Modafinil oral lyophilizate
a technology of oral lyophilizate and modafinil, which is applied in the direction of pill delivery, digestive system, metabolism disorder, etc., can solve the problems of inconvenient use of additives and preserving agents, inability to meet patients' diet restrictions, and inability to tolerate liquid dosage forms containing saccharose or sodium, etc., to achieve the effect of convenient swallowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples
Material and Methods
[0187] The modafinil particles of size 20-40 μm are commercially available (Cephalon, Inc., West Chester, Pa.). The modafinil particles of size 1-10 μm were prepared by micronizing modafinil particles of commercial grade 20-40 μm using a micronizer type Jet mill (Microjet Mill, Switzerland). The modafinil particles of size 200-315 μm were obtained by screening an API modafinil batch (Active Pharmaceutical Ingredient) through a 200 μm screen and a 315 μm screen. Modafinil lot with bounded particles size may be prepared by methods discloses in WO 2004 / 006905.
[0188] The glycerol palmitostearate was purchased as Precirol ATO 5® from Gattefossé (France).
Measurement of the Dissolution Kinetics of Oral Lyophilizates (Dissolution Assay)
[0189] The modafinil dissolution rate of oral lyophilizates was measured according to the following protocol.
[0190] The solutions to be analyzed were prepared by introducing an oral lyophilizate into 1L of HCl 0.1 M kept at a temper...
examples 1 to 4
[0205]
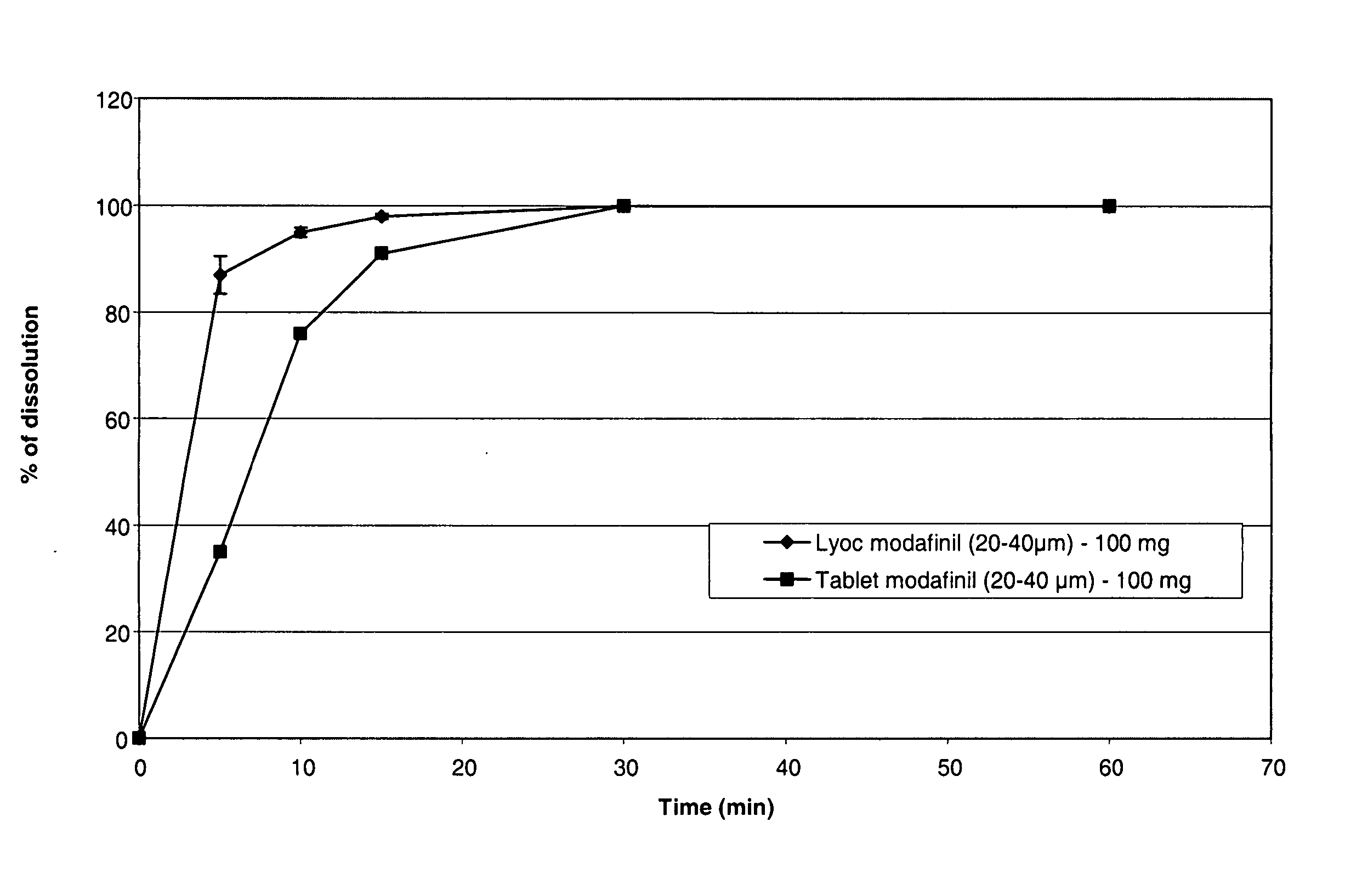
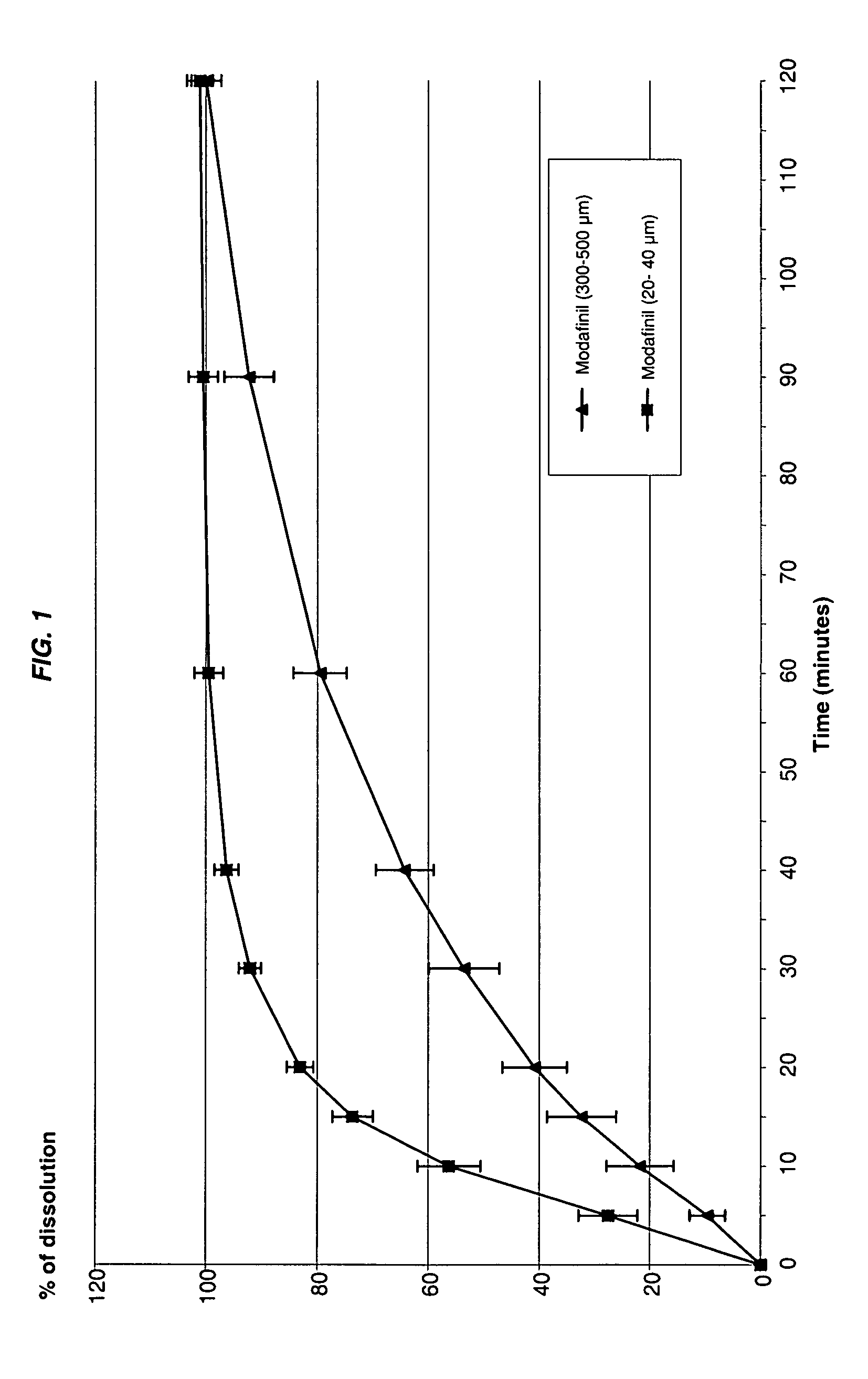
TABLE IExamples1234Modafinil particle size1-10μm20-40μm200-315μm300-500μmModafinil280mg280mg300mg300mgCopovidone0.7mg0.7mg0.7mg0.7mgDextran 7015mg15mg15mg15mgAspartam7mg7mg7mg7mgAcesulfam K10mg10mg10mg10mgFlavor (pineapple)15mg15mg15mg15mgCitric acid5mg5mg5mg5mgMannitol367.3mg367.3mg347.3mg347.3mgWater380mg380mg380mg380mgUnit weight700mg700mg700mg700mgTasteBitterBitterMaskedMaskedDissolution at 30 mn100%96%85%53%
[0206] As apparent from Table I, oral lyophilizates of modafinil with small particle size (1-10 μm or 20-40 μm) exhibited a bitter taste while an equivalent formulation prepared with large particle size (superior to 200 μm) is have a masked taste. However, the dissolution of oral lyophilizates with large particles is notably slower (FIG. 1).
examples 5 and 6
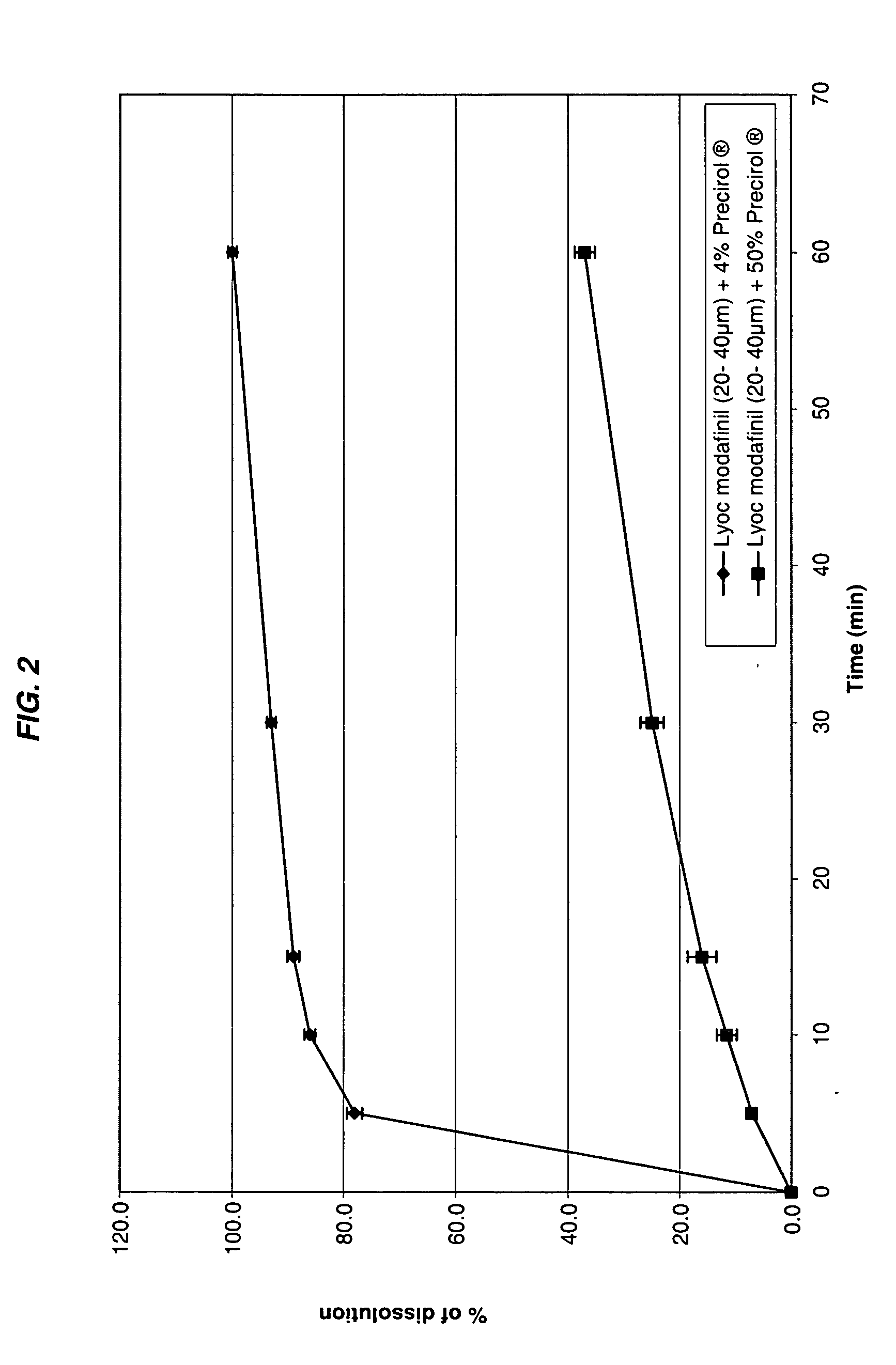
[0207] Oral lyophilizates were prepared as Example 1 according to the formulations set out in Table II and using modafinil particles of 20-40 μm coated with 4% and 50% by weight of glycerylpalmitostearate with respect to modafinil weight, respectively.
[0208] These coated modafinil particles were prepared by melting a specified quantity of glycerylpalmitostearate (Precirol® ATO5 from Gattefossé) in an appropriate vessel (a planetary or a turbosphere mixer) at about 60° C.-65° C. Modafinil of the specified particle size was then introduced and mixed until homogeneization. The obtained granules were screened through a 1 mm screen and stored at room temperature. They are used according to the same procedure as uncoated particles.
TABLE IIExamples56Modafinil (96%)Modafinil (50%)Precirol ®Precirol ®ATO 5 (4%)ATO 5 (50%)Modafinil (20-40 μm)300mg 140mgPrecirol ® ATO 512.5mg140mgCopovidone0.7mg0.7mgDextran 7015mg15mgAspartam7mg7mgAcesulfam K10mg10mgFlavor (pineapple)15mg15mgCitric acid5mg5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| median diameter | aaaaa | aaaaa |
| median diameter | aaaaa | aaaaa |
| median diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com