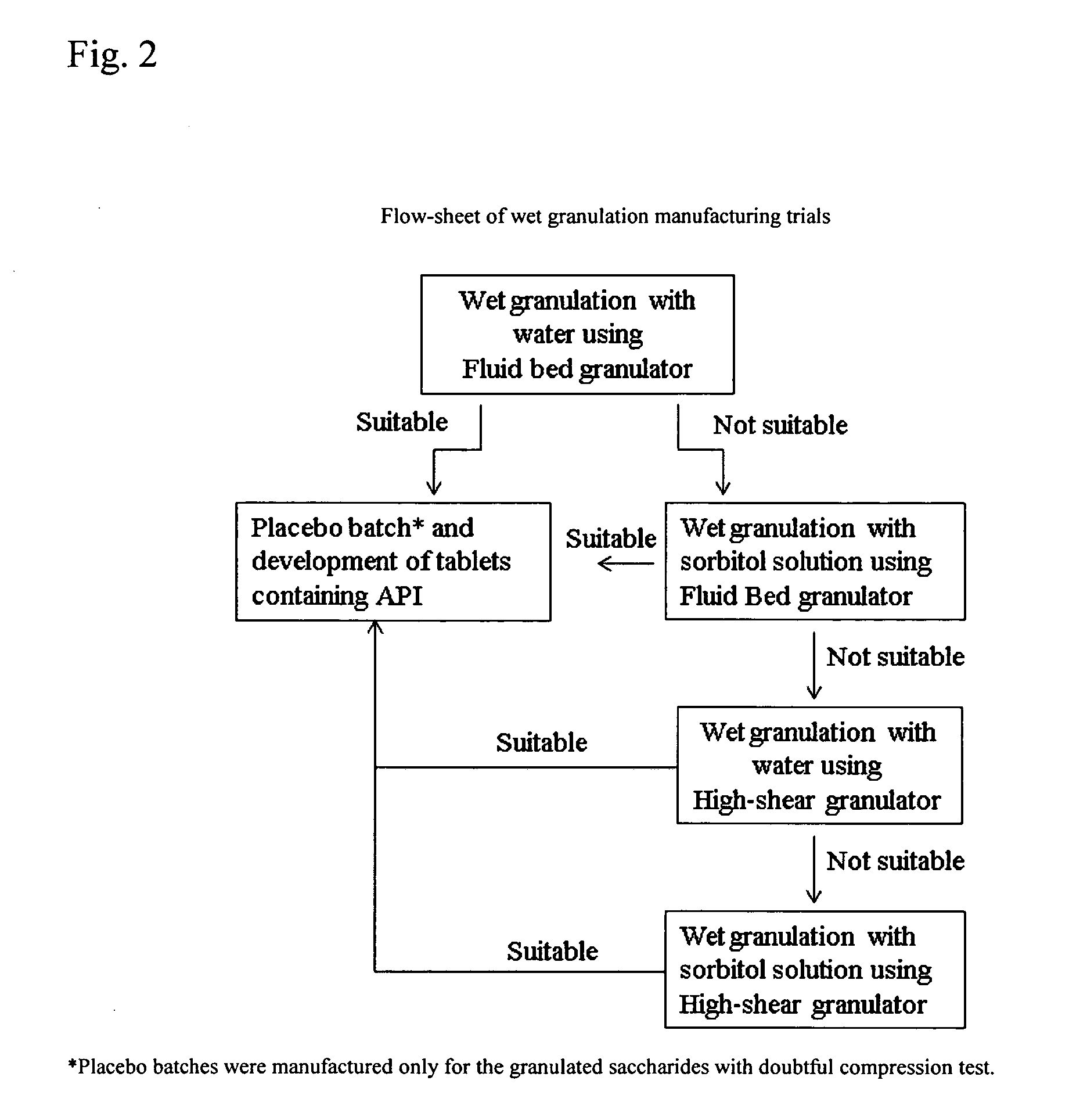
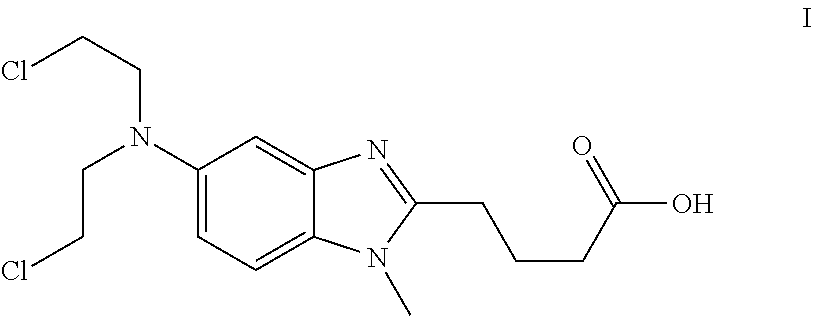
Patents
Literature
80 results about "Cyclic oligosaccharide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fragrance compositions
The present invention relates to a composition comprising:(a) a fragrance oil wherein the fragrance oil comprises:(i) greater than about 50%, by weight of the fragrance oil, of perfume raw materials with high odour impact perfume raw materials which have an odour detection threshold of less than, or equal to, about 50 parts per billion;(ii) less than about 5%, by weight of the fragrance oil, of top note perfume raw materials wherein the top note perfume raw materials have a boiling point of less than about 250° C. at 1 atmosphere pressure(b) an entrapment material which is selected from the group consisting of polymers;capsules, microcapsules, and nanocapsules; liposomes; pro-perfumes; film formers; absorbents; cyclic oligosaccharides and mixtures thereof.(c) greater than about 50%, by weight, of a volatile solvent.The present invention provides compositions wherein the fragrance character remains detectable for greater than about 2 hours, preferably greater than about 4 hours, more preferably greater than about 6 hours, after the composition has been applied to the substrate.
Owner:PROCTER & GAMBLE CO
Cosmetic compositions
InactiveUS6893647B1Long-lasting fragranceCosmetic preparationsImpression capsLong lastingCyclic oligosaccharide
According to the present invention there is provided fragrance compositions comprising (a) fragrance; and (b) cyclic oligosaccharides having one or more unsubstituted alkyl substituents; wherein the weight ratio of (a) to (b) is at least about 1:1. The compositions of the present invention provide a long-lasting fragrance while at the same time having a ‘burst’ of fragrance on application.
Owner:THE PROCTER & GAMBLE COMPANY
Fragrance compositions
A composition comprising:(a) a fragrance oil comprising:(i) top note perfume raw material, or mixture of perfume raw materials, with a boiling point of less than, or equal to, about 250° C. at 1 atmosphere pressure;(ii) middle or base note perfume raw material, or mixture of perfume raw materials, with a boiling point of greater than 250° C. at 1 atmosphere pressure;(b) an entrapment material which is selected from the group consisting of polymers; capsules, microcapsules and nanocapsules; liposomes; pro-perfumes selected from more than 1 type of pro-chemistry; film formers; absorbents; cyclic oligosaccharides and mixtures thereof;(c) greater than 50% volatile solvent;wherein the weight ratio of the top note perfume raw materials to middle or base note perfume raw materials within the fragrance oil is in the range from about 1:20 to about 20:1.The present invention provides compositions wherein the light, fresh, fruity, citrus, green or delicate floral top note fragrance character remains detectable for greater than about 2 hours, preferably greater than about 4 hours, more preferably greater than about 6 hours, after the composition has been applied to the substrate.
Owner:PROCTER & GAMBLE CO
Fragrance compositions
A composition comprising:(a) a fragrance oil wherein the fragrance oil comprises greater than 0.5% of a top note perfume raw material, or mixture of top note perfume raw materials, with a boiling point of less than, or equal to, 250° C. at 1 atmosphere pressure;(b) an entrapment material which is selected from the group consisting of polymers; capsules, microcapsules and nanocapsules; liposomes; film formers; absorbents; cyclic oligosaccharides and mixtures thereof;(c) greater than 50% volatile solvent;wherein the perfume raw material and the entrapment material exist in an associated form on the substrate and wherein the weight ratio of the top note perfume raw material to the entrapment material within the associated form is in the range from about 1:20 to about 20:1.The present invention provides compositions wherein the light, fresh, fruity, citrus, green or delicate floral top note fragrance character remains detectable for greater than about 2 hours, preferably greater than about 4 hours, more preferably greater than about 6 hours, after the composition has been applied to the substrate.
Owner:PROCTER & GAMBLE CO
Fragrance compositions
A composition comprising:(a) from about 0.01% to about 99%, by weight, of a fragrance oil, wherein the fragrance oil comprises about 5% or greater, by weight of fragrance oil, of a top note perfume raw material, or mixture of top note perfume raw materials, and wherein the top note perfume raw materials have a boiling point of less than, or equal to, about 250° C. at 1 atmosphere pressure;(b) an entrapment material which is selected from the group consisting of polymers; capsules, microcapsules and nanocapsules; liposomes; pro-perfumes selected from more than 1 type of pro-chemistry; film formers; absorbents; cyclic oligosaccharides and mixtures thereof;(c) greater than about 50% volatile solvent.The present invention provides compositions wherein the light, fresh, fruity, citrus, green or delicate floral top note fragrance character remains detectable for greater than about 2 hours, preferably greater than about 4 hours, more preferably greater than about 6 hours, after the composition has been applied to the substrate.
Owner:THE PROCTER & GAMBLE COMPANY
Fragrance compositions
The present invention relates to a composition comprising:(a) a fragrance oil wherein the fragrance oil comprises:(i) one or more perfume raw materials with a high odour impact which have an odour detection threshold of less than, or equal to about 50 parts per billion(ii) less than about 4%, by weight of the fragrance oil, of top note perfume raw materials where in the top note perfume raw materials have a boiling point of less than 250° C. at 1 atmosphere pressure(b) an entrapment material which is selected from the group consisting of polymers; capsules, microcapsules, and nanocapsules; liposomes; film formers; absorbents; cyclic oligosaccharides and mixtures thereof;wherein the perfume raw material and the entrapment material exist in an associated form on the substrate and wherein the weight ratio of high odour impact perfume raw materials which have an odour detection threshold of less than, or equal to, about 50 parts per billion to entrapment material within the associated form falls in the range of about 1:20 to about 20:1.
Owner:THE PROCTER & GAMBLE COMPANY
Fragrance compositions
A composition comprising: (a) a fragrance oil wherein the fragrance oil comprises greater than 0.5% of a top note perfume raw material, or mixture of top note perfume raw materials, with a boiling point of less than, or equal to, 250° C. at 1 atmosphere pressure; (b) an entrapment material which is selected from the group consisting of polymers; capsules, microcapsules and nanocapsules; liposomes; film formers; absorbents; cyclic oligosaccharides and mixtures thereof; (c) greater than 50% volatile solvent; wherein the perfume raw material and the entrapment material exist in an associated form on the substrate and wherein the weight ratio of the top note perfume raw material to the entrapment material within the associated form is in the range from about 1:20 to about 20:1. The present invention provides compositions wherein the light, fresh, fruity, citrus, green or delicate floral top note fragrance character remains detectable for greater than about 2 hours, preferably greater than about 4 hours, more preferably greater than about 6 hours, after the composition has been applied to the substrate.
Owner:THE PROCTER & GAMBLE COMPANY
Fragrance compositions
A composition comprising: (a) a fragrance oil comprising: (i) top note perfume raw material, or mixture of perfume raw materials, with a boiling point of less than, or equal to, about 250° C. at 1 atmosphere pressure; (ii) middle or base note perfume raw material, or mixture of perfume raw materials, with a boiling point of greater than 250° C. at 1 atmosphere pressure; (b) an entrapment material which is selected from the group consisting of polymers; capsules, microcapsules and nanocapsules; liposomes; pro-perfumes selected from more than 1 type of pro-chemistry; film formers; absorbents; cyclic oligosaccharides and mixtures thereof; (c) greater than 50% volatile solvent; wherein the weight ratio of the top note perfume raw materials to middle or base note perfume raw materials within the fragrance oil is in the range from about 1:20 to about 20:1. The present invention provides compositions wherein the light, fresh, fruity, citrus, green or delicate floral top note fragrance character remains detectable for greater than about 2 hours, preferably greater than about 4 hours, more preferably greater than about 6 hours, after the composition has been applied to the substrate.
Owner:HELTOVICS GABOR +3
Fragrance compositions
A composition comprising: (a) a fragrance oil comprising: (i) top note perfume raw material, or mixture of perfume raw materials, with a boiling point of less than, or equal to, about 250° C. at 1 atmosphere pressure; (ii) middle or base note perfume raw material, or mixture of perfume raw materials, with a boiling point of greater than 250° C. at 1 atmosphere pressure; (b) an entrapment material which is selected from the group consisting of polymers; capsules, microcapsules and nanocapsules; liposomes; pro-perfumes selected from more than 1 type of pro-chemistry; film formers; absorbents; cyclic oligosaccharides and mixtures thereof; (c) greater than 50% volatile solvent; wherein the weight ratio of the top note perfume raw materials to middle or base note perfume raw materials within the fragrance oil is in the range from about 1:20 to about 20:1. The present invention provides compositions wherein the light, fresh, fruity, citrus, green or delicate floral top note fragrance character remains detectable for greater than about 2 hours, preferably greater than about 4 hours, more preferably greater than about 6 hours, after the composition has been applied to the substrate.
Owner:THE PROCTER & GAMBLE COMPANY
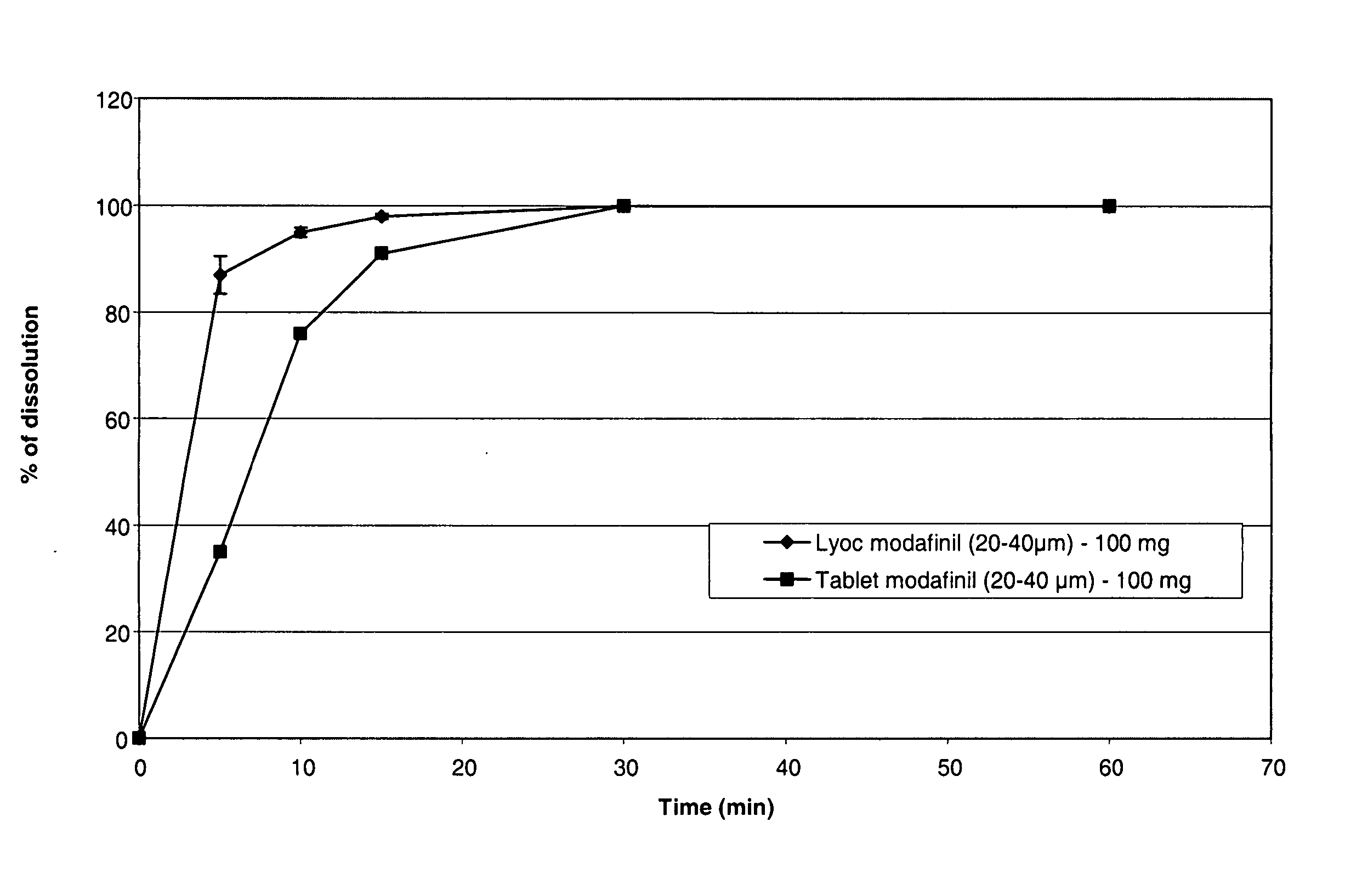
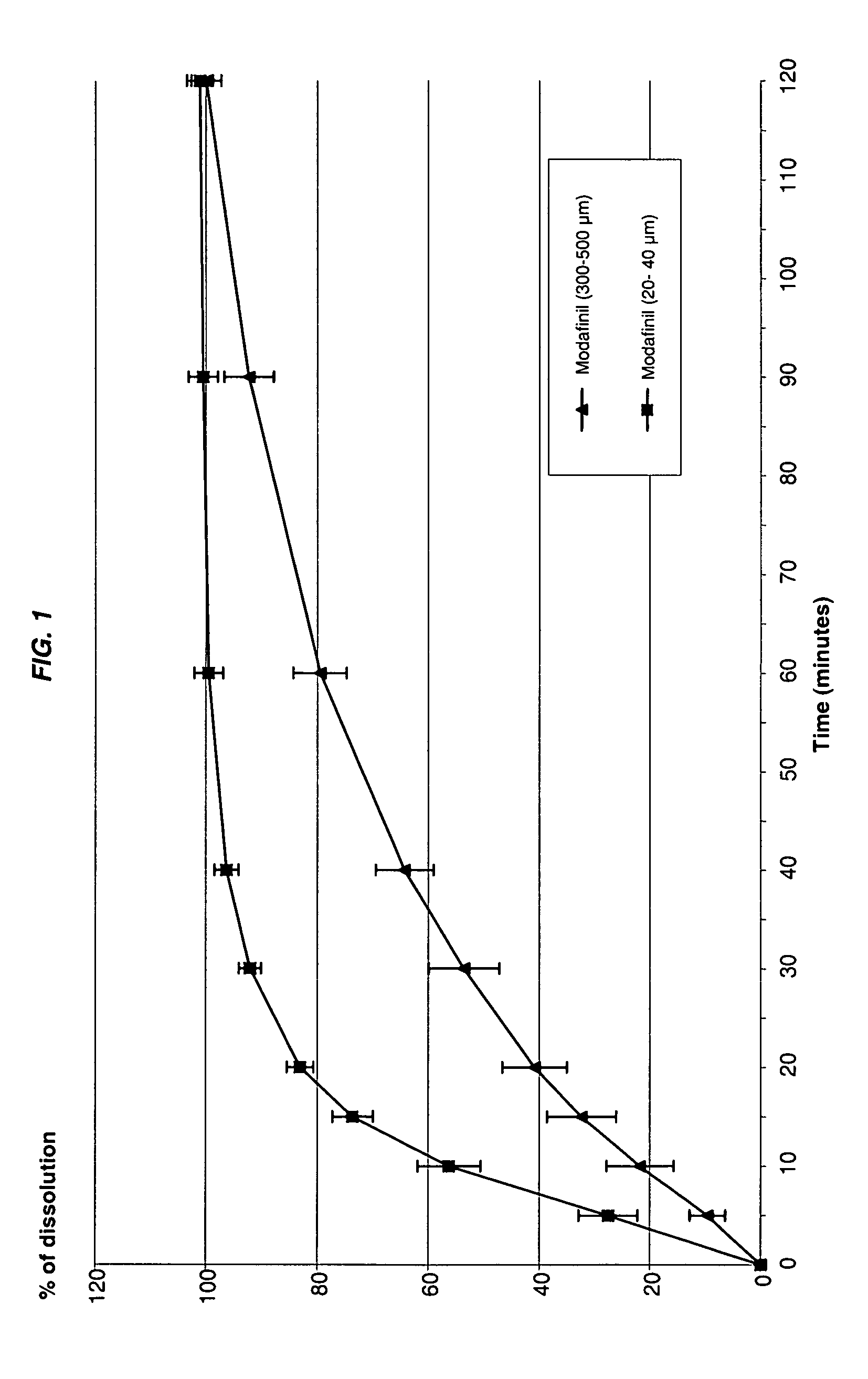
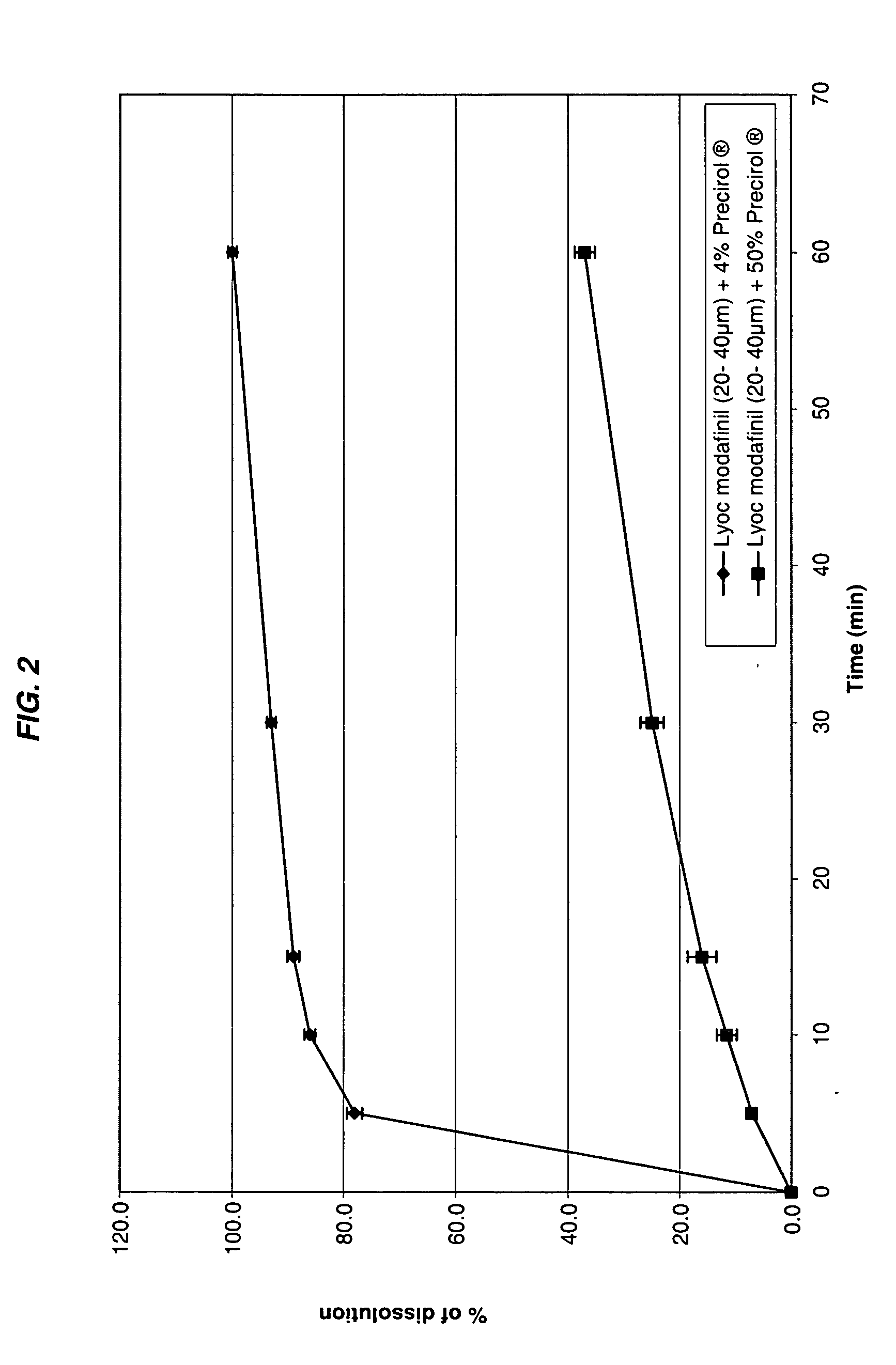
Modafinil oral lyophilizate
The invention concerns an oral lyophilizate comprising modafinil particles having a median diameter of about 10 to about 1000 μm in association with an appropriate amount of at least one excipient selected from the group consisting of fatty acid esters of glycerol, cyclic oligosaccharides, sweeteners or mixtures thereof.
Owner:CEPHALON FRANCE
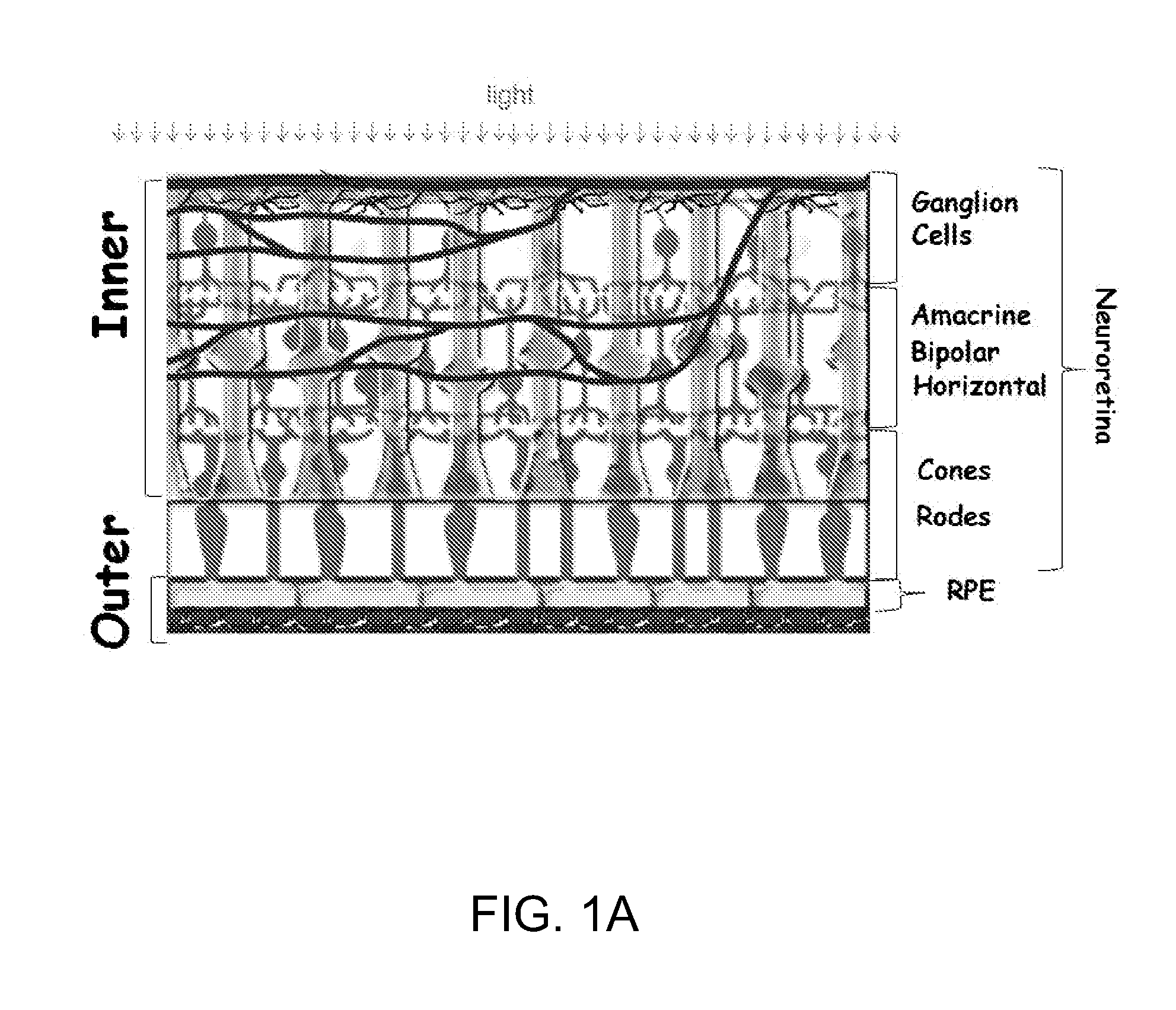
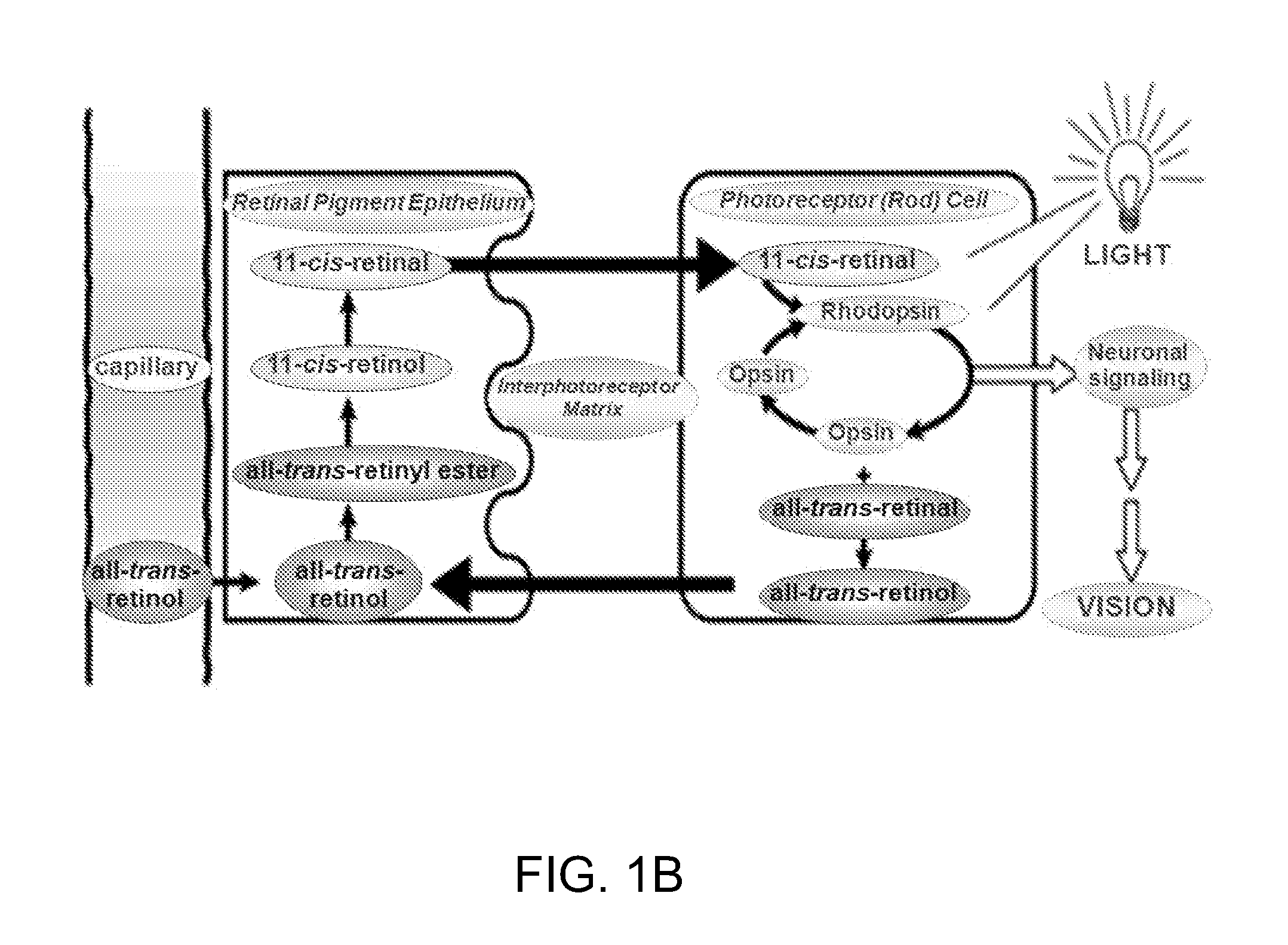
Treatments for retinal disorders
ActiveUS20140038918A1Little and no toxicityLower blood lipid levelsOrganic active ingredientsBiocideRetinitis pigmentosaRetinal Disorder
The present invention relates to the use of cyclic oligosaccharides as chemical complexants of lipofuscin bisretinoids (A2E) to prevent and treat eye (i.e., retinal or macular) disease. Monomeric, dimeric, multimeric, or polymeric oligosaccharide rings act as pharmacologic agents to prevent and treat ophthalmologic disorders triggered by the accumulation of lipofuscin in the retinal pigment epithelium (RPE), which occurs as a consequence of either genetic disorders, such as Stargardt Disease (SD) and Best Disease (BD), or aging, such as Age-Related Macular Degeneration (AMD), or other diseases, such as retinitis pigmentosa, and cone-rod dystrophy.
Owner:CORNELL UNIVERSITY
Methods of fragrancing a surface
The present invention provides a method of applying a fragrance to a surface such as skin and / or hair. The method comprises applying to one area of the surface a first composition wherein the first composition comprises at least one first fragrance oil; at least one first cyclic oligosaccharide, wherein the at least one first fragrance oil and the at least one first cyclic oligosaccharide form a complex, which complex is dissolved or dispersed in the first composition; and a first solvent, wherein the first solvent has a dielectric constant at 25° C. of greater than or equal to 43. The method also comprises simultaneously or sequentially, in either order, applying to the one area or an area adjacent thereto a second composition wherein the second composition comprises at least one second fragrance oil; and a second solvent wherein the at least one second fragrance oil is soluble or is dispersed at 25° C. in the second solvent.
Owner:PROCTER & GAMBLE CO
Process for hydrotreating a hydrocarbon cut with a boiling point of more than 250°c in the presence of a sulphide catalyst prepared using a cyclic oligosaccharide
ActiveUS20130186806A1Improve catalytic performanceHigh catalytic activityRefining with metalsCatalyst regeneration/reactivationBoiling pointSulfide
Preparation of a catalyst having at least one metal from group VIII, at least one metal from group VIB and at least one support; in succession:i) one ofi1) contacting a pre-catalyst with metal from group VIII, metal from group VIB and support with a cyclic oligosaccharide naming at least 6 α-(1,4)-bonded glucopyranose subunits;i2) contacting support with a solution containing a precursor of metal from group VIII, a precursor of said metal from group VIB and a cyclic oligosaccharide composed of at least 6 α-(1,4)-bonded glucopyranose subunits; ori3) contacting support with a cyclic oligosaccharide composed of at least 6 α-(1,4)-bonded glucopyranose subunits followed by contacting solid derived therefrom with a precursor of metal from group VIII and a precursor of metal from group VIB.
Owner:INST FR DU PETROLE
Fine Particle-Containing Composition and Manufacturing Method Therefor
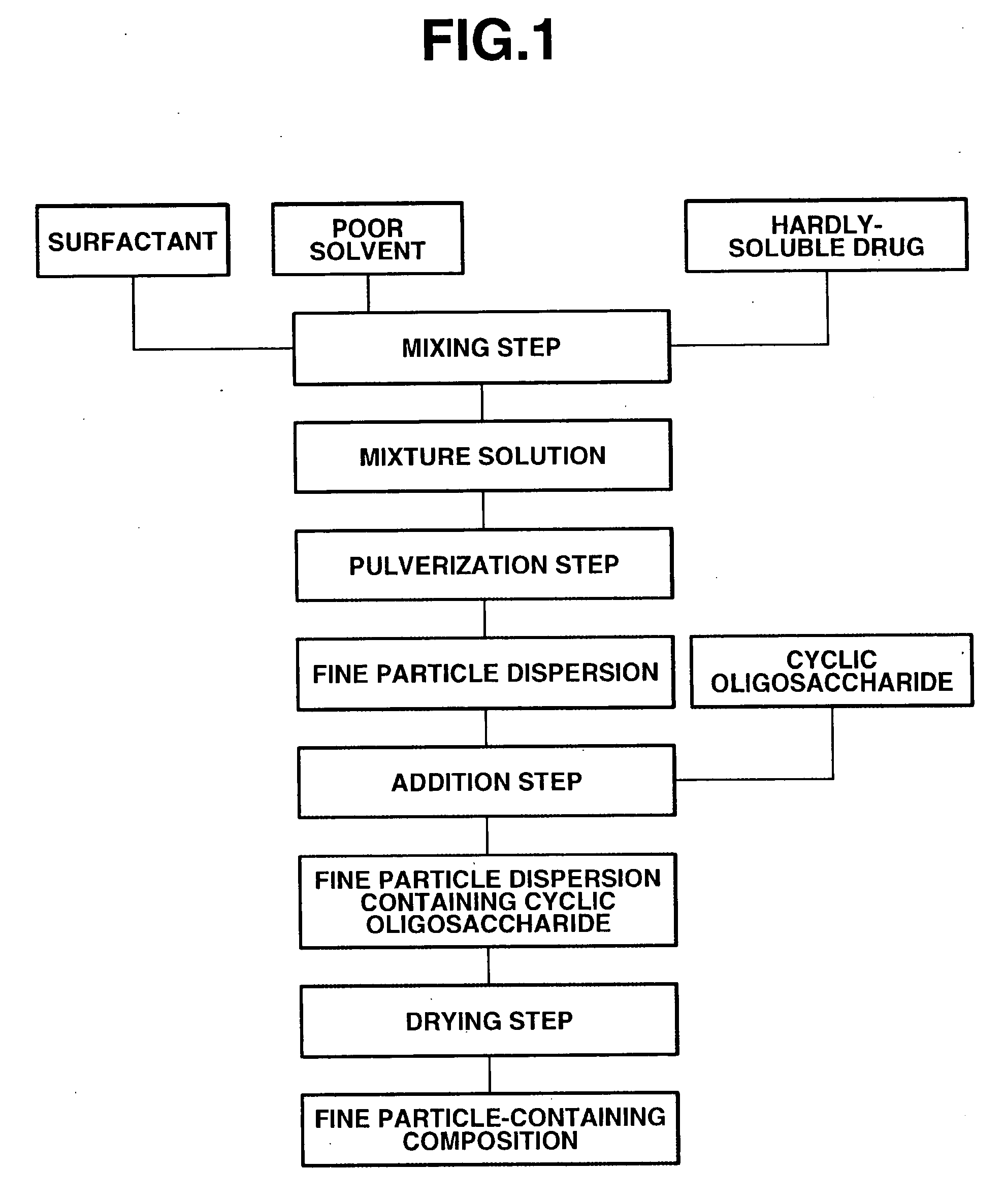
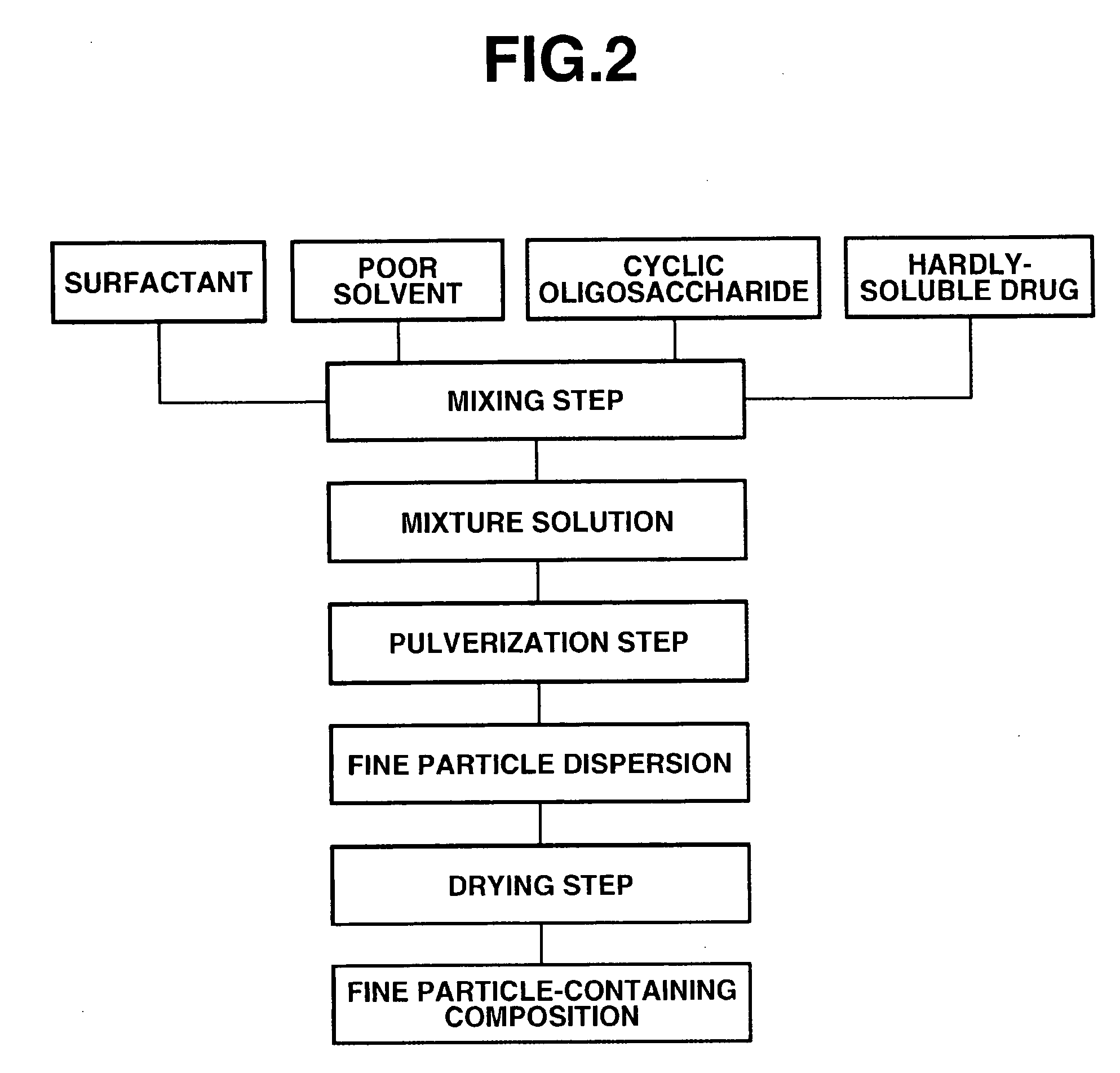
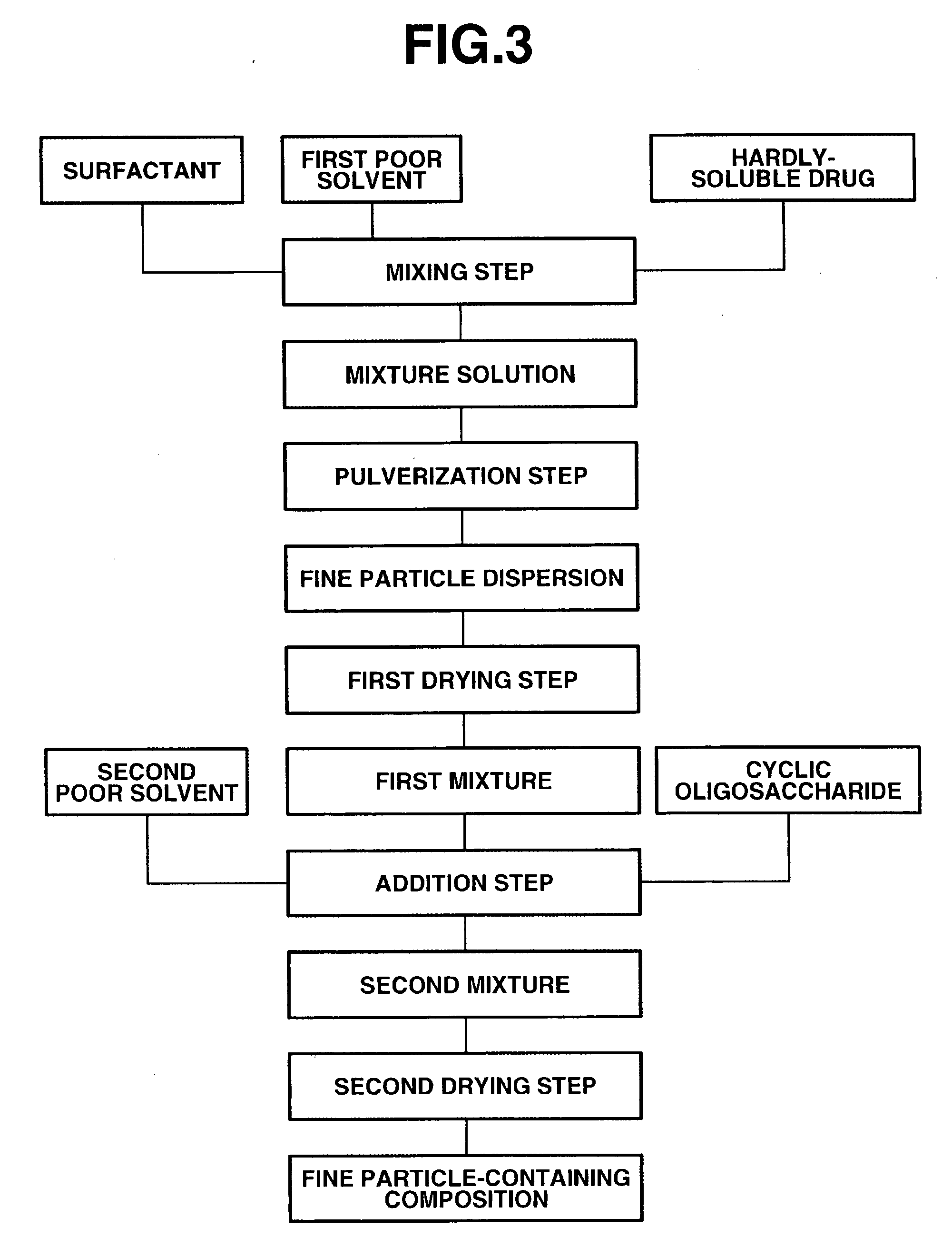
The present invention provides a composition containing fine particles of a hardly-soluble drug which is stable and is not affected by storage environment conditions, as well as a manufacturing method therefor. The present invention provides a fine particle-containing composition containing fine particles of the hardly-soluble drug, a surfactant and a cyclic oligosaccharide, wherein an average particle size of the fine particles is at least 50 nm but not more than 1000 nm. The present invention also provides a method for manufacturing a fine particle-containing composition, comprising (I) a mixing step in which the hardly-soluble drug, the surfactant and a poor solvent are mixed to obtain a liquid mixture, (II) a pulverization step in which the liquid mixture is pulverized with a wet disperser to obtain a dispersion of fine particles, (III) an addition step in which the cyclic oligosaccharide is added to the dispersion of fine particles, and (IV) a drying step in which the dispersion of fine particles containing the cyclic oligosaccharide is dried.
Owner:EISIA R&D MANAGEMENT CO LTD
Fragrance compositions
The present invention relates to a composition comprising: (a) a fragrance oil wherein the fragrance oil comprises: (i) greater than about 50%, by weight of the fragrance oil, of perfume raw materials with high odour impact perfume raw materials which have an odour detection threshold of less than, or equal to, about 50 parts per billion; (ii) less than about 5%, by weight of the fragrance oil, of top note perfume raw materials wherein the top note perfume raw materials have a boiling point of less than about 250° C. at 1 atmosphere pressure (b) an entrapment material which is selected from the group consisting of polymers; capsules, microcapsules, and nanocapsules; liposomes; pro-perfumes; film formers; absorbents; cyclic oligosaccharides and mixtures thereof. (c) greater than about 50%, by weight, of a volatile solvent. The present invention provides compositions wherein the fragrance character remains detectable for greater than about 2 hours, preferably greater than about 4 hours, more preferably greater than about 6 hours, after the composition has been applied to the substrate.
Owner:THE PROCTER & GAMBLE COMPANY
Process for synthesizing c5+ hydrocarbons in the presence of a catalyst prepared using at least one cyclic oligosaccharide
ActiveUS20130184361A1Hydrocarbon from carbon oxidesOrganic compound preparationSimple Organic CompoundsPtru catalyst
C5+ hydrocarbon synthesis by contracting a synthesis gas with a catalyst naming at least one metal from group VIII deposited on a support formed by at least one oxide, said catalyst being prepared using a process of at least:i) contracting at least the support with at least one solution containing at least one precursor of metal from group VIII;ii) contracting at least the support with at least one organic compound formed from at least one cyclic oligosaccharide composed of at least 6 α-(1,4)-bonded glucopyranose subunits;iii) at least one calcining to obtain at least the metal from group VIII in the oxide form;i) and ii) being carried out separately, in any order, or simultaneously.
Owner:INST FR DU PETROLE
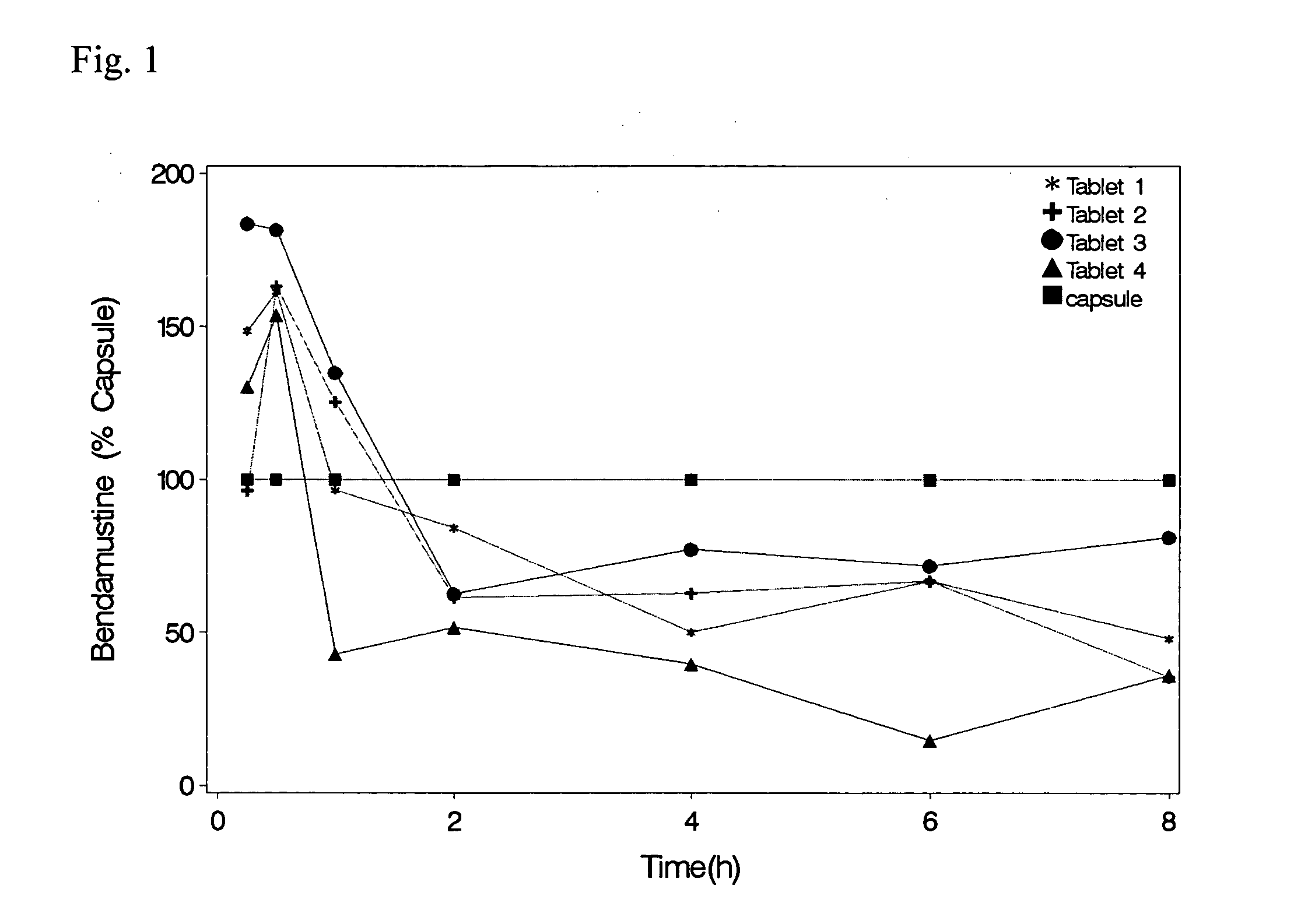
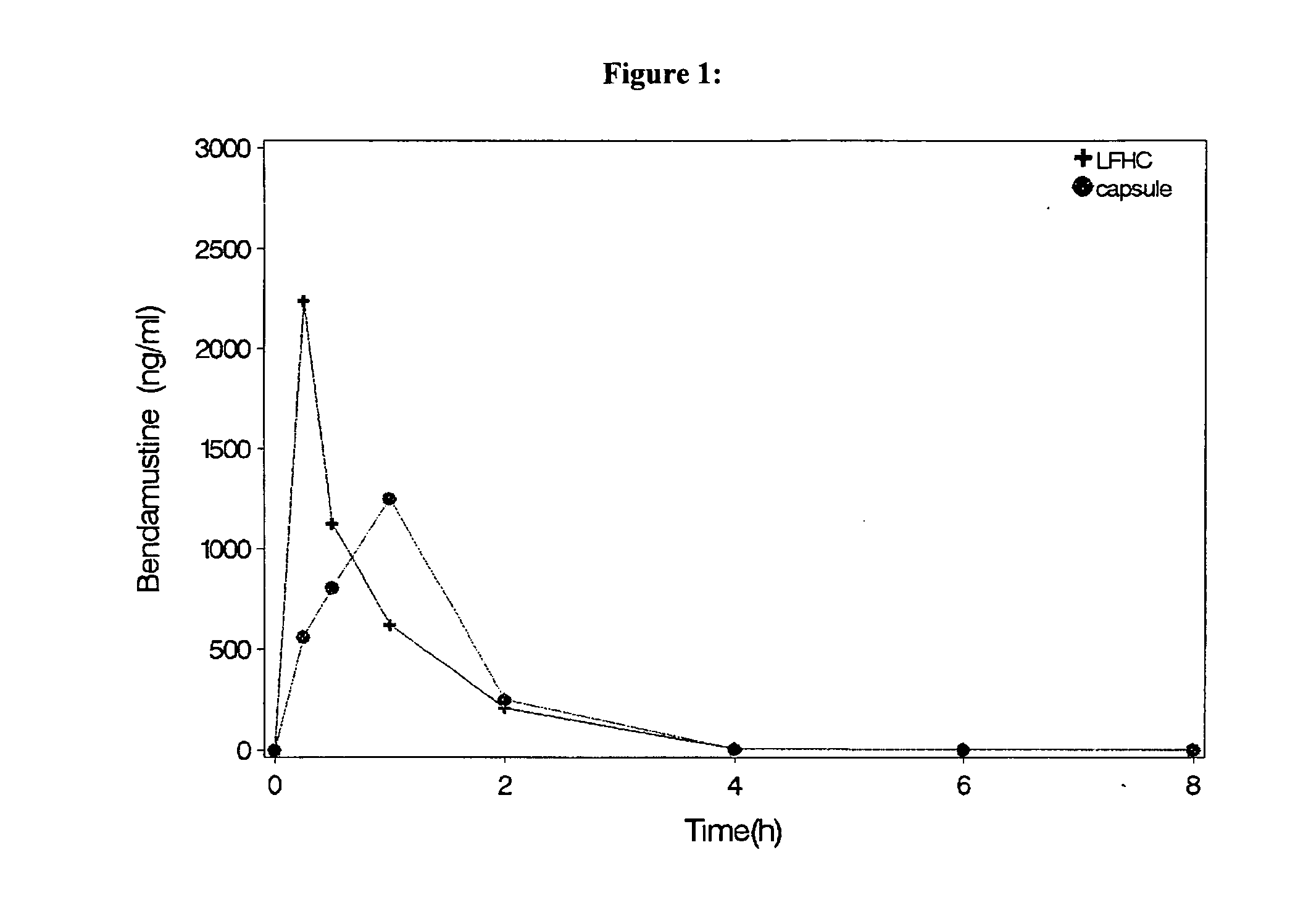
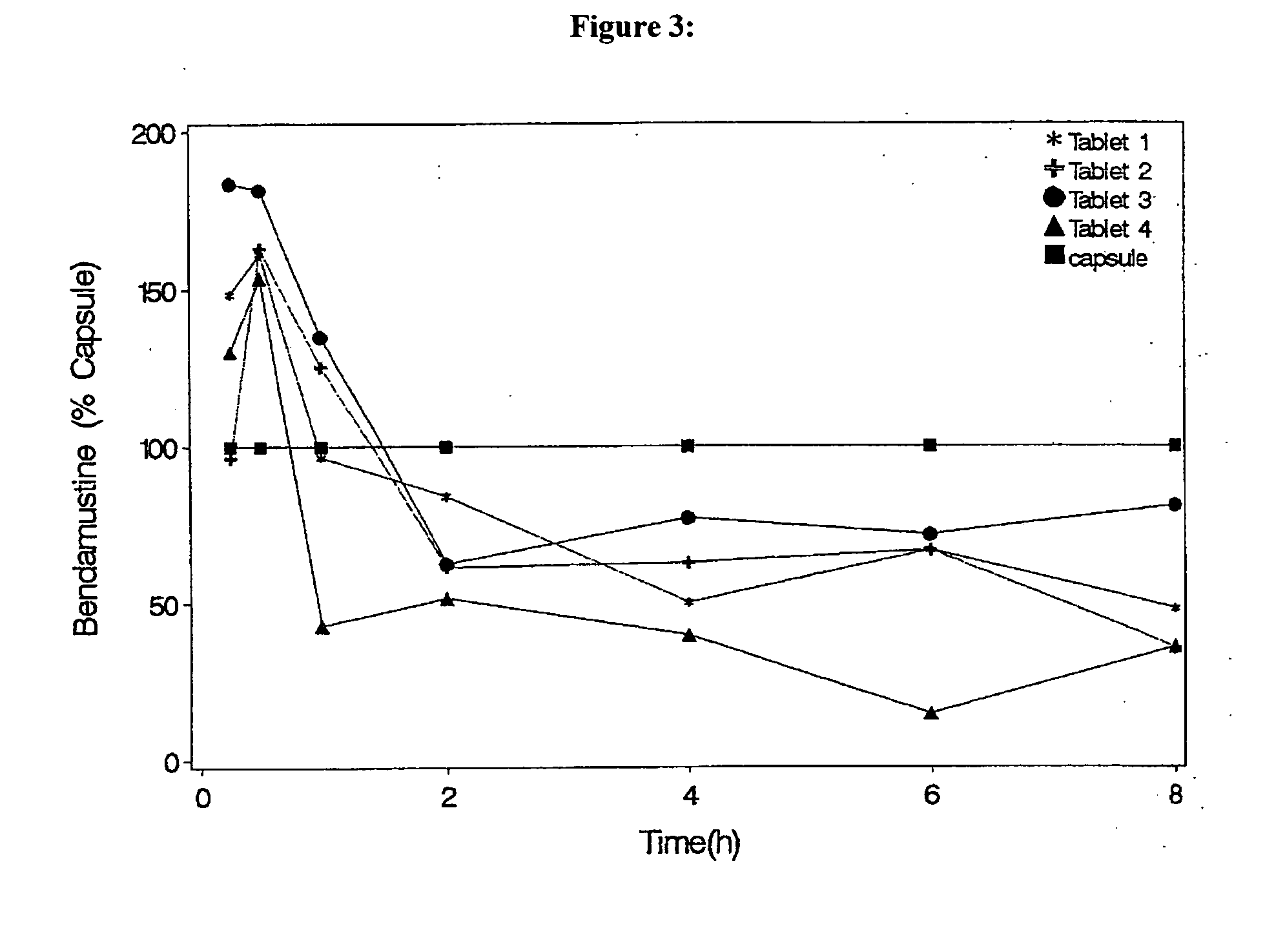
Solid Dosage Forms Of Bendamustine
InactiveUS20120003309A1High maximum concentrationBiocideHeavy metal active ingredientsBULK ACTIVE INGREDIENTExcipient
In the present invention there is provided a pharmaceutical composition in a solid dosage form suitable for oral administration, the composition comprising bendamustine or a pharmaceutically acceptable ester, salt or solvate thereof as an active ingredient, and at least one pharmaceutically acceptable excipient, which is a pharmaceutically acceptable saccharide selected from the group consisting of one or more of a monosaccharide, a disaccharide, an oligosaccharide, a cyclic oligosaccharide, a polysaccharide and a saccharide alcohol, wherein the ratio by weight of the active ingredient to the saccharide excipient(s) is in the range of 1:1-5.
Owner:ASTELLAS DEUTLAND
Stabilized radiopharmaceutical kits
InactiveUS6428768B1Extended shelf lifeRadioactive preparation carriersGroup 3/13 element organic compoundsCombinatorial chemistryCyclic oligosaccharide
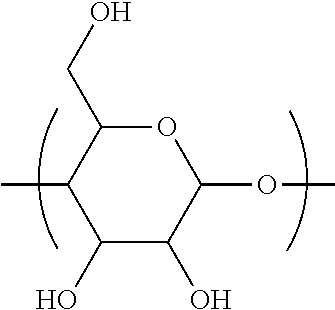
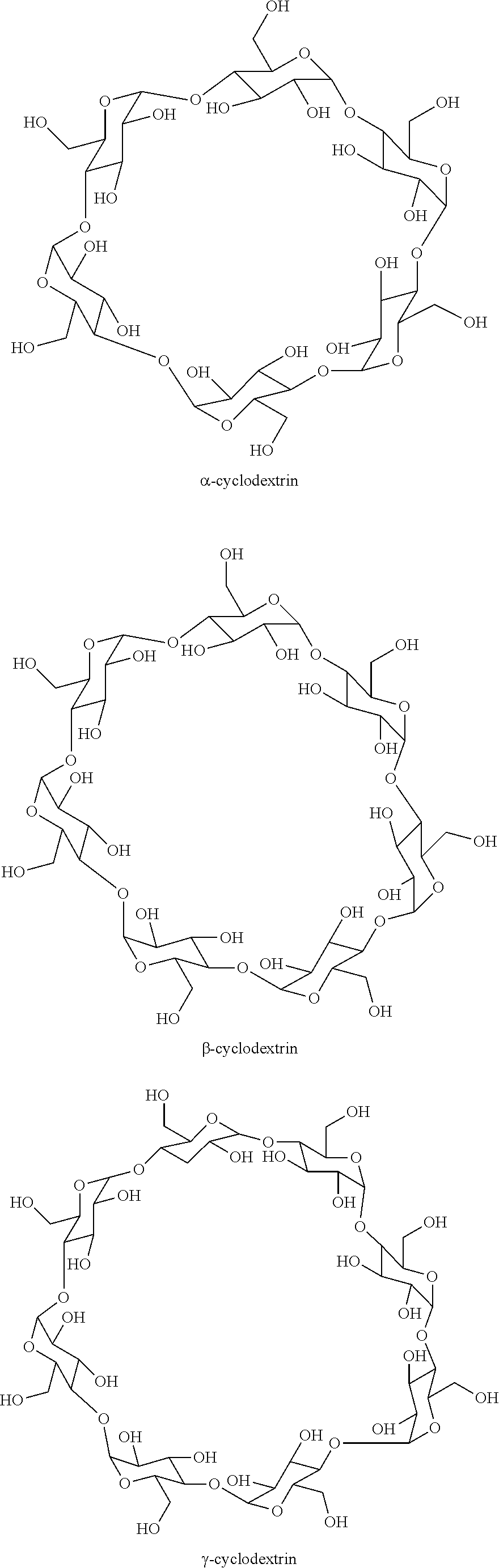
The present invention relates to the stabilization of radiopharmaceutical preparations and to the stabilization of components of radiopharmaceutical kits. In particular, the present invention relates to stabilization of lyophilized components of radiopharmaceutical kits by the addition of a cyclic oligosaccharide, such as, a modified or unmodified cyclodextrin, to the kit.
Owner:MALLINCKRODT INC
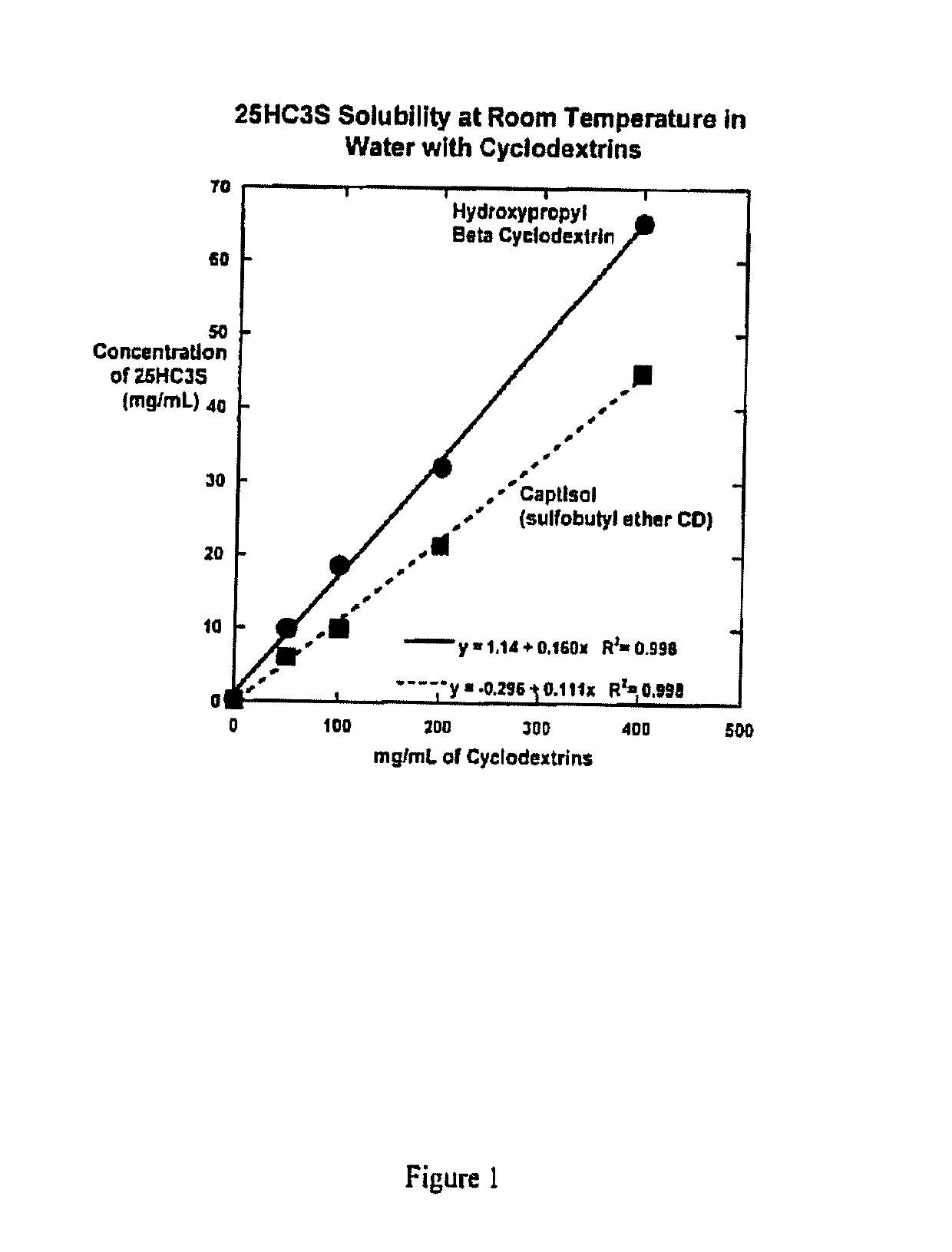
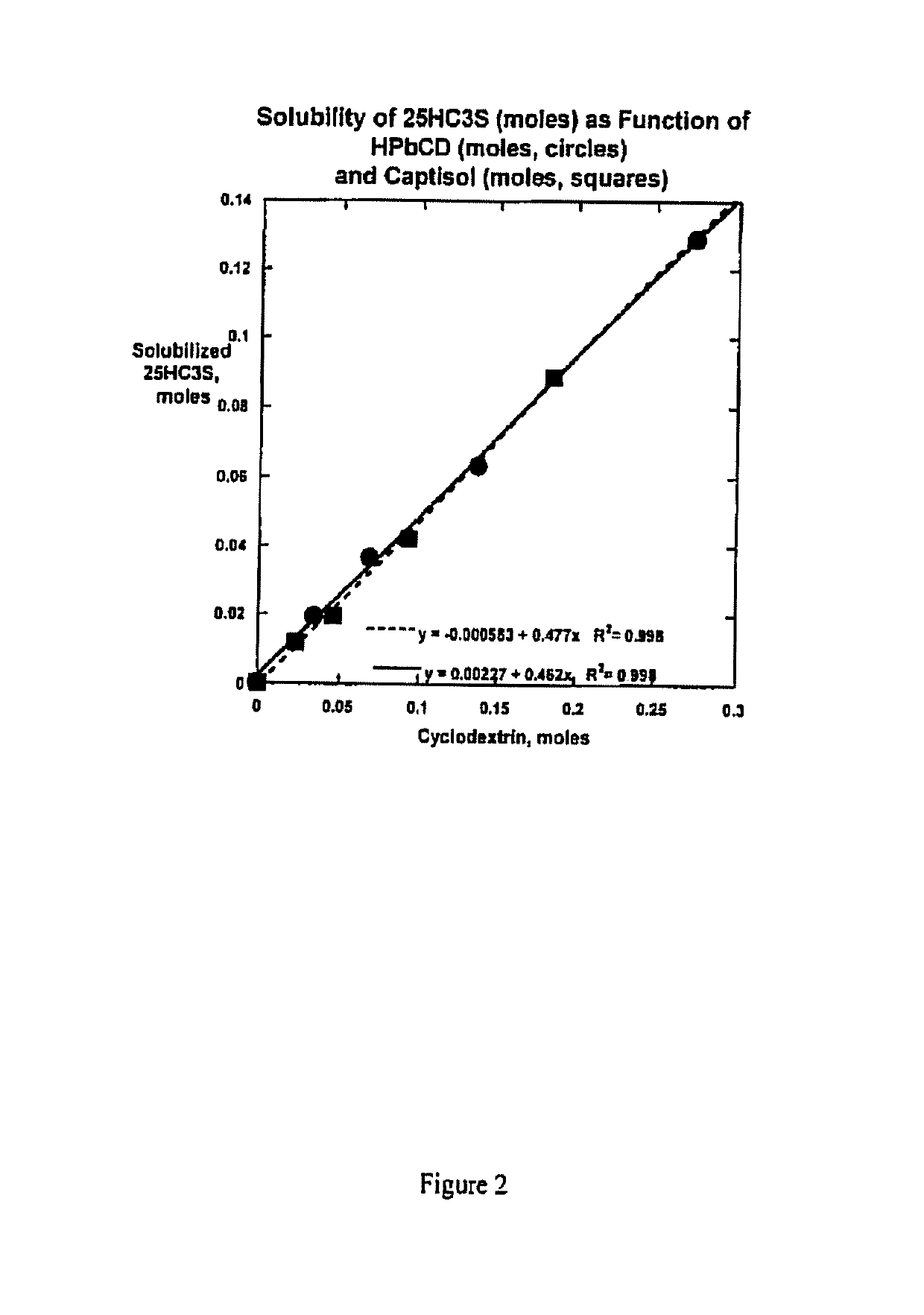
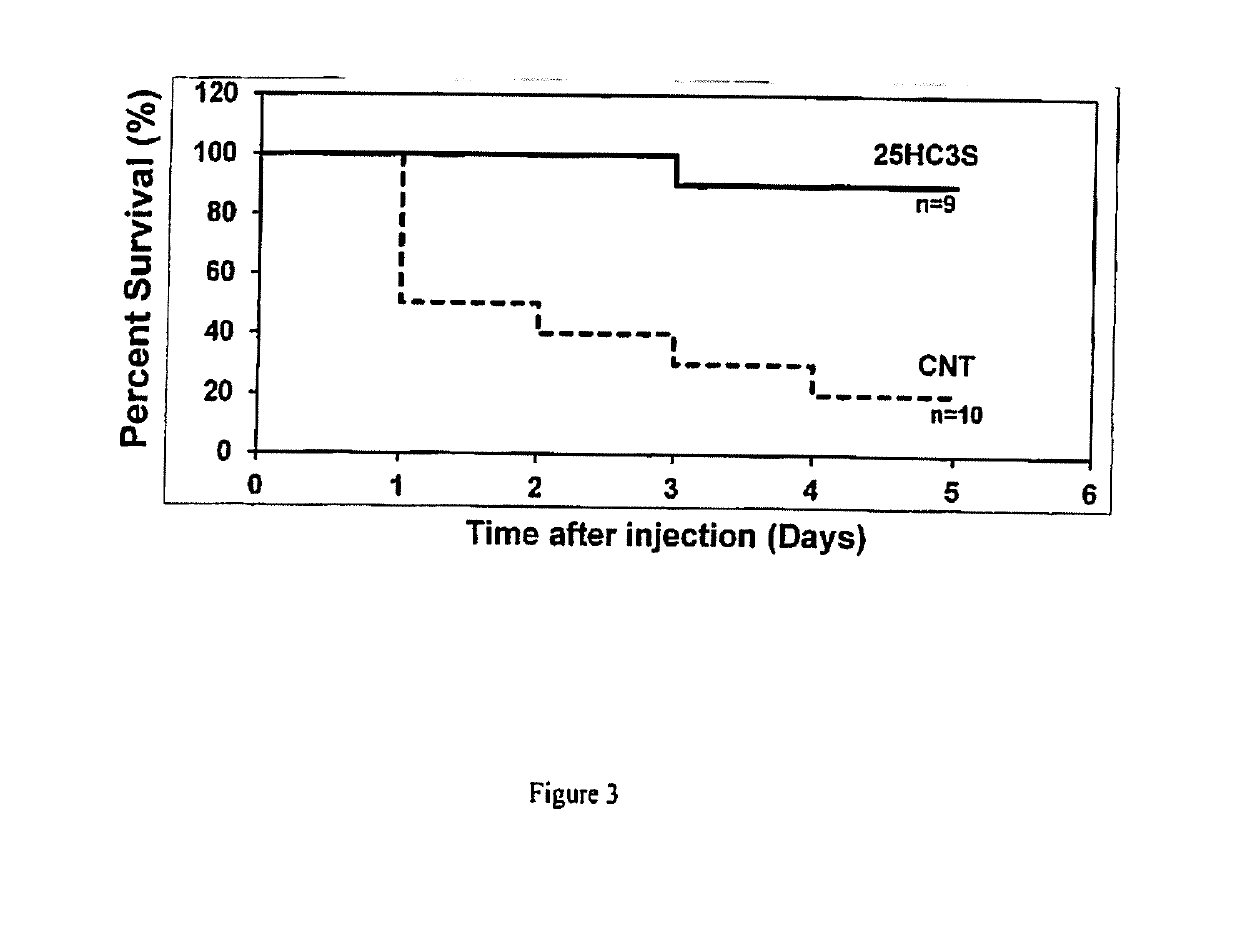
Compositions comprising 5-cholesten-3, 25-diol, 3-sulfate (25hc3s) or pharmaceutically acceptable salt thereof and at least one cyclic oligosaccharide
Compositions comprising 5-cholesten-3, 25-diol, 3-sulfate (25HC3S) or pharmaceutically acceptable salt thereof and at least one cyclic oligosaccharide, e.g., a cyclodextrin (CD), are provided. The compositions may be used to prevent and / or treat a variety of diseases and conditions, including organ failure (e.g. acute liver failure), high cholesterol / high lipids, and various inflammatory diseases and conditions.
Owner:VIRGINIA COMMONWEALTH UNIV +2
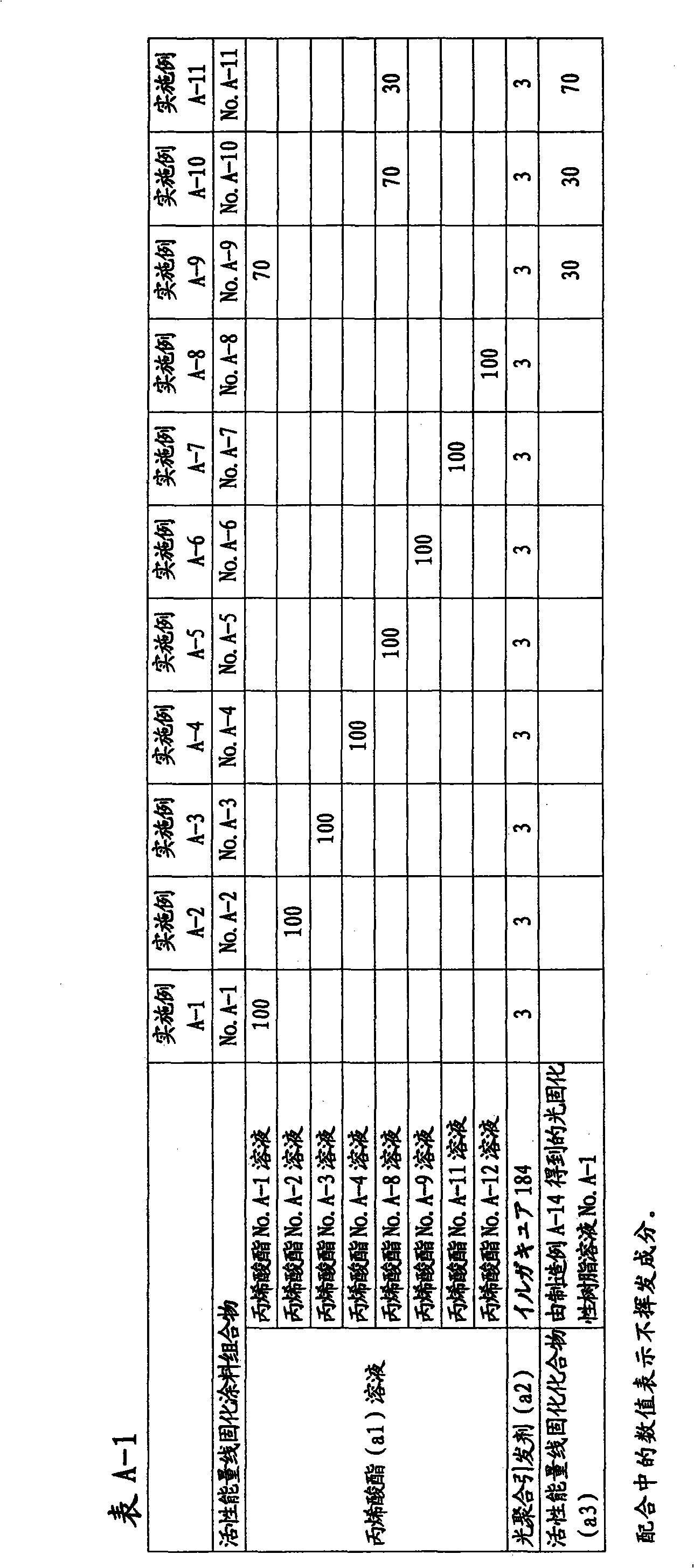
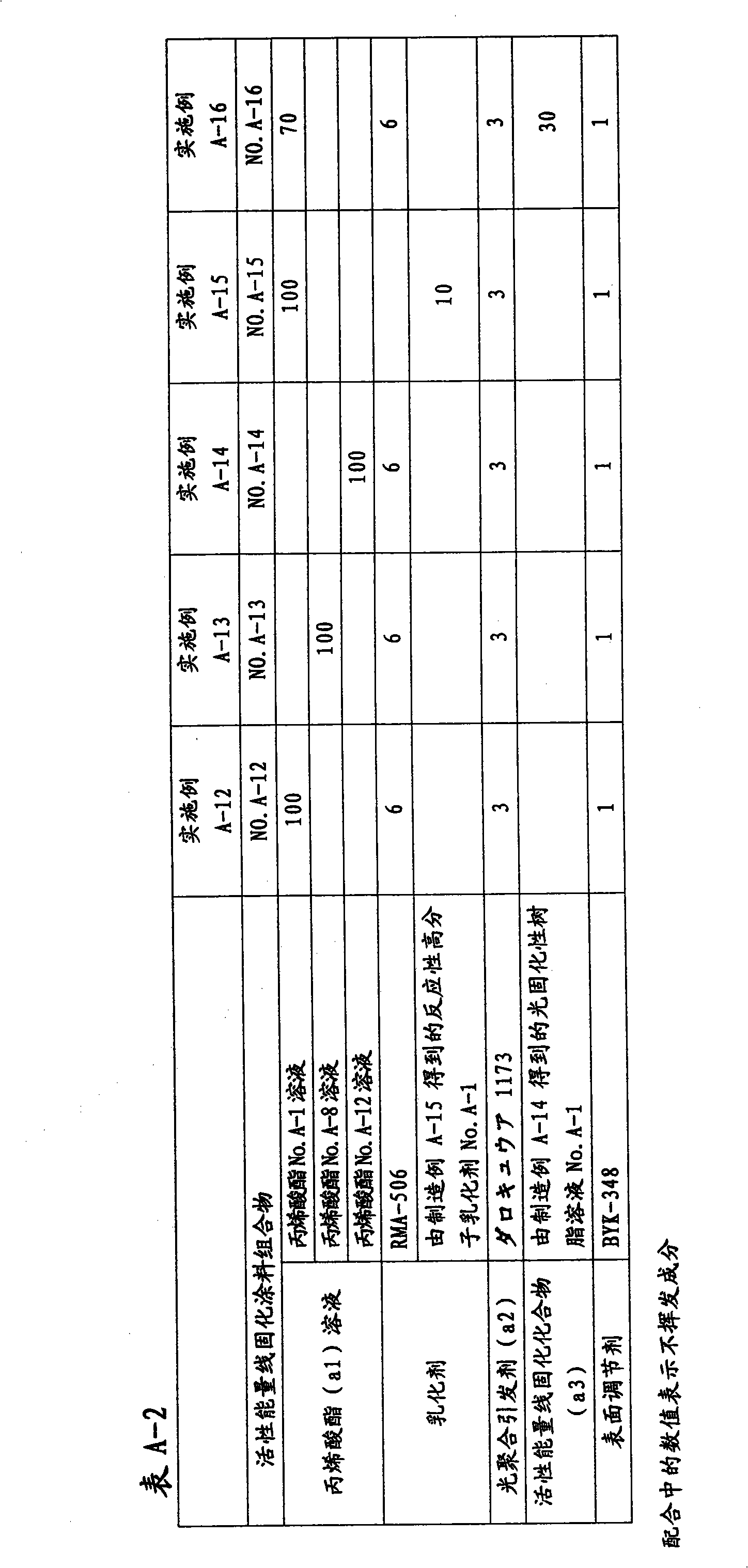
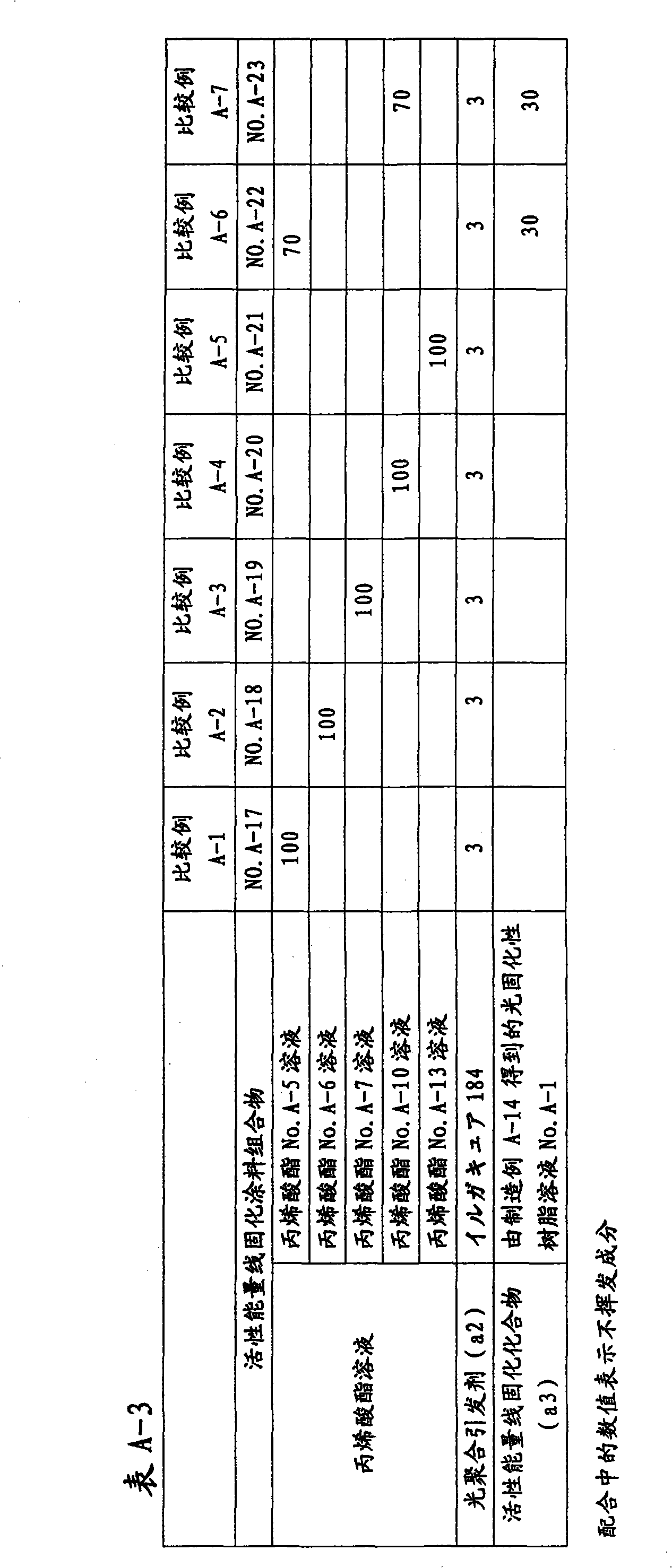
Active energy ray-curable coating composition, method for formation of coating film, and coated article
InactiveCN101945955AEmission reductionReduce pollutionPretreated surfacesEmulsion paintsWeather resistanceHardness
Disclosed is an active energy ray-curable coating composition which is characterized by comprising: (a1) an acrylic acid ester of a non-cyclic oligosaccharide or a derivative thereof, which has an weight average molecular weight of 400 to 2,000 and contains 3.0 to 12.0 acryloyl groups on average per molecule; and (a2) a photopolymerization initiator. The active energy ray-curable coating composition is a bio-derived active energy ray-curable coating composition, and can form a coating film having excellent finished appearance, pencil hardness, scratch resistance, weather resistance and solvent resistance.
Owner:KANSAI PAINT CO LTD
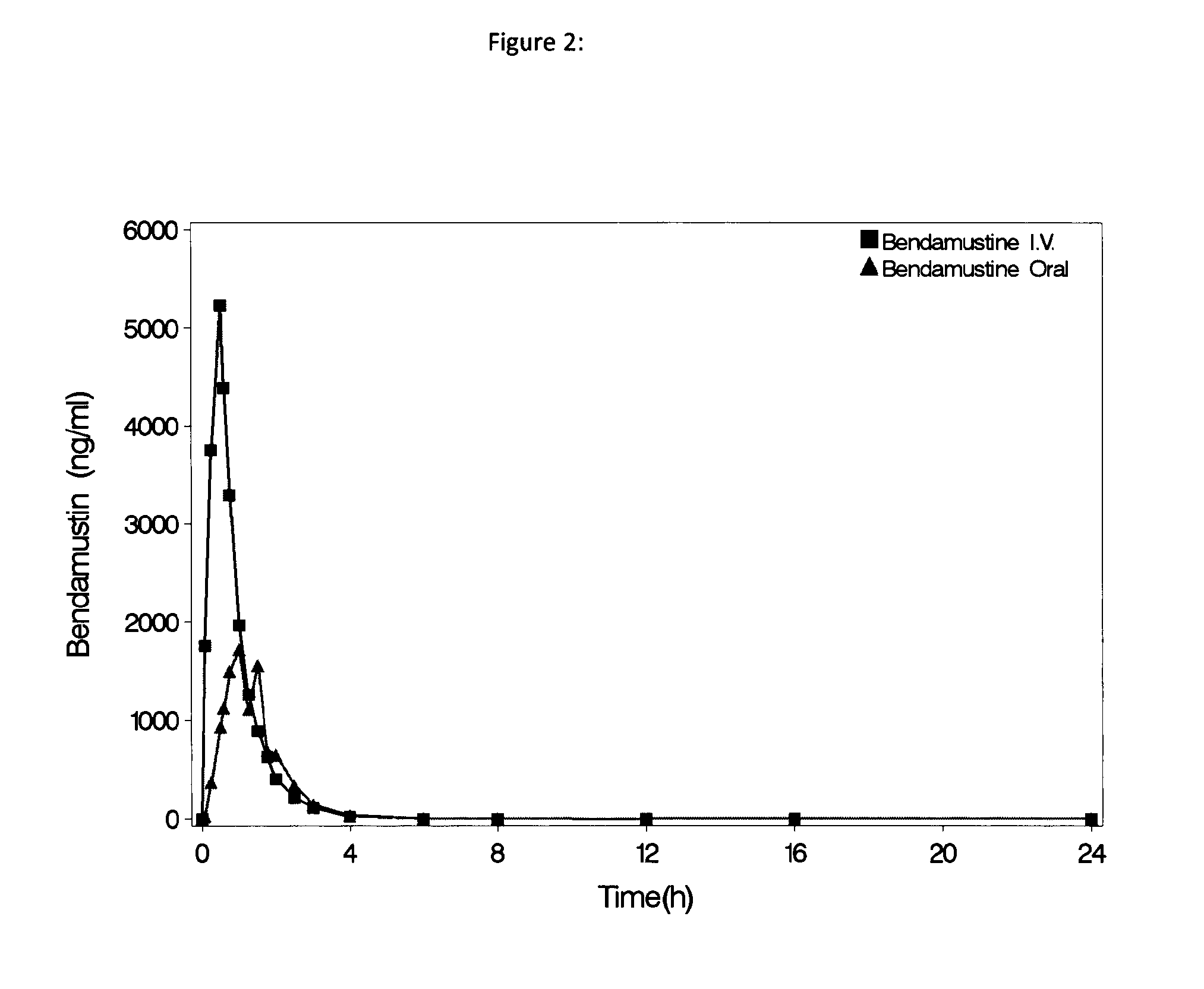
Oral Dosage Forms of Bendamustine and Therapeutic Use Thereof
InactiveUS20130209558A1Improve stabilitySuperior dissolution profileBiocideHeavy metal active ingredientsDiseaseOral treatment
In the present invention there is provided a pharmaceutical composition for oral administration which comprises bendamustine or a pharmaceutically acceptable, ester, salt or solvate thereof as an active ingredient, and a pharmaceutically acceptable excipient and which shows a dissolution of the bendamustine of at least 60% in 20 minutes, 70% in 40 minutes and 80% in 60 minutes, as measured with a paddle apparatus at 50 rpm according to the European Pharmacopoeia in 500 ml of a dissolution medium at a pH of 1.5, and wherein the pharmaceutically acceptable excipient is either a pharmaceutically acceptable non-ionic surfactant, selected from the group consisting of a polyethoxylated castor oil or derivative thereof and a block copolymer of ethylene oxide and propylene oxide or a pharmaceutically acceptable saccharide selected from the group consisting of one or more of a monosaccharide, a disaccharide, an oligosaccharide, a cyclic oligosaccharide, a polysaccharide and a saccharide alcohol, wherein the ratio by weight of the active ingredient to the saccharide excipient(s) is in the range of 1:1-5. The invention further relates to the above pharmaceutical composition for use for the oral treatment of a medical condition which is selected from chronic lymphocytic leukemia, acute lymphocytic leukaemia, chronic myelocytic leukaemia, acute myelocytic leukaemia, Hodgkin's disease, non-Hodgkin's lymphoma, multiple myeloma, breast cancer, ovarian cancer, small cell lung cancer and non-small cell lung cancer. The invention moreover relates to the above pharmaceutical composition for the above use wherein the dosage regimen comprises at least the administration of a dose of 100 to 600 mg / m2 / per person of bendamustine on day 1 and day 2, optionally a dose of 50 to 150 mg / m2 i.v. or orally of a corticosteroid on days 1 to 5, and optionally a suitable dose of a further active agent selected from the group consisting of an antibody specific for CD20, an anthracyclin derivative, a vinca alkaloid or a platin derivative; and the repetition of said dosage regimen 4 to 15 times after intervals of two to four weeks.
Owner:ASTELLAS DEUTLAND
Mulberry leaf green juice powder and preparation method of mulberry leaf green juice solid beverage
InactiveCN106107972AFit for drinkingFast nutrient absorptionFood thermal treatmentFood dryingVitamin CBlood sugar
The invention discloses mulberry leaf green juice powder and a preparation method of a mulberry leaf green juice solid beverage. The method comprises the steps of screening, cleaning, scalding, drying and low-temperature airflow crushing. The mulberry leaf green juice powder can fully utilize the nutrient substances contained in the mulberry leaves, is convenient to eat, and can be widely applied to the fields of food and natural pigments. The invention also provides a preparation method of the mulberry leaf green juice solid beverage. By taking the mulberry leaf green juice powder having the functions of reducing blood sugar, reducing blood pressure and reducing blood fat as a main raw material, the mulberry leaf green juice solid beverage plays an extremely high function through the mutual effect of the mulberry leaf green juice powder with green tea powder, barley seedling green juice powder, kelp powder, cyclic oligosaccharide, lactose, vitamin C, etc. on the basis of the original functions of the mulberry leaves, and is easy to drink.
Owner:JIANGXI SERICULTURE & TEA RES INST
Adhesive composition, adhesive, and adhesive sheet
ActiveCN103360997AFavorable inventory burdenFavorable production efficiencyMacromolecular adhesive additivesEster polymer adhesivesPolymer sciencePolyrotaxane
An adhesive composition, an adhesive, and an adhesive sheet are suitable for optical components such as a polarized sheet and have excellent stress relaxation property. The adhesive composition comprises monomer units which form a polymer, a (methyl) acrylate polymer (A) which has carboxyl-contained monomers and has weight-average molecular weight of being 500,000 to 3 million, and a reactive polyrotaxane compound (B) containing cyclic oligosaccharide having polymerized double bonds.
Owner:LINTEC CORP
Disinfectant and/or bactericidal aqueous compositions
The present invention provides a disinfectant and / or bactericidal aqueous composition containing an olanexidine acid addition salt, and at least one polyoxyethylene-based nonionic surfactant selected from the group consisting of polyoxyethylene higher alkyl ethers and polyoxyethylene alkylphenyl ethers; a disinfectant and / or bactericidal aqueous composition containing an olanexidine acid addition salt at a concentration of 0.05 to 0.5 W / V %, and an alcohol at a concentration of 20 to 80 W / V %, and not containing any surfactants; and a disinfectant and / or bactericidal aqueous composition containing an olanexidine acid addition salt, and at least one member selected from the group consisting of ester-based nonionic surfactants and cyclic oligosaccharides.
Owner:OTSUKA PHARM CO LTD
Fragrance compositions
The present invention relates to a composition comprising: (a) a fragrance oil wherein the fragrance oil comprises: (i) one or more perfume raw materials with a high odour impact which have an odour detection threshold of less than, or equal to about 50 parts per billion (ii) less than about 5%, by weight of the fragrance oil, of top note perfume raw materials where in the top note perfume raw materials have a boiling point of less than 250° C. at 1 atmosphere pressure (b) an entrapment material which is selected from the group consisting of polymers; capsules, microcapsules, and nanocapsules; liposomes; film formers; absorbents; cyclic oligosaccharides and mixtures thereof; wherein the perfume raw material and the entrapment material exist in an associated form on the substrate and wherein the weight ratio of high odour impact perfume raw materials which have an odour detection threshold of less than, or equal to, about 50 parts per billion to entrapment material within the associated form falls in the range of about 1:20 to about 20:1. The present invention provides compositions wherein the fragrance character remains detectable for greater than about 2 hours, preferably greater than about 4 hours, more preferably greater than about 6 hours, after the composition has been applied to the substrate.
Owner:THE PROCTER & GAMBLE COMPANY
Methods of fragrancing a surface
The present invention provides a method of applying a fragrance to a surface such as skin and / or hair. The method comprises applying to one area of the surface a first composition wherein the first composition comprises at least one first fragrance oil; at least one first cyclic oligosaccharide, wherein the at least one first fragrance oil and the at least one first cyclic oligosaccharide form a complex, which complex is dissolved or dispersed in the first composition; and a first solvent or a mixture thereof, wherein the first solvent or the mixture thereof has a dielectric constant at 25° C. of greater than or equal to 43, preferably greater than or equal to 45. The method also comprises simultaneously or sequentially, in either order, applying to the one area or an area adjacent thereto a second composition wherein the second composition comprises at least one second fragrance oil; and a second solvent, or a mixture thereof, wherein the at least one second fragrance oil is soluble or is dispersed at 25° C. in the second solvent or mixture thereof. The invention also provides a delivery package and a kit suitable for use in the above-mentioned method of applying a fragrance to a surface. The invention facilitates the achievement of unique lasting fragrance character combinations arising from the combination of the individual fragrance character profiles of the first and second compositions.
Owner:THE PROCTER & GAMBLE COMPANY
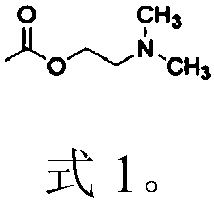
Intelligent fiber or product thereof with environmental responsibility and preparation method thereof
The invention discloses an intelligent fiber or a product thereof with environmental responsibility and a preparation method thereof. The intelligent fiber or the product thereof with environmental responsibility is prepared by connecting a fiber body or a fiber product and granules with environmental responsibility by virtue of chemical bonds; and the surfaces of the granules are connected with at least one of the following functional groups: acylamino, pyridyl, a group shown as a formula 1, amino, carboxyl, cyclodextrin, epoxy group, hydroxide radical and adamantly. The granules with environmental responsibility are combined with the fiber so as to obtain a fiber material with environmental responsibility; and a series of intelligent fiber materials with different bodies are prepared. The invention provides an intelligent fiber material with environmental responsibility on multiple environmental factors respectively or simultaneously, an intelligent fiber fabric and a simple and universal preparation method thereof suitable for large-scale production.
Owner:BEIJING INSTITUTE OF CLOTHING TECHNOLOGY
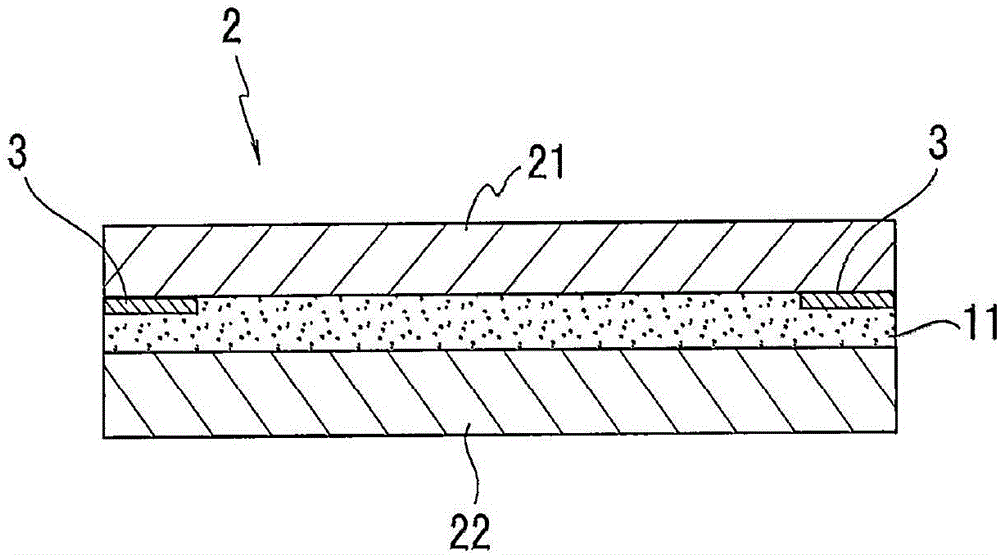
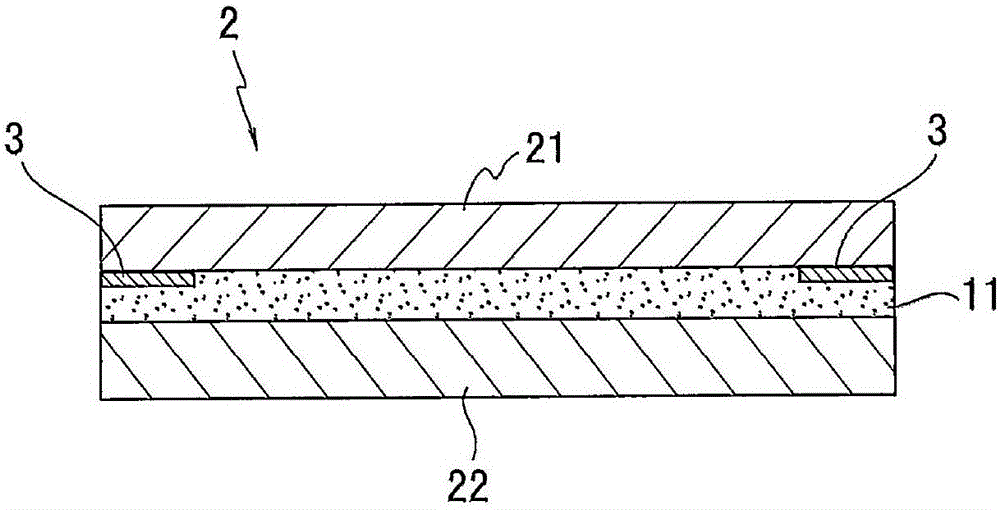
Adhesive composition, adhesive, adhesive sheet, and display body
PendingCN106433523AExcellent step followabilityGood anti-foamNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesPolyrotaxaneMeth-
The invention provides an adhesive composition, an adhesive, an adhesive sheet, and a display body. The adhesive composition has an excellent performance on preventing foaming. The adhesive composition comprises (meth)acrylate polymer (A), which comprises a monomer unit composed of hydroxyl monomers; polyrotaxane (B), which comprises hydroxyl taken as the reactive groups and cyclic oligosaccharide as the cyclic molecules; an isocyanate crosslinking agent (C); and a reaction promoter (D), which promotes the carbamate reactions.
Owner:LINTEC CORP
Adhesive composition, adhesive, adhesive sheet, and display body
ActiveCN106433522AExcellent step followabilityHigh film strengthNon-macromolecular adhesive additivesCyclodextrin adhesivesMeth-Polyrotaxane
The invention provides an adhesive composition, an adhesive, an adhesive sheet, and a display body. The adhesive composition can exert high coating strength. The adhesive composition comprises (meth)acrylate polymer (A), which comprises a monomer unit composed of hydroxyl monomers and fatty ring monomers; polyrotaxane (B), which comprises cyclic oligosaccharide as the cyclic molecules; and an isocyanate crosslinking agent (C).
Owner:LINTEC CORP
Hydro-alcoholic cosmetic compositions with a delayed release
According to the present invention there is provided a use of a cyclic oligosaccharide for delaying the release of a volatile solvent from a composition, preferably a cosmetic composition, which comprises at least 50% volatile solvent.There is also provided a use of a cyclic oligosaccharide for reducing the initial harsh solvent odour impact, for example ethanolic / alcoholic odour impact of alcoholic or hydro-alcoholic compositions.
Owner:PROCTER & GAMBLE CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com