Patents
Literature
136 results about "Retinal Disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
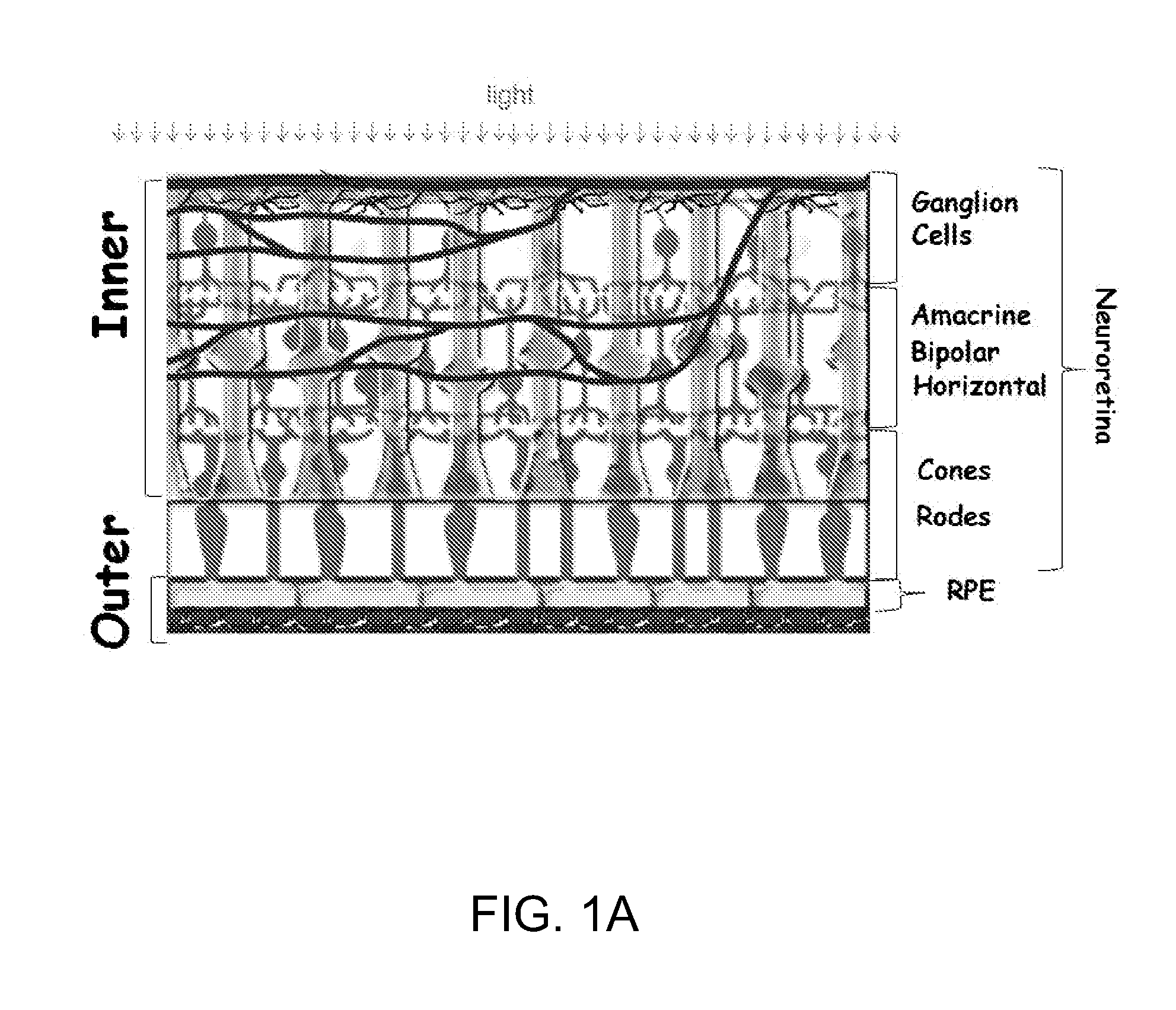
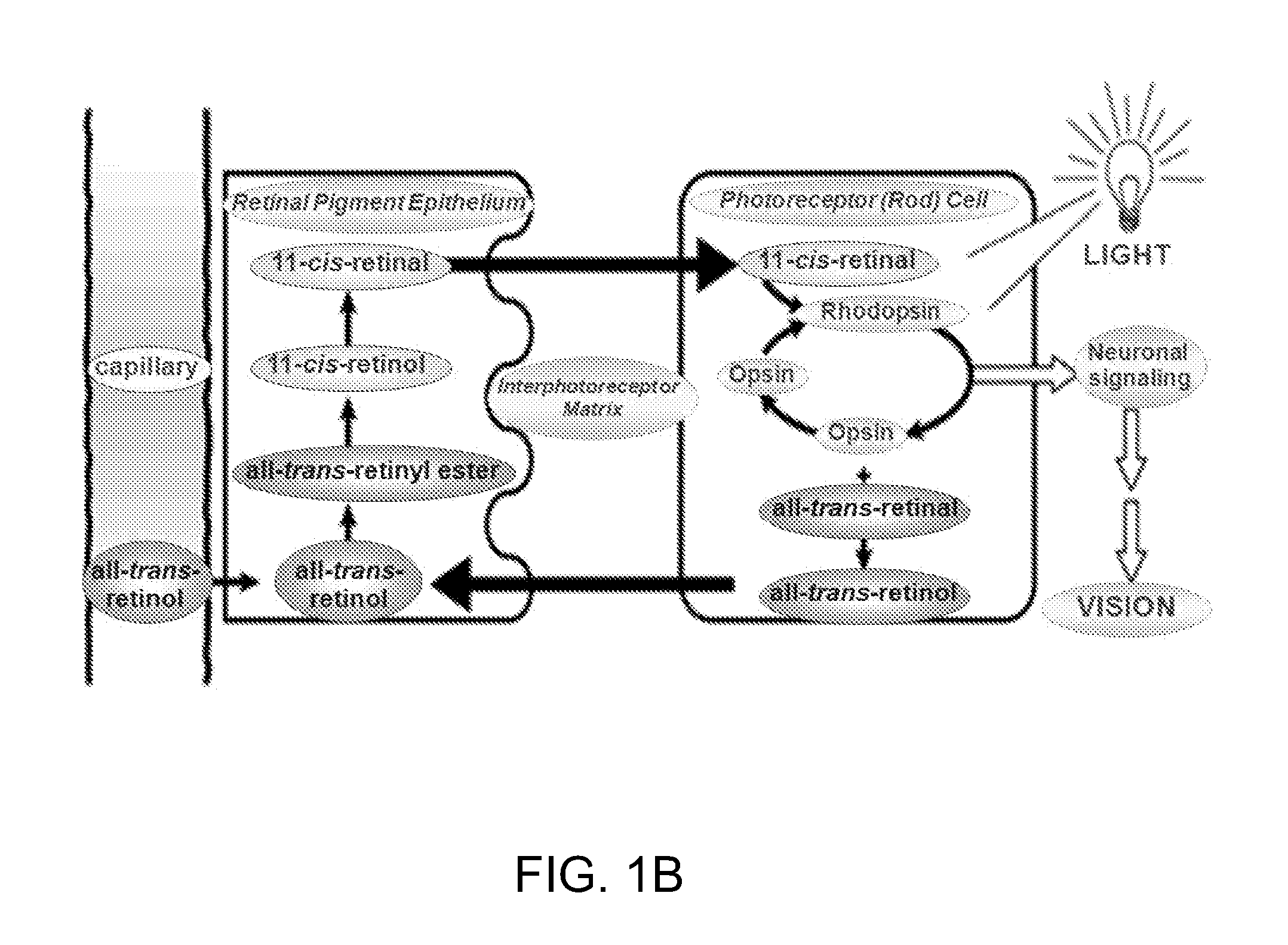
An abnormal structure or function of the retina and its associated tissues.
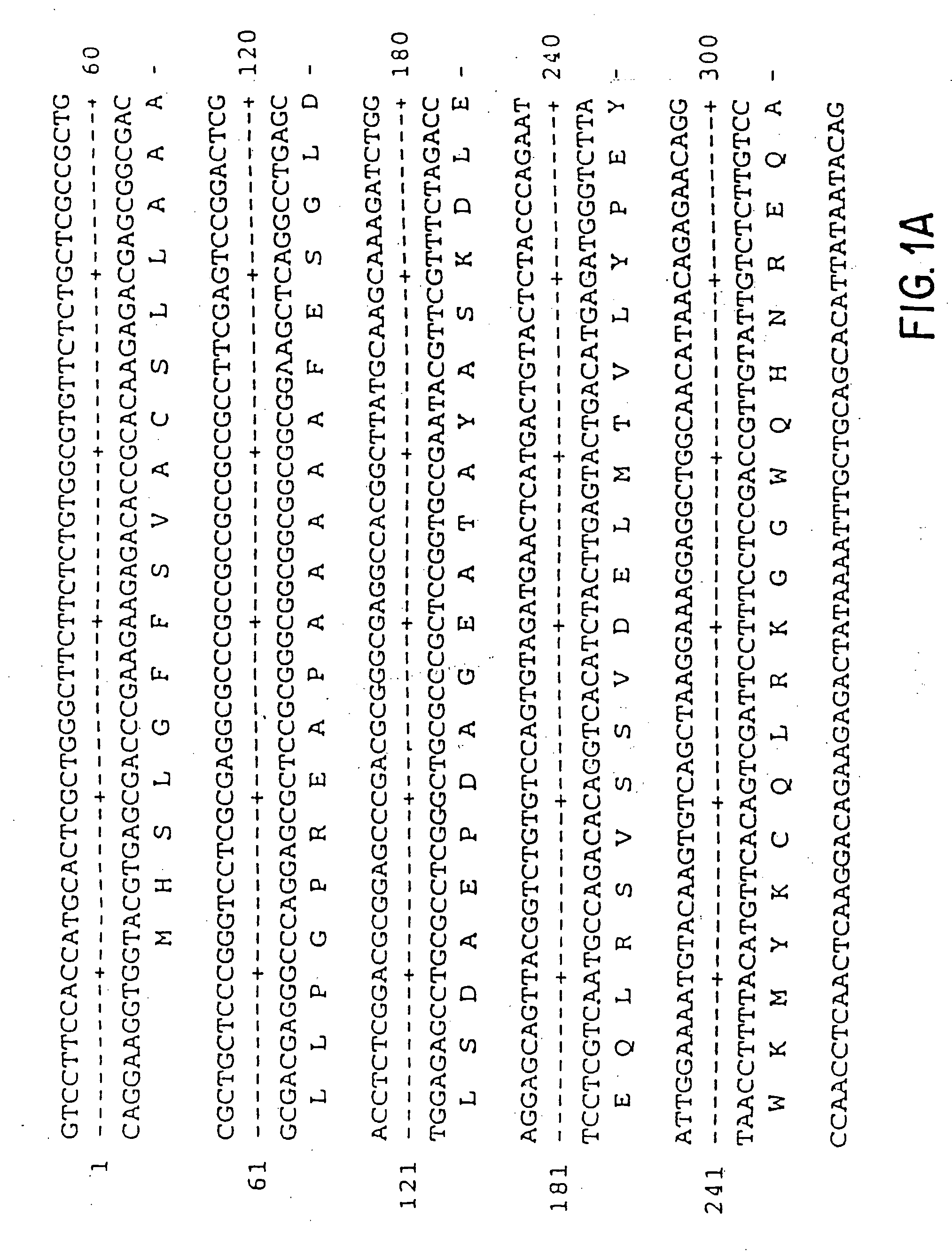
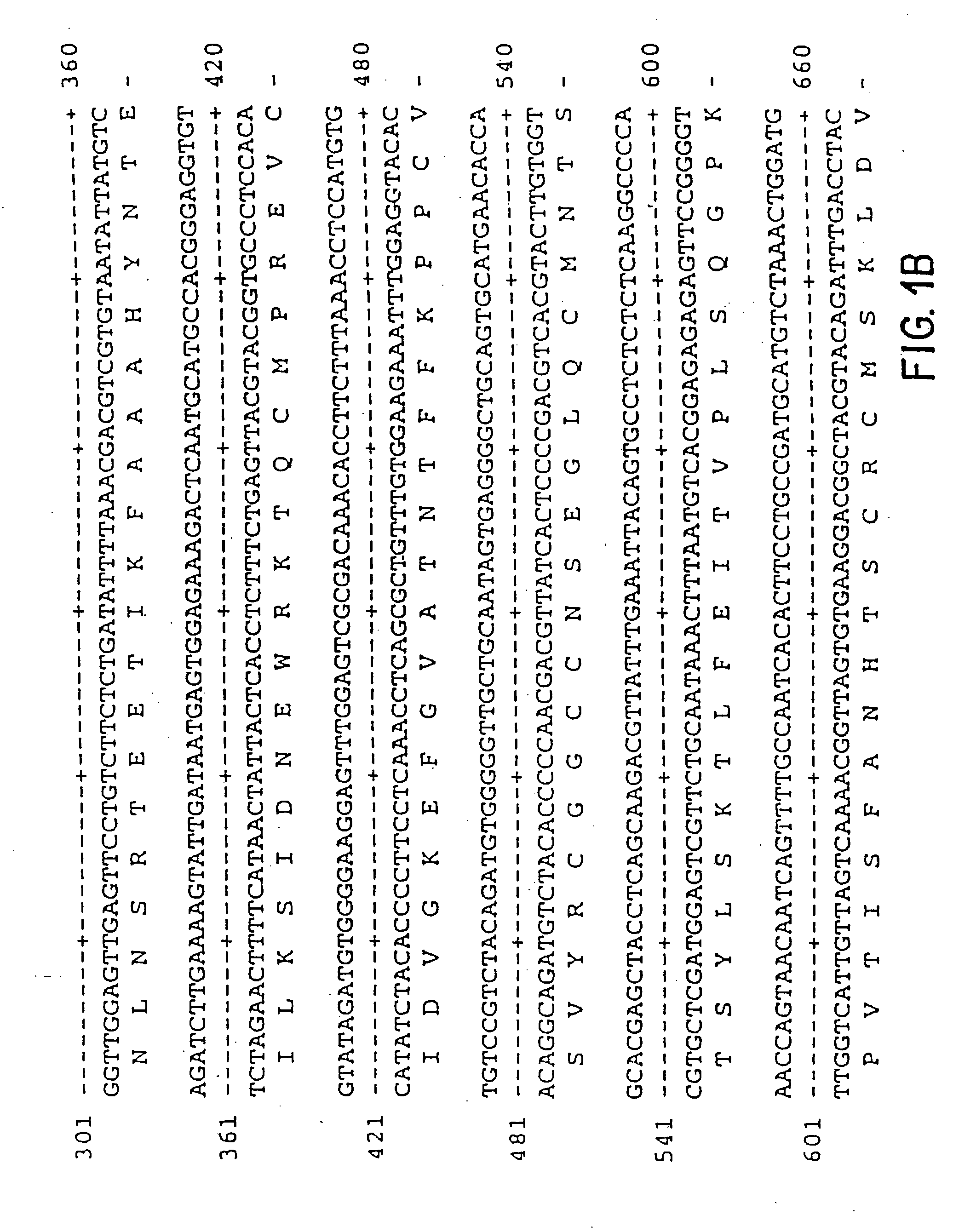
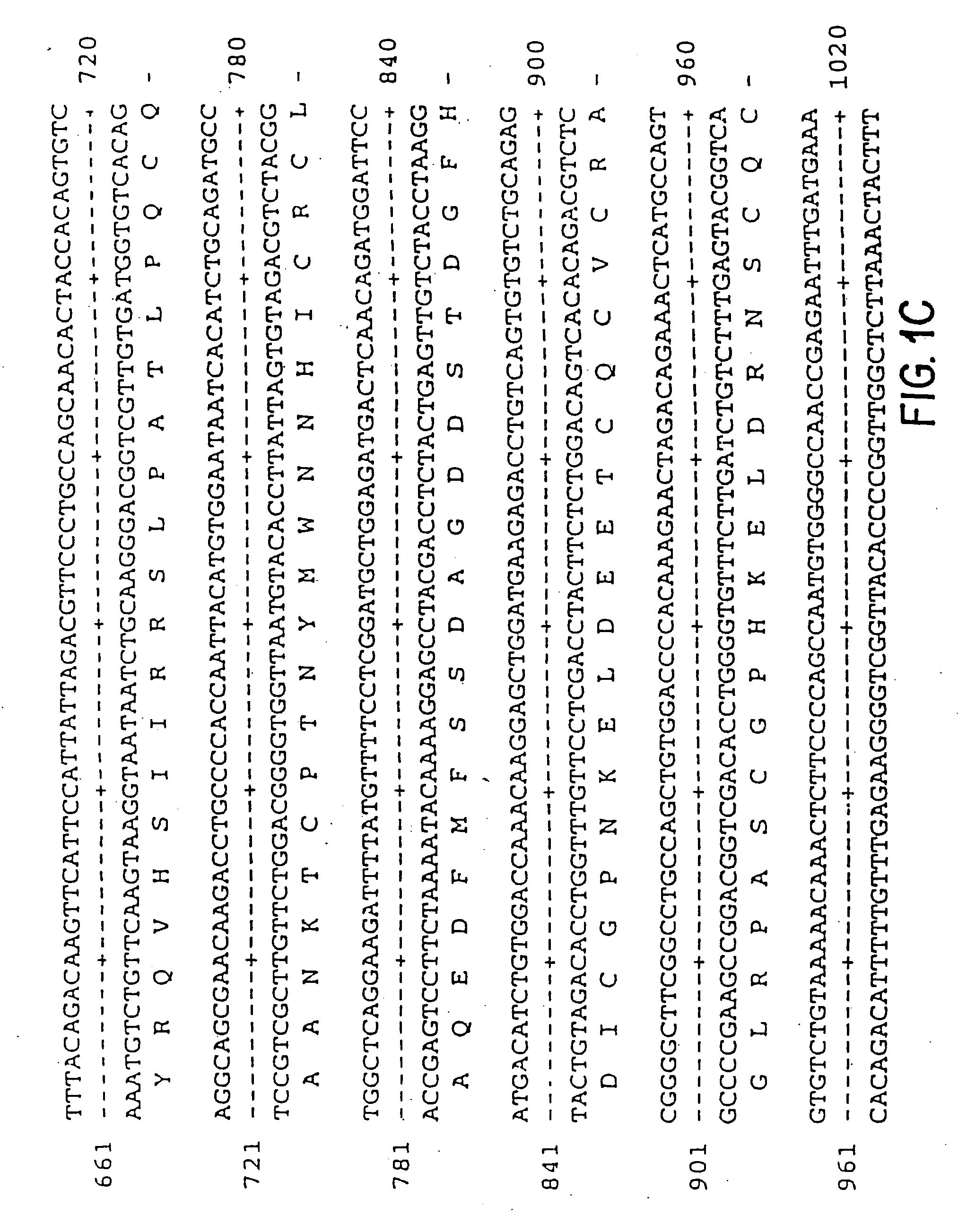
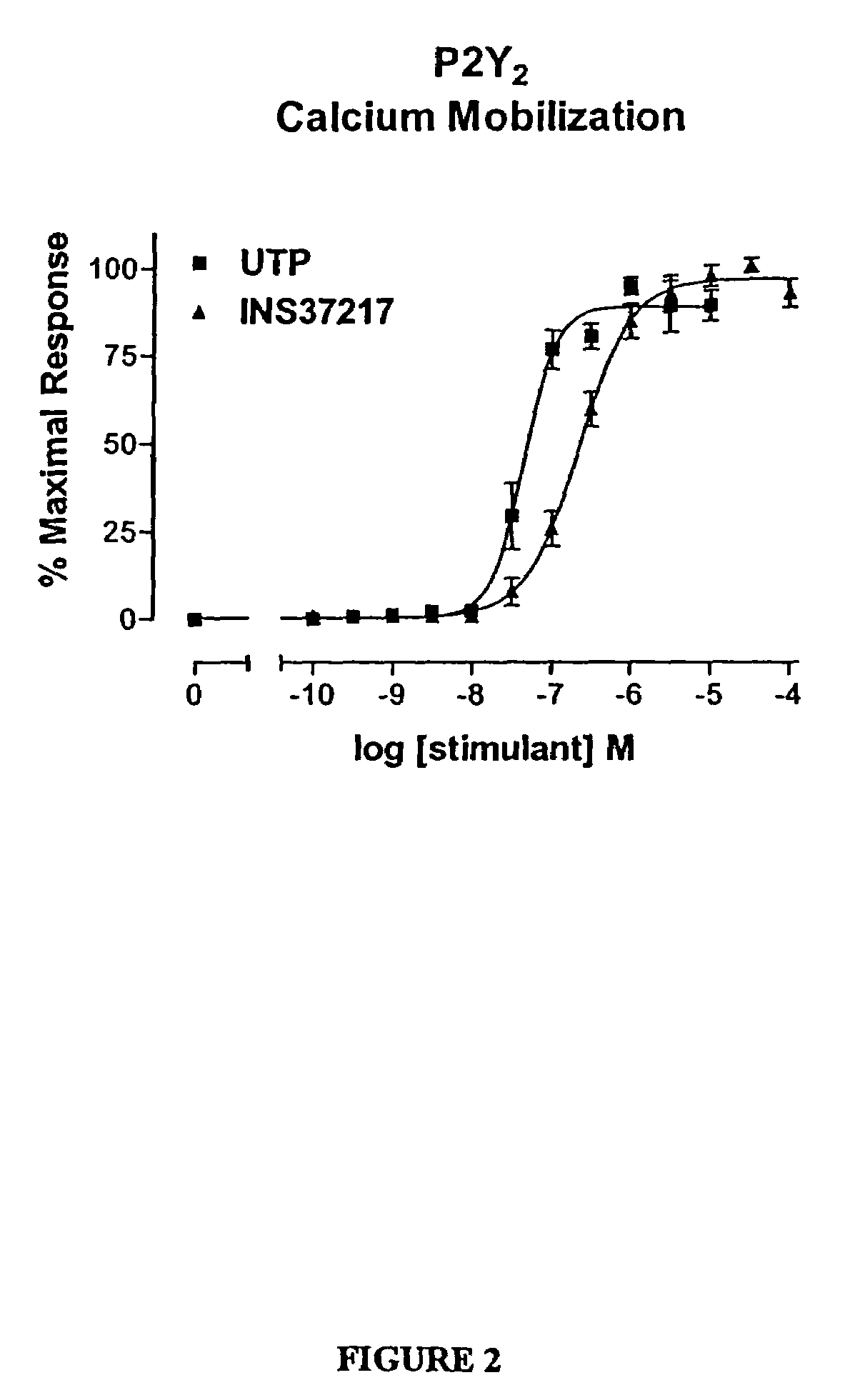
Pharmaceutical formulation comprising dinucleoside polyphosphates and salts thereof
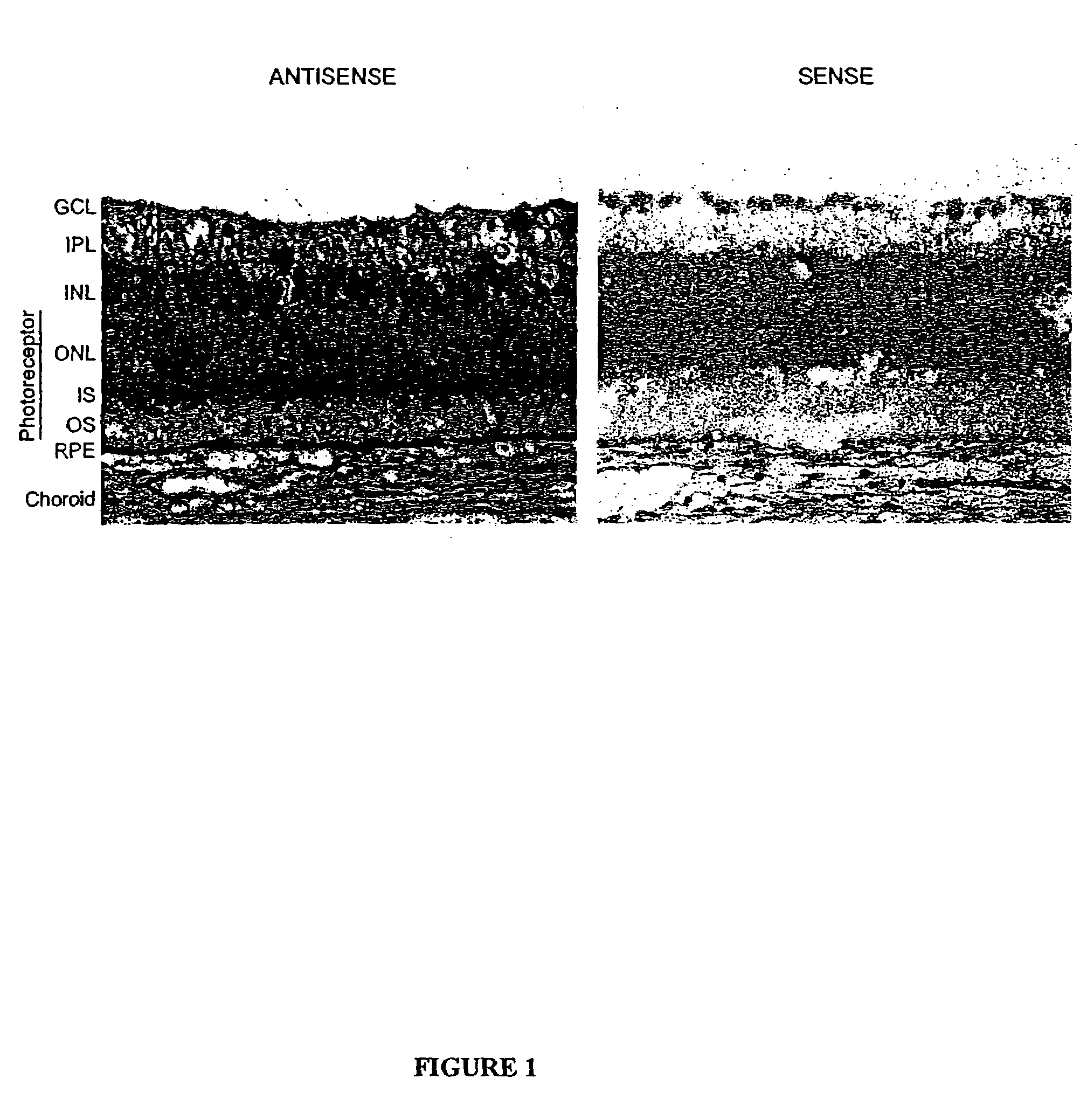
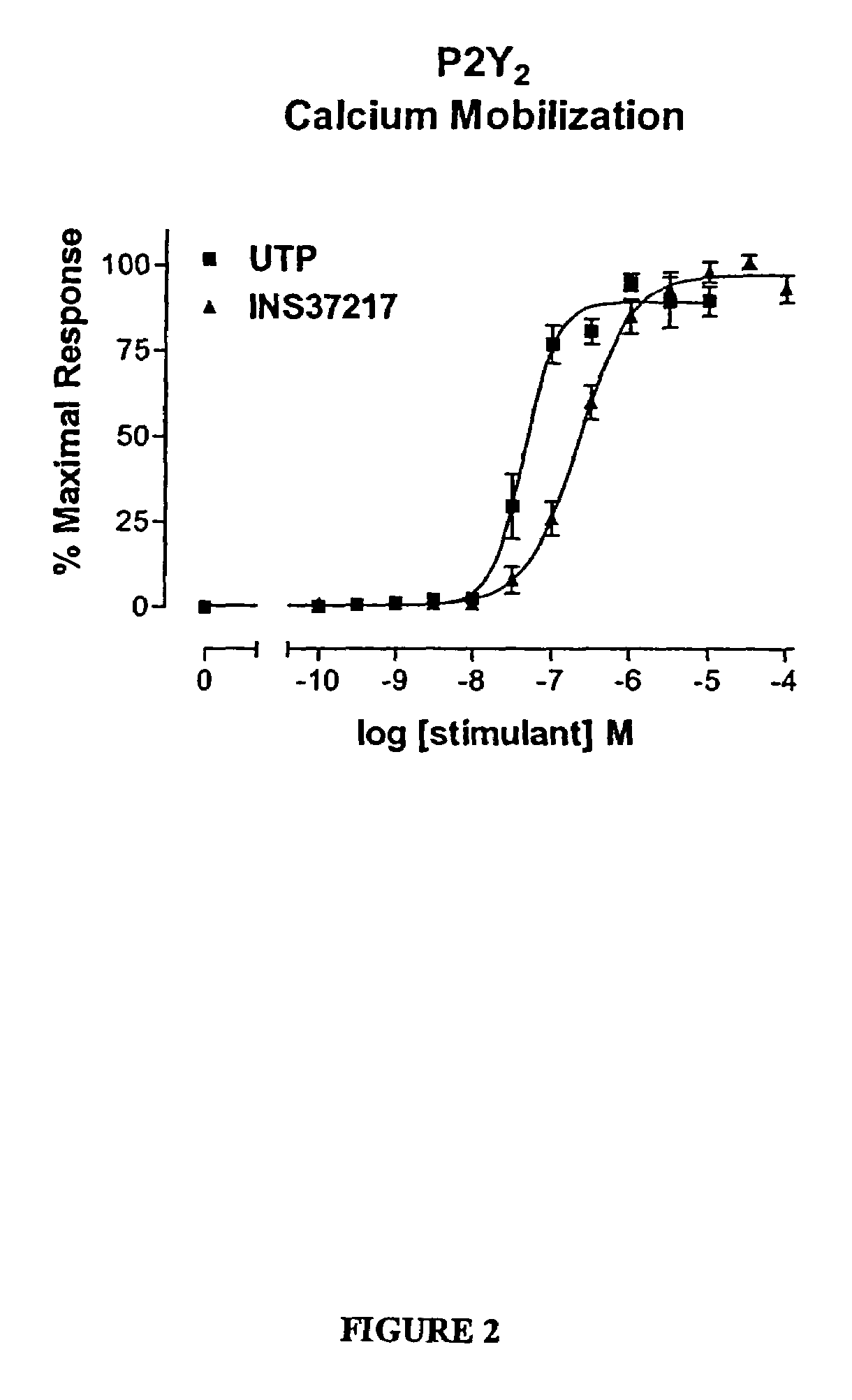
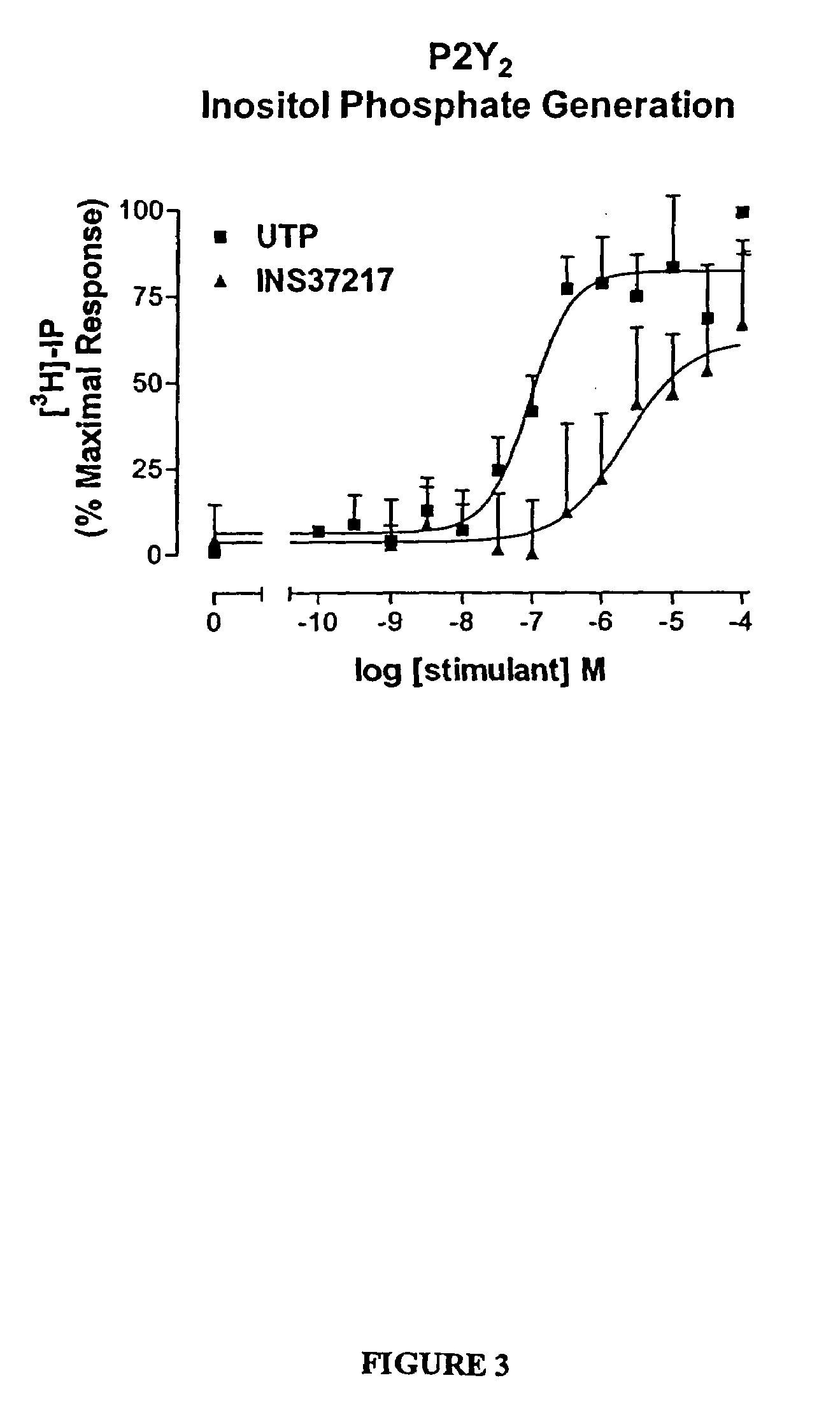
The present invention provides a method of treating edematous retinal disorders. The method comprises administration of a pharmaceutical formulation comprising a hydrolysis-resistant P2Y receptor agonist to stimulate the removal of pathological extraneous fluid from the subretinal and retinal spaces and thereby reduce the accumulation of said fluid associated with retinal detachment and retinal edema. The P2Y receptor agonist can be administered with therapeutic and adjuvant agents commonly used to treat edematous retinal disorders. The pharmaceutical formulation useful in this invention comprises a P2Y receptor agonist with enhanced resistance to extracellular hydrolysis, such as dinucleoside polyphosphate compounds, or hydrolysis-resistant mononucleoside triphosphate salts. The present invention also provides P1-(2′-deoxycytidine 5′-)P4-(uridine 5′-)tetraphosphate, tetra-(alkali metal) salts such as tetrasodium, tetralithium, tetrapotassium, and mixed (tetra-alkali metal) salts. The present further provides a pharmaceutical formulation comprising a P1-(2′-deoxycytidine 5′-)P4-(uridine 5′-)tetraphosphate, tetra-(alkali metal) salt, in a pharmaceutically acceptable carrier.
Owner:MERCK SHARP & DOHME LLC
Sustained release implants and methods for subretinal delivery of bioactive agents to treat or prevent retinal disease
InactiveUS20060257451A1Inhibit progressQuantity maximizationSenses disorderEye implantsChoroid membraneOphthalmology
The invention relates to sustained release implants and to methods for treating eyes, particularly the eyes of mammals having eye disorders or diseases. By using the implants and methods described herein, the delivery of the one or more bioactive agents can be localized at a desired treatment site, particularly the choroid and the retina.
Owner:SURMODICS INC
Compounds for the treatment of posterior segment disorders and diseases
The use of certain urea compounds, for the treatment of retinal disorders associated with pathologic ocular angiogenesis and / or neovascularization is disclosed.
Owner:ALCON RES LTD
Improved raav vectors and methods for transduction of photoreceptors and rpe cells
ActiveUS20160369299A1More efficiencyImprove efficiencyVectorsGenetic material ingredientsDiseaseIn vivo
Disclosed are capsid-modified rAAV particles and expression vectors, as well as compositions and pharmaceutical formulations that comprise them. Also disclosed are methods of preparing and using novel capsid-protein-mutated particle or rAAV vector constructs in a variety of diagnostic and therapeutic applications including, inter alia, as delivery agents for diagnosis, treatment, or amelioration of one or more diseases, disorders, or dysfunctions of the mammalian eye. Also disclosed are methods for subretinal delivery of therapeutic gene constructs to mammalian photoreceptors and retinal pigment epithelial cells, as well as use of the disclosed compositions in the manufacture of medicaments for a variety of in vitro and / or in vivo applications including the treatment of a variety of inherited retinal diseases.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
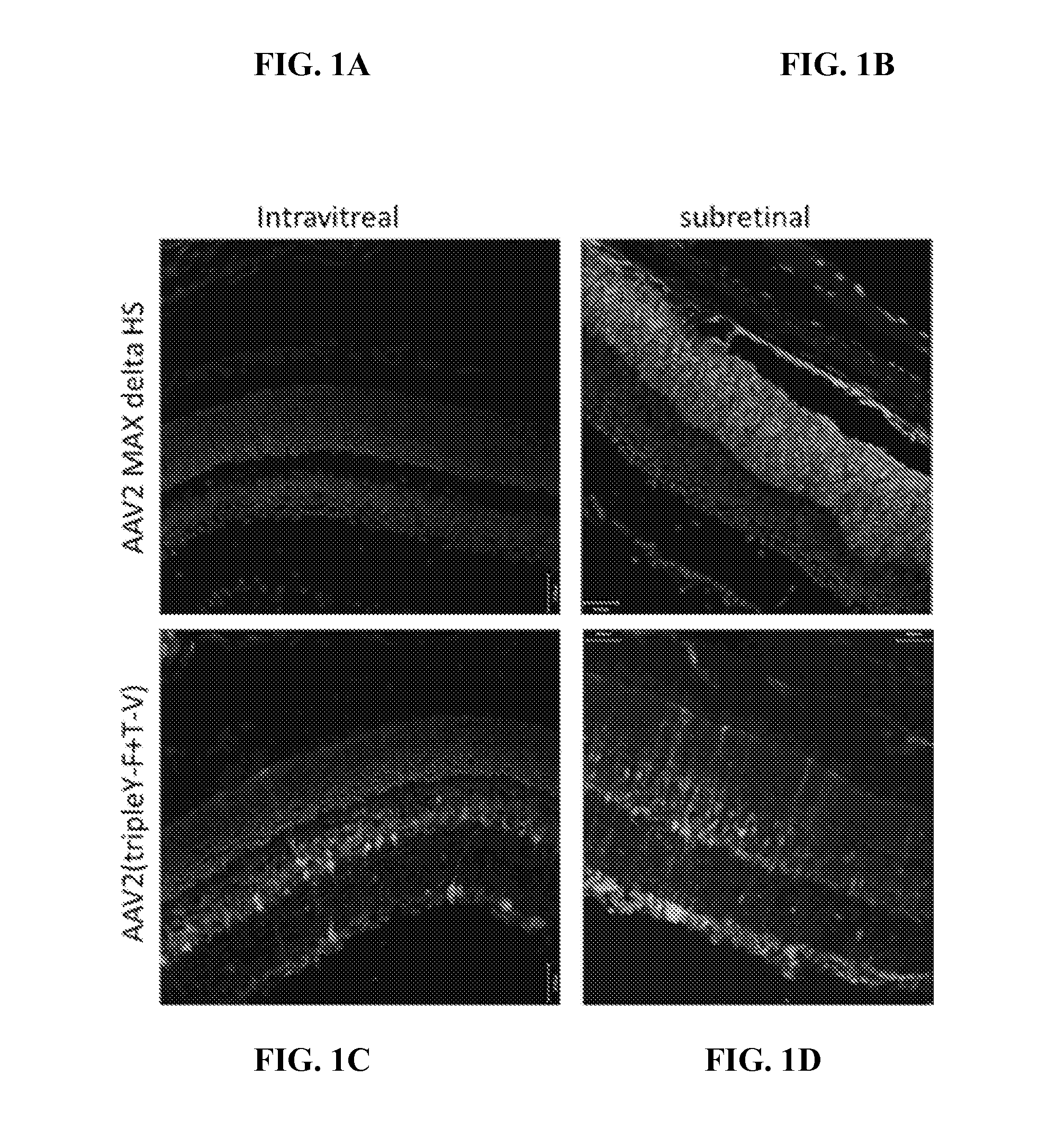
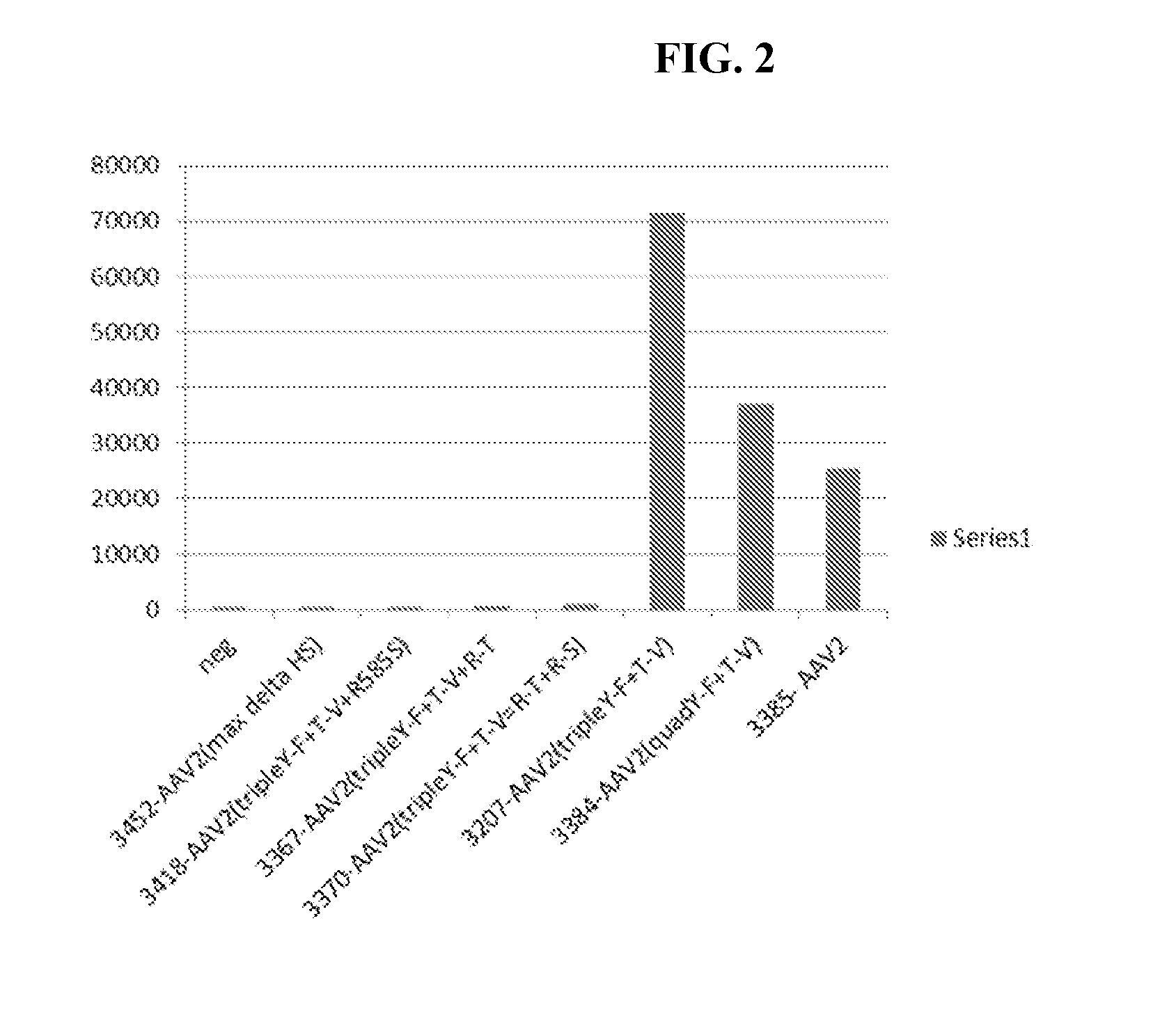
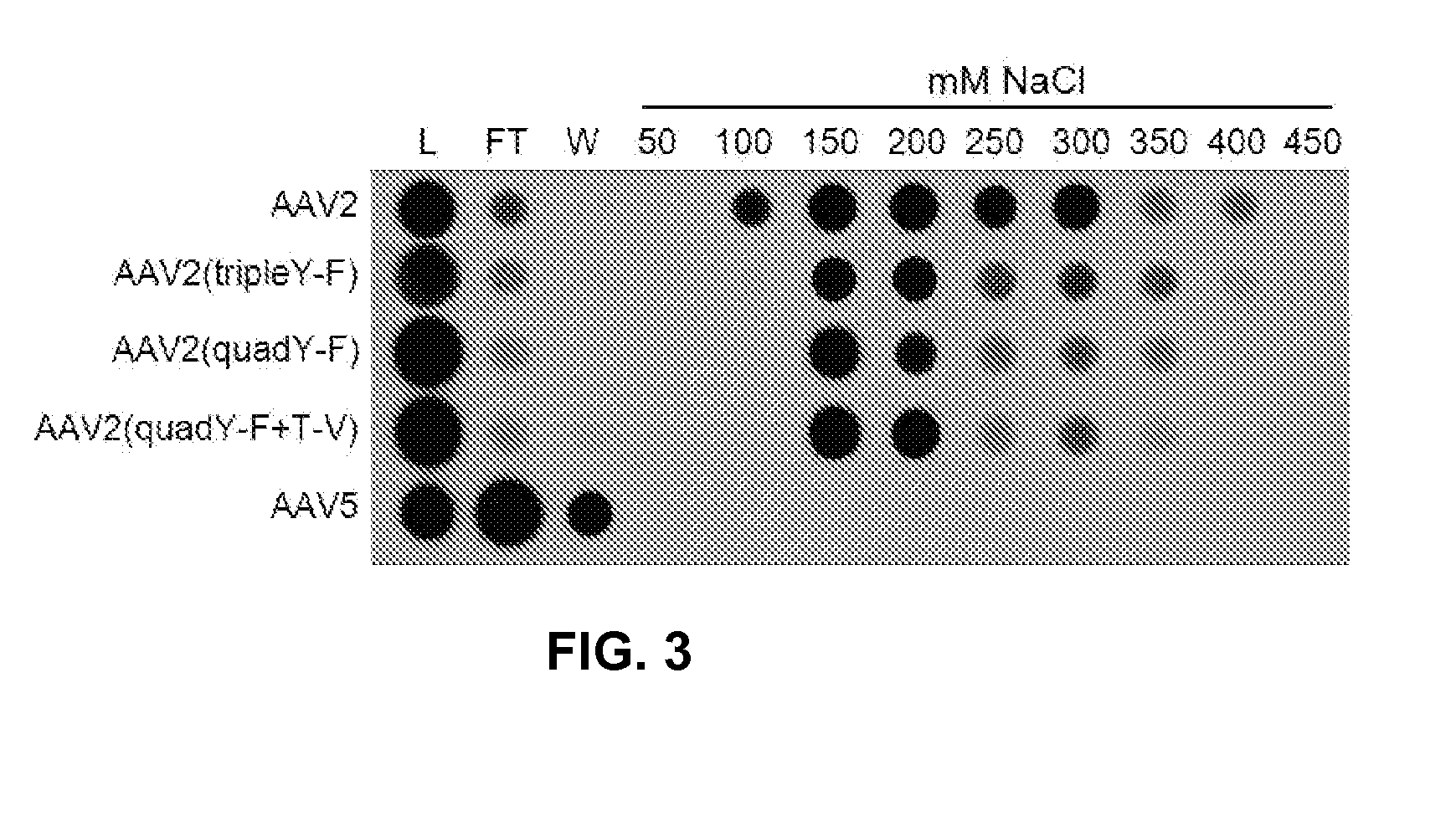
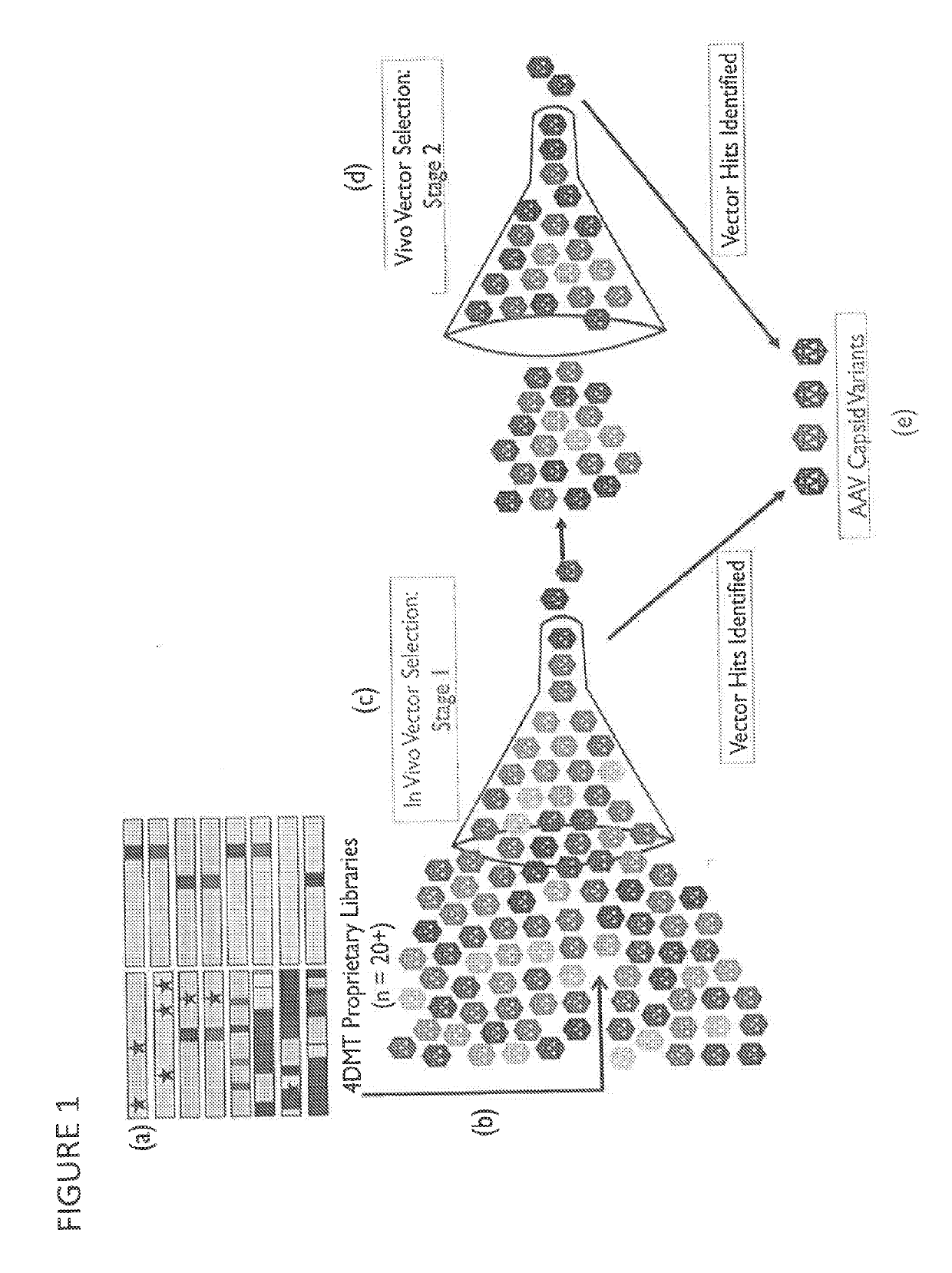
Adeno-associated virus variant capsids and methods of use thereof
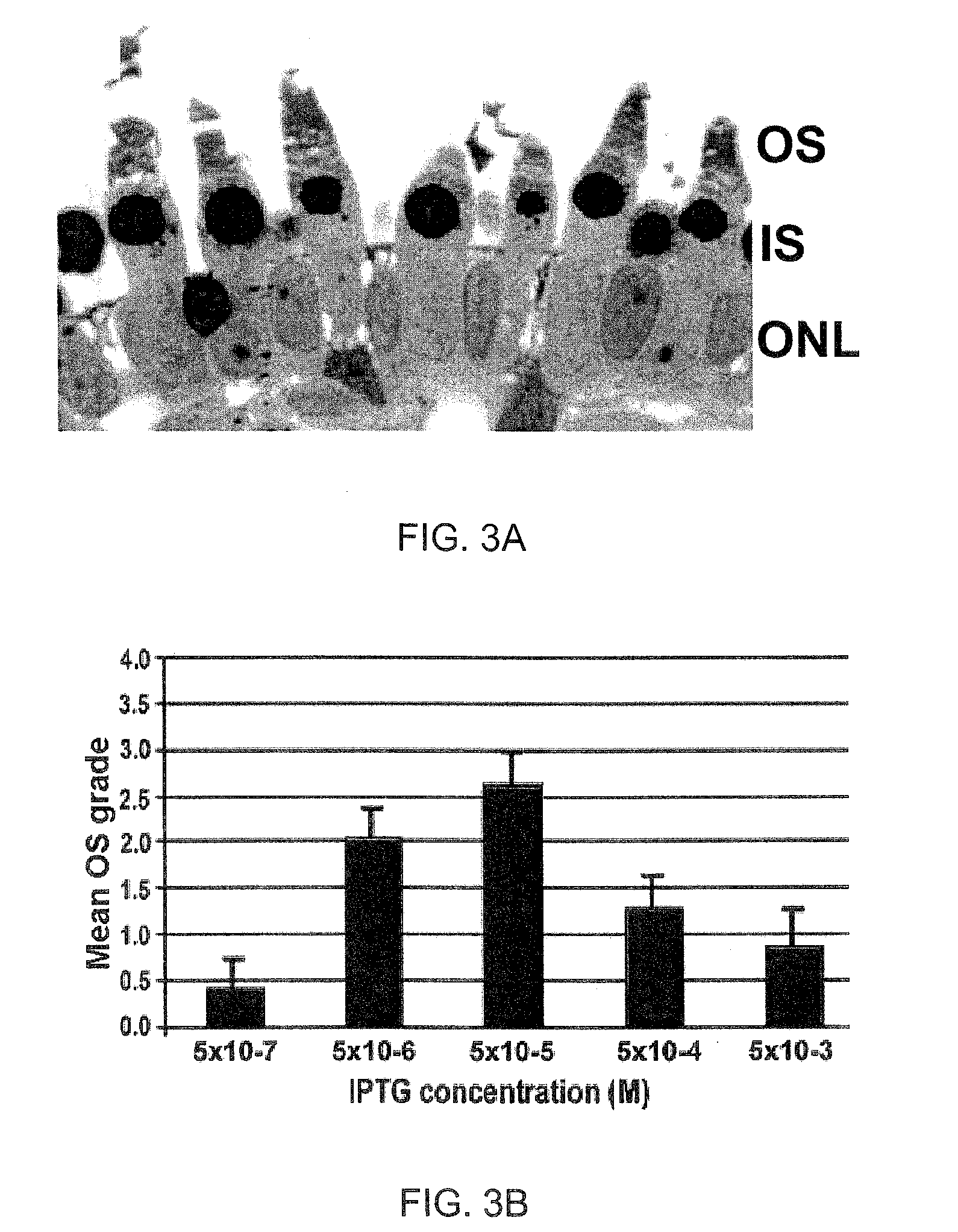
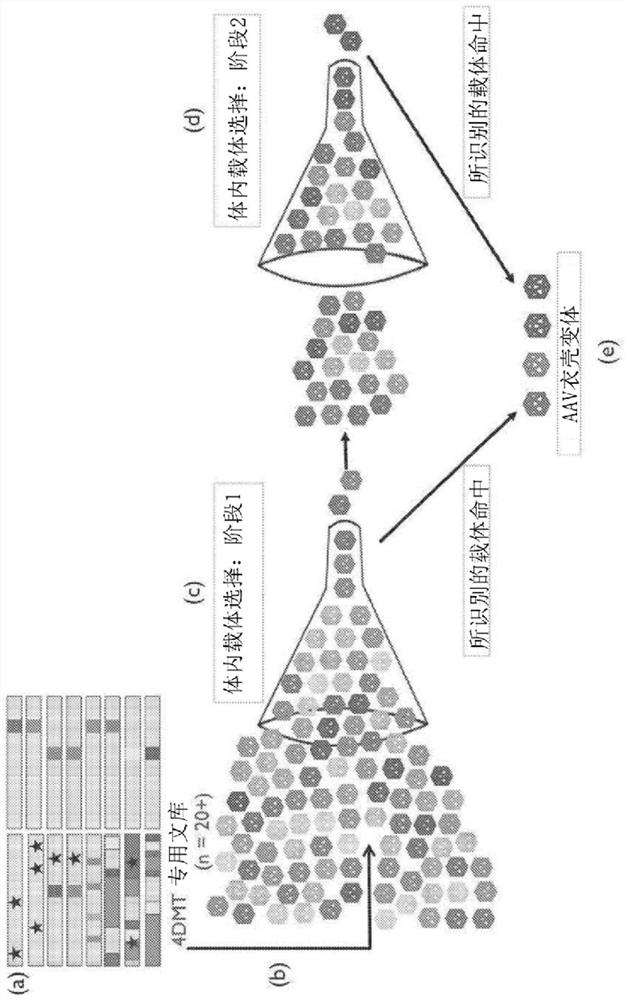
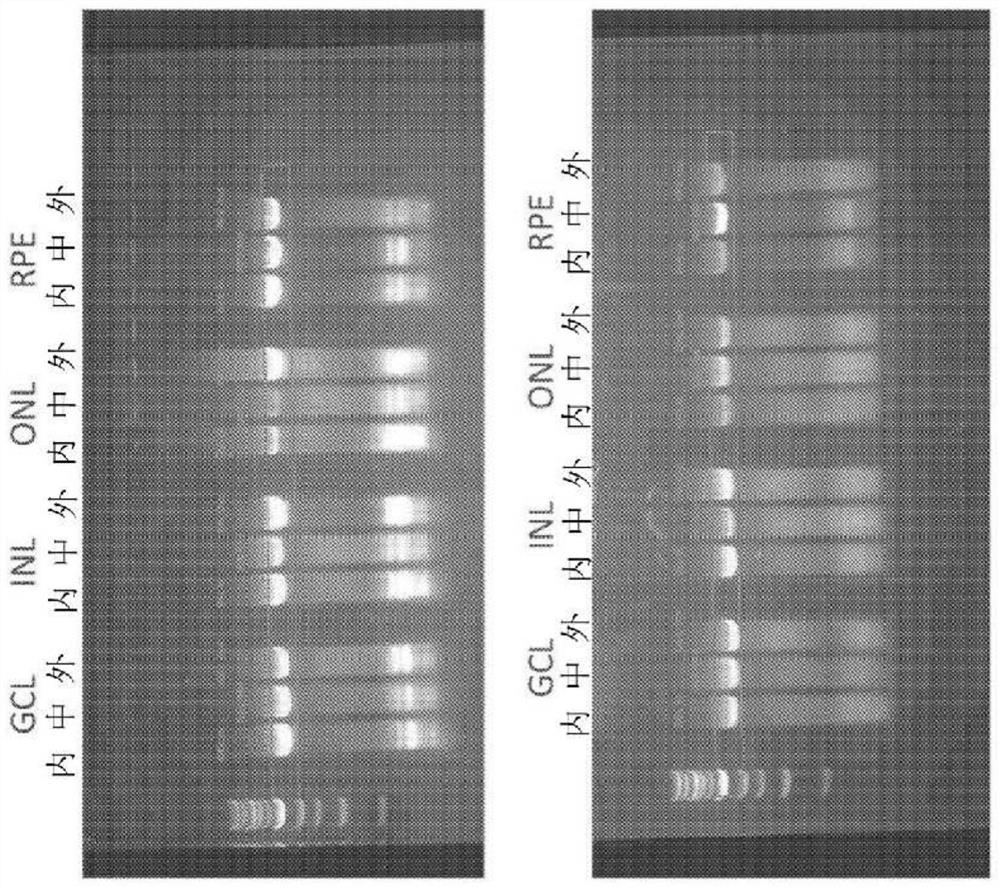
Provided herein are variant adeno-associated virus (AAV) capsid proteins having one or more modifications in amino acid sequence relative to a parental AAV capsid protein, which, when present in an AAV virion, confer increased infectivity of one or more types of retinal cells as compared to the infectivity of the retinal cells by an AA V virion comprising the unmodified parental capsid protein. Also provided are recombinant AAV virions and pharmaceutical compositions thereof comprising a variant AAV capsid protein as described herein, methods of making these rAAV capsid proteins and virions, and methods for using these rAAV capsid proteins and virions in research and in clinical practice, for example in, e.g., the delivery of nucleic acid sequences to one or more cells of the retina for the treatment of retinal disorders and diseases.
Owner:4D MOLECULAR THERAPEUTICS INC
Circular preferential hyperacuity perimetry video game to monitor macular and retinal diseases
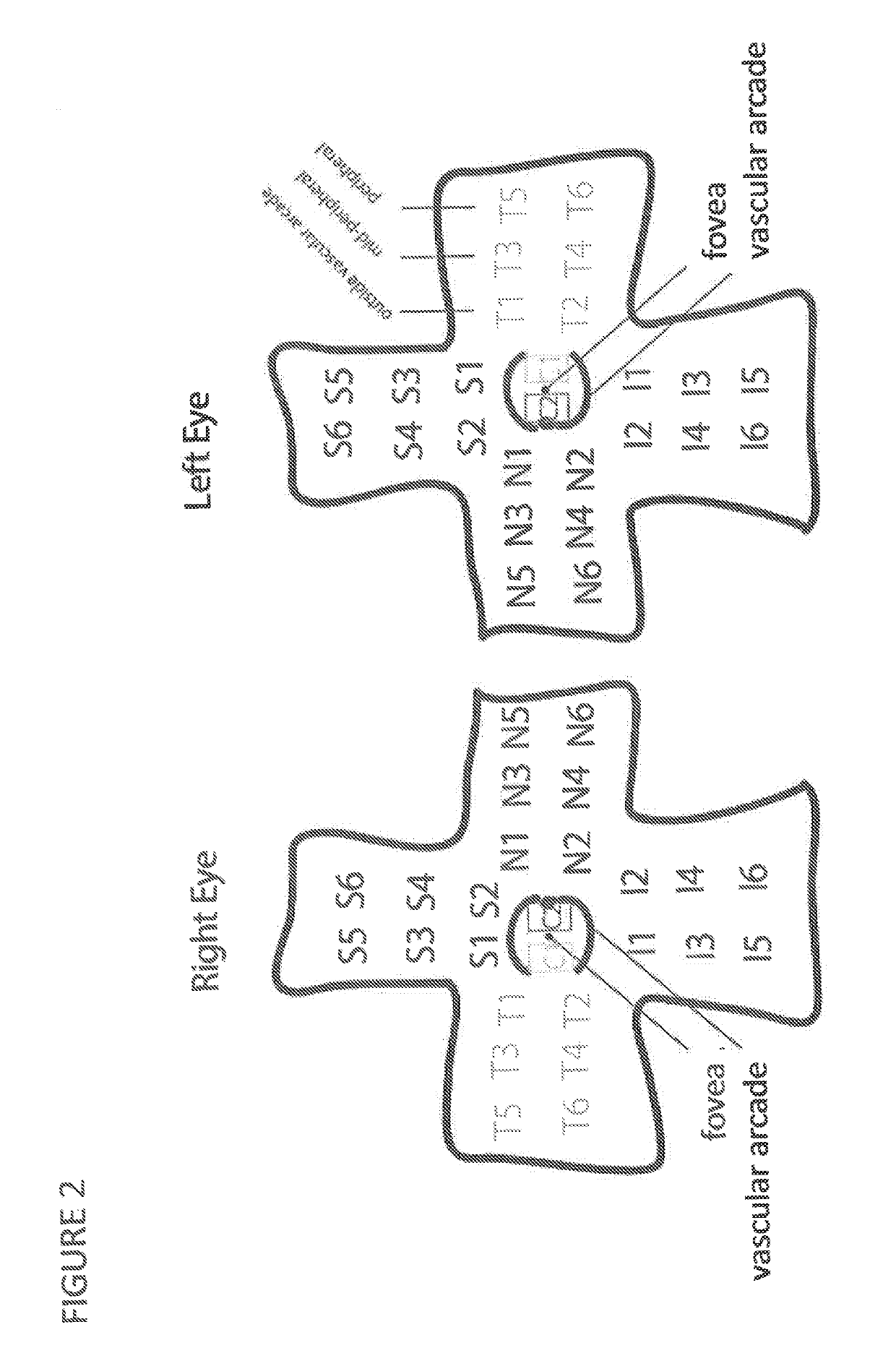
Systems and methods for providing a video game to map macular visual acuity comprising a test where a fixation point is ensured by brief simultaneous presentation of central and pericentral targets. The game may be implemented on a hardware platform including a video display, a user input device, and a video camera. The camera is used to monitor ambient light level and the distance between the device and the eyes of the test subject. The game serves as a macular acuity perimeter that produces a map of the acuity of an eye that may be compared with normative data. The type of acuity tested is preferably Vernier acuity, but resolution acuity can also be tested. The test results are transmitted to a health care professional by telecommunications means to facilitate the diagnosis or monitoring of age-related macular degeneration or other relevant eye diseases.
Owner:GOBIQUITY INC
Use of vascular endothelial growth factor to treat photoreceptor cells
The present invention is directed to VEGF-2 polynucleotides and polypeptides and methods of using such polynucleotides and polypeptides. In particular, provided are methods of treating retinal disorders with VEGF-2 polynucleotides and polypeptides.
Owner:HUMAN GENOME SCI INC
Multi-phasic microphotodetector retinal implant with variable voltage and current capability and apparatus for insertion
InactiveUS20020099420A1Higher capacitive effectLower work functionTelevision system detailsHead electrodesDiseaseRetinal implant
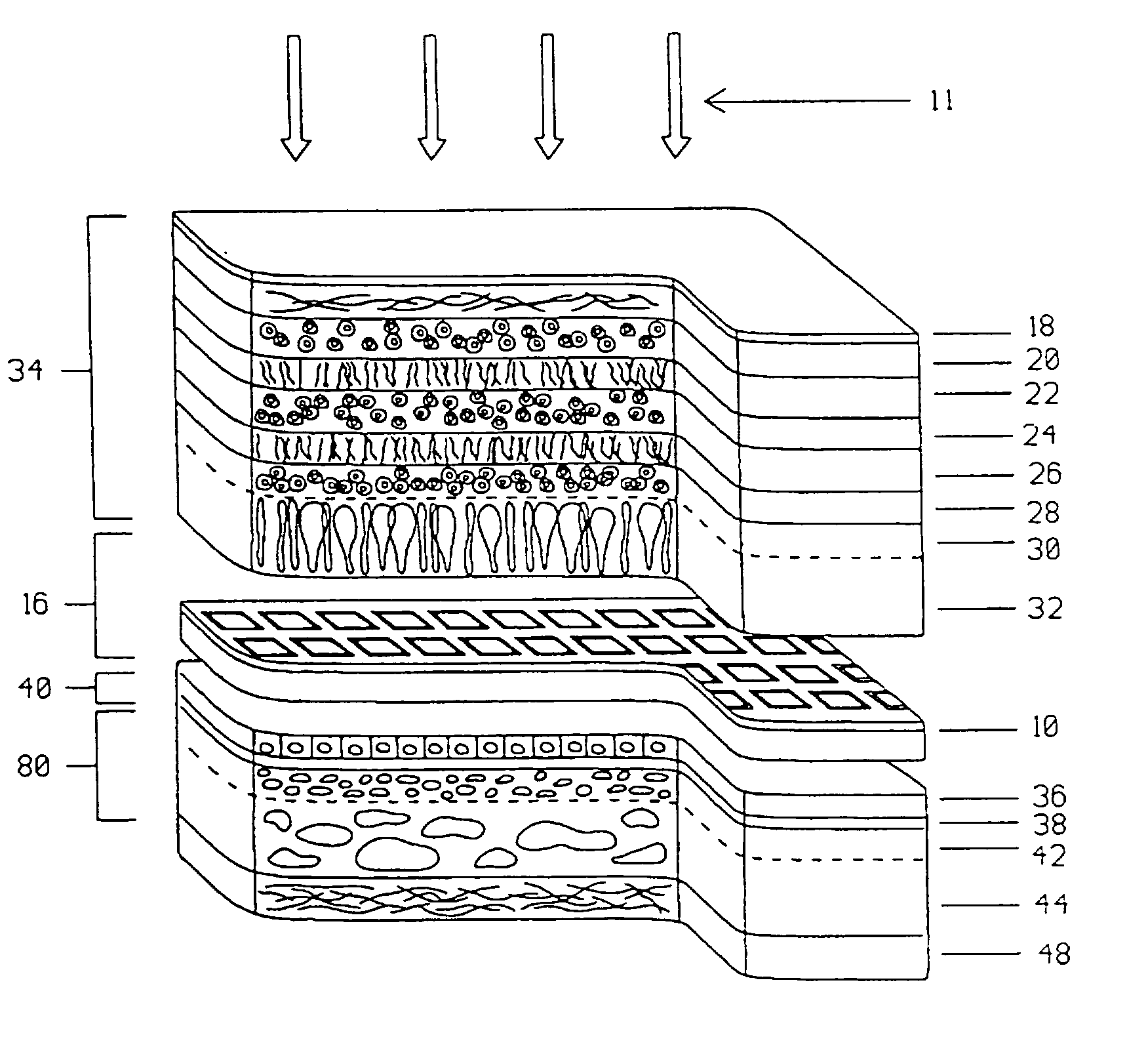
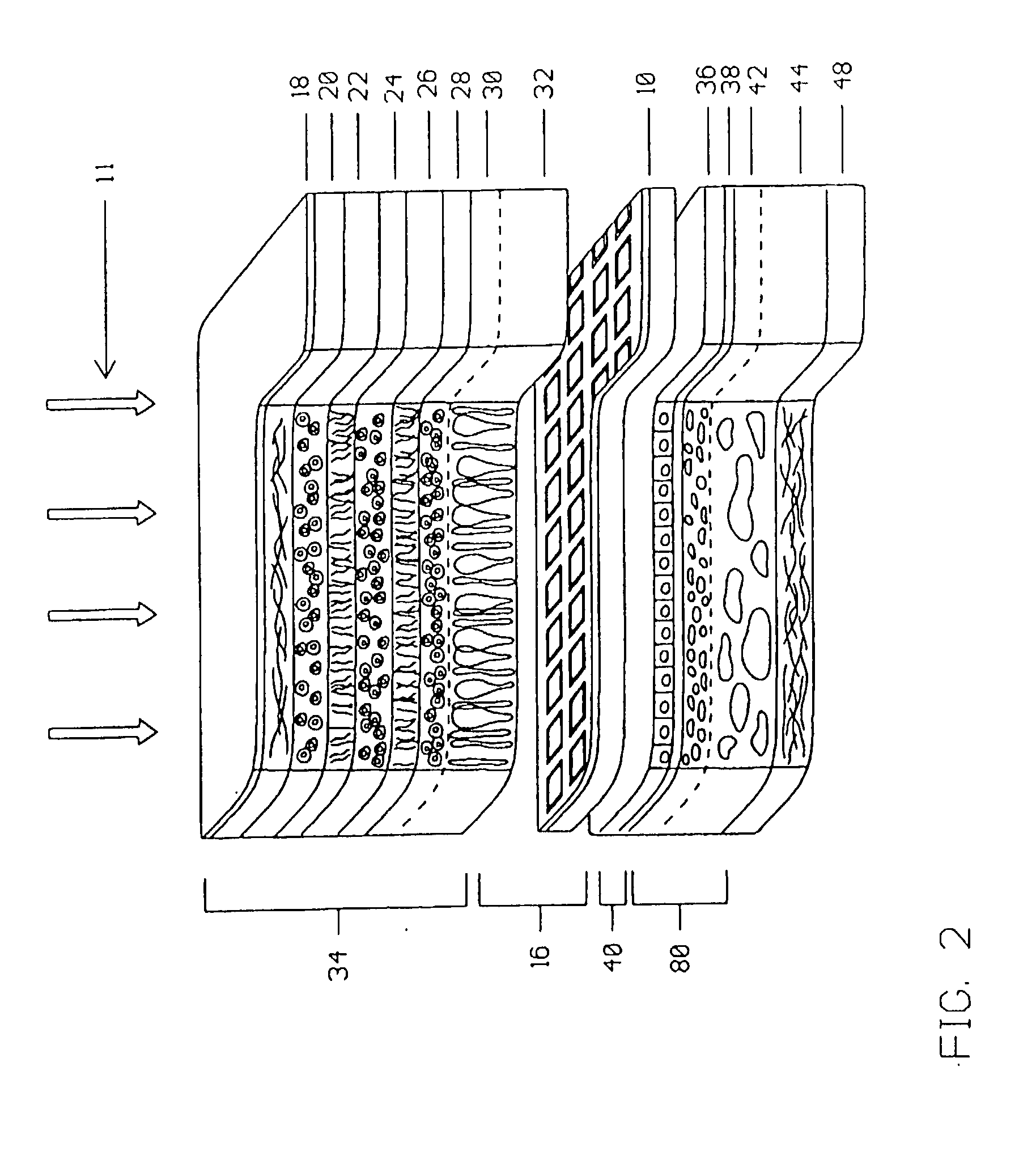
A visible and infrared light powered retinal implant is disclosed that is implanted into the subretinal space for electrically inducing formed vision in the eye. The retinal implant includes a stacked microphotodetector arrangement having an image sensing pixel layer and a voltage and current gain adjustment layer for providing variable voltage and current gain to the implant so as to obtain better low light implant performance than the prior art, and to compensate for high retinal stimulation thresholds present in some retinal diseases. A first light filter is positioned on one of the microphotodetectors in each of the image sensing pixels of the implant, and a second light filter is positioned on the other of the microphotodetectors in the image sensing pixel of the implant, each of the microphotodetectors of the pixel to respond to a different wavelength of light to produce a sensation of darkness utilizing the first wavelength, and a sensation of light using the second wavelength, and a third light filter is positioned on a portion of the voltage and current gain adjustment layer that is exposed to light, to allow adjustment of the implant voltage and current gain of the device by use of a third wavelength of light.
Owner:OPTOBIONICS
Treatments for retinal disorders
ActiveUS20140038918A1Little and no toxicityLower blood lipid levelsOrganic active ingredientsBiocideRetinitis pigmentosaRetinal Disorder
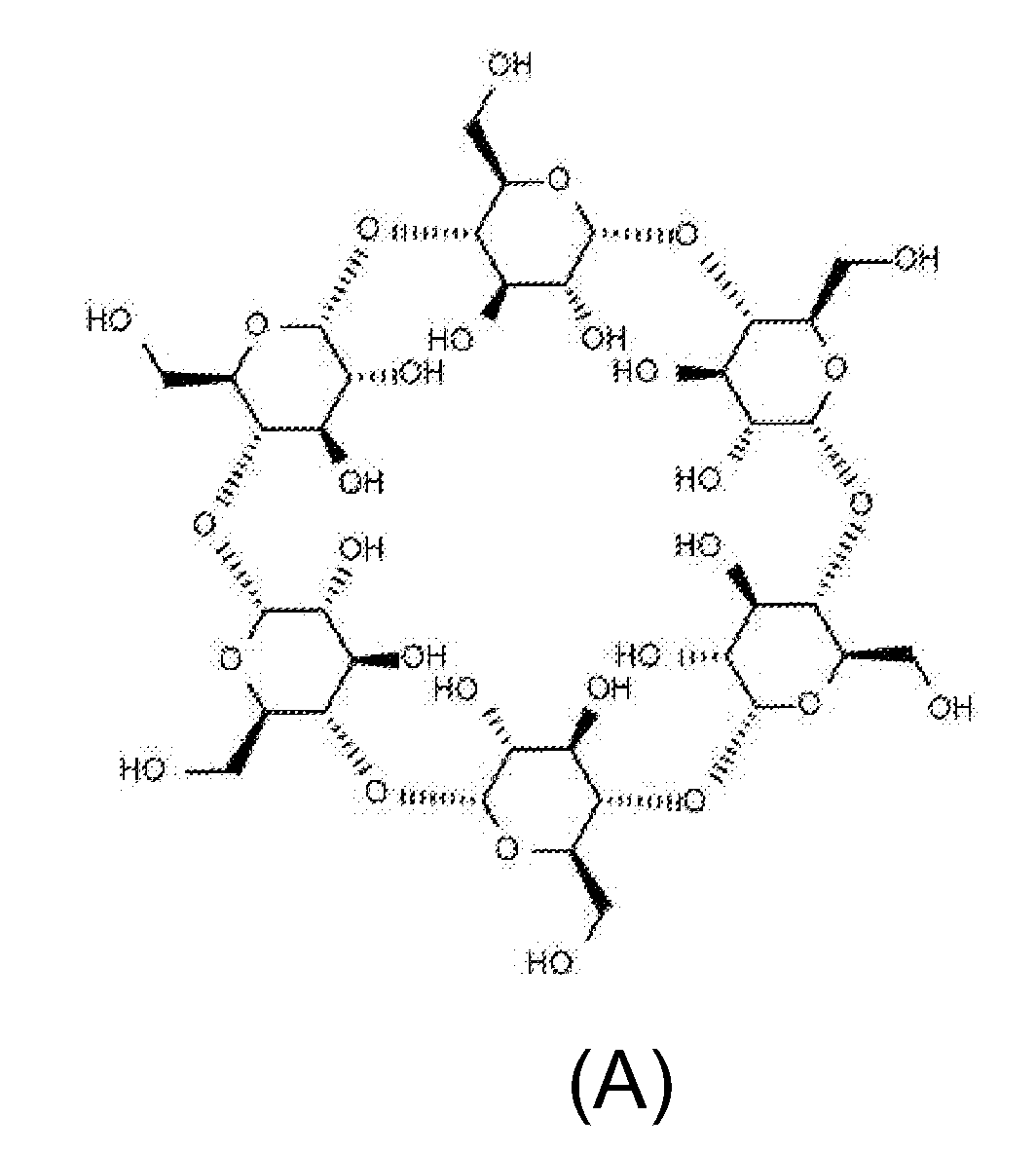
The present invention relates to the use of cyclic oligosaccharides as chemical complexants of lipofuscin bisretinoids (A2E) to prevent and treat eye (i.e., retinal or macular) disease. Monomeric, dimeric, multimeric, or polymeric oligosaccharide rings act as pharmacologic agents to prevent and treat ophthalmologic disorders triggered by the accumulation of lipofuscin in the retinal pigment epithelium (RPE), which occurs as a consequence of either genetic disorders, such as Stargardt Disease (SD) and Best Disease (BD), or aging, such as Age-Related Macular Degeneration (AMD), or other diseases, such as retinitis pigmentosa, and cone-rod dystrophy.
Owner:CORNELL UNIVERSITY
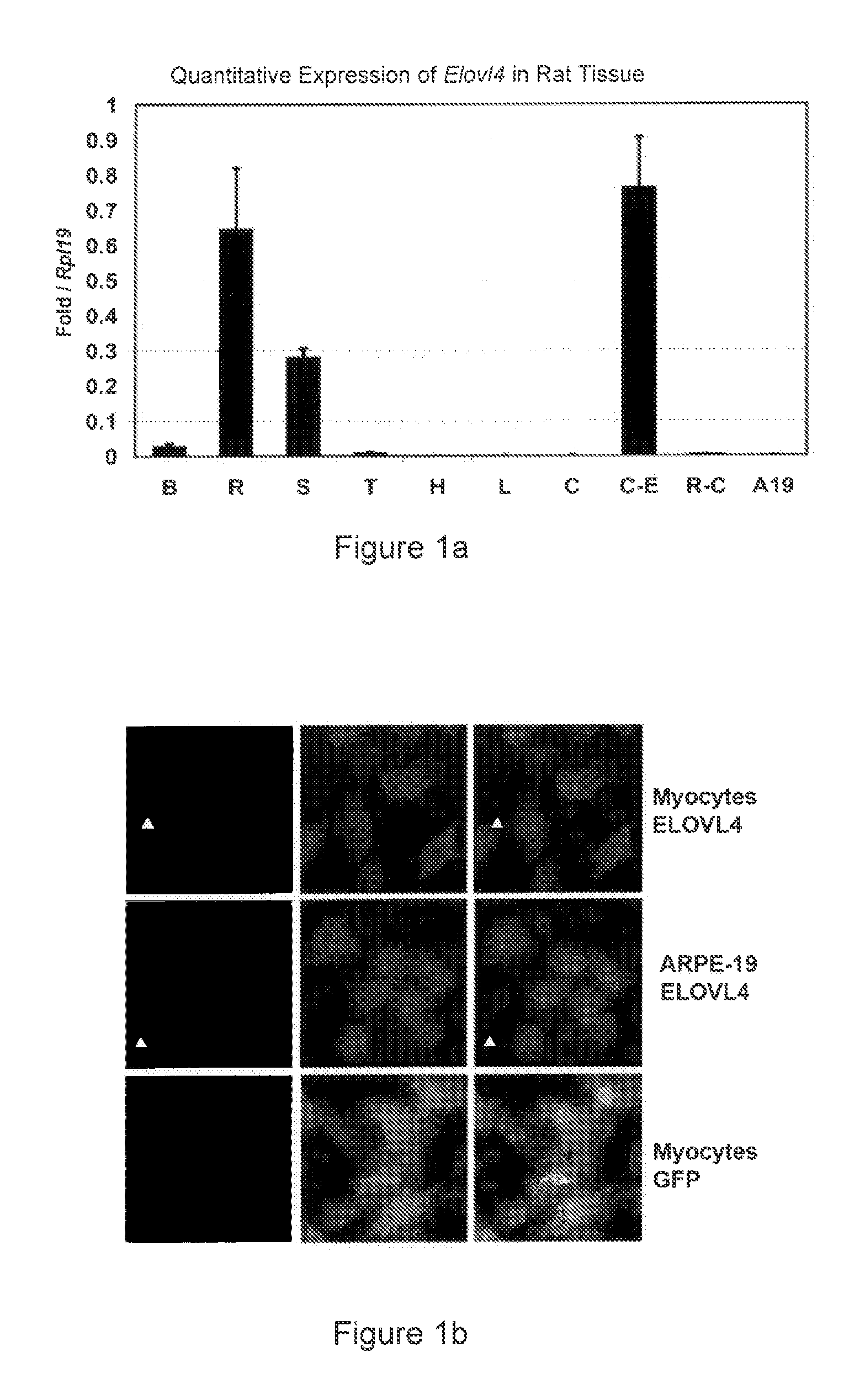
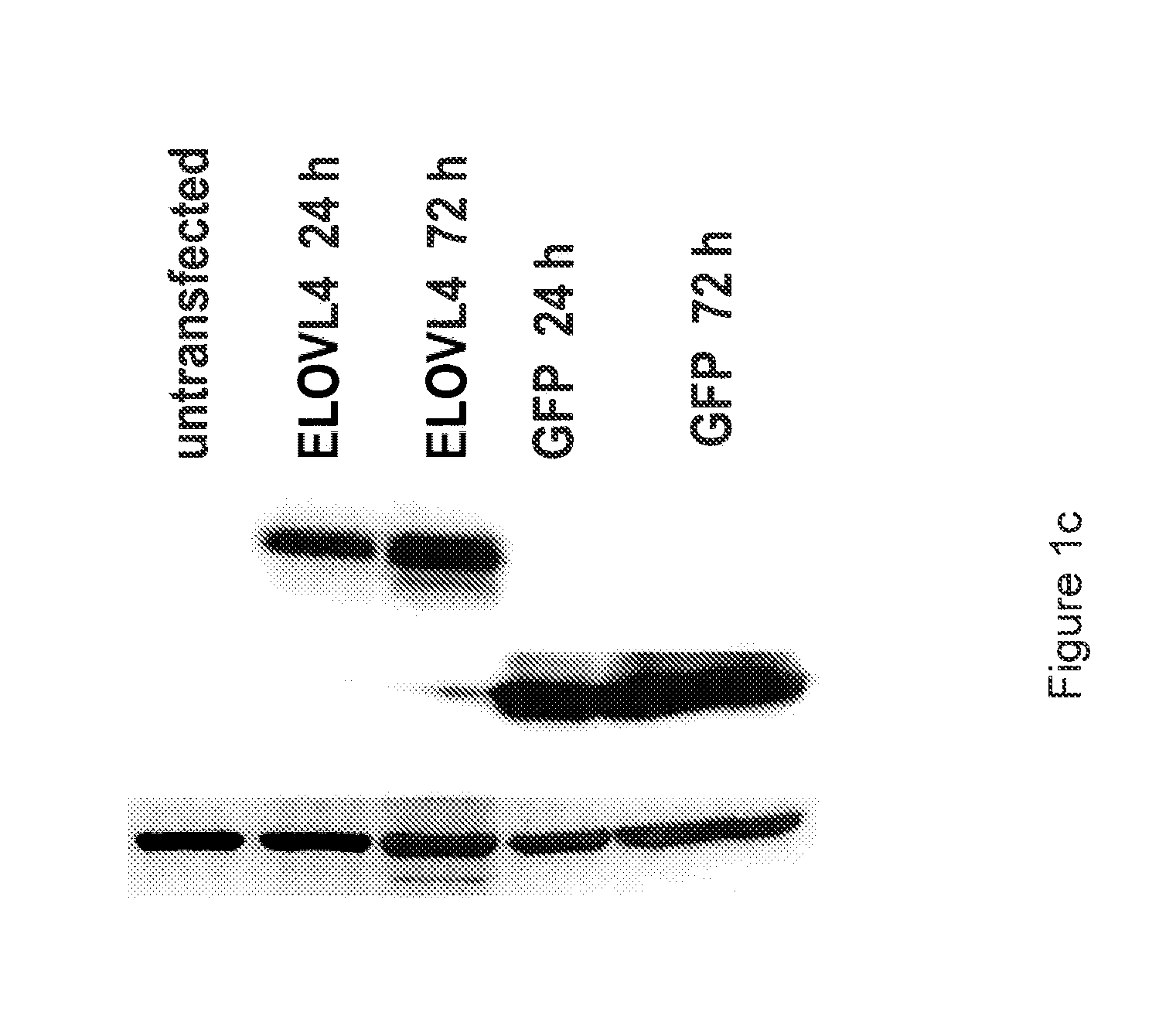
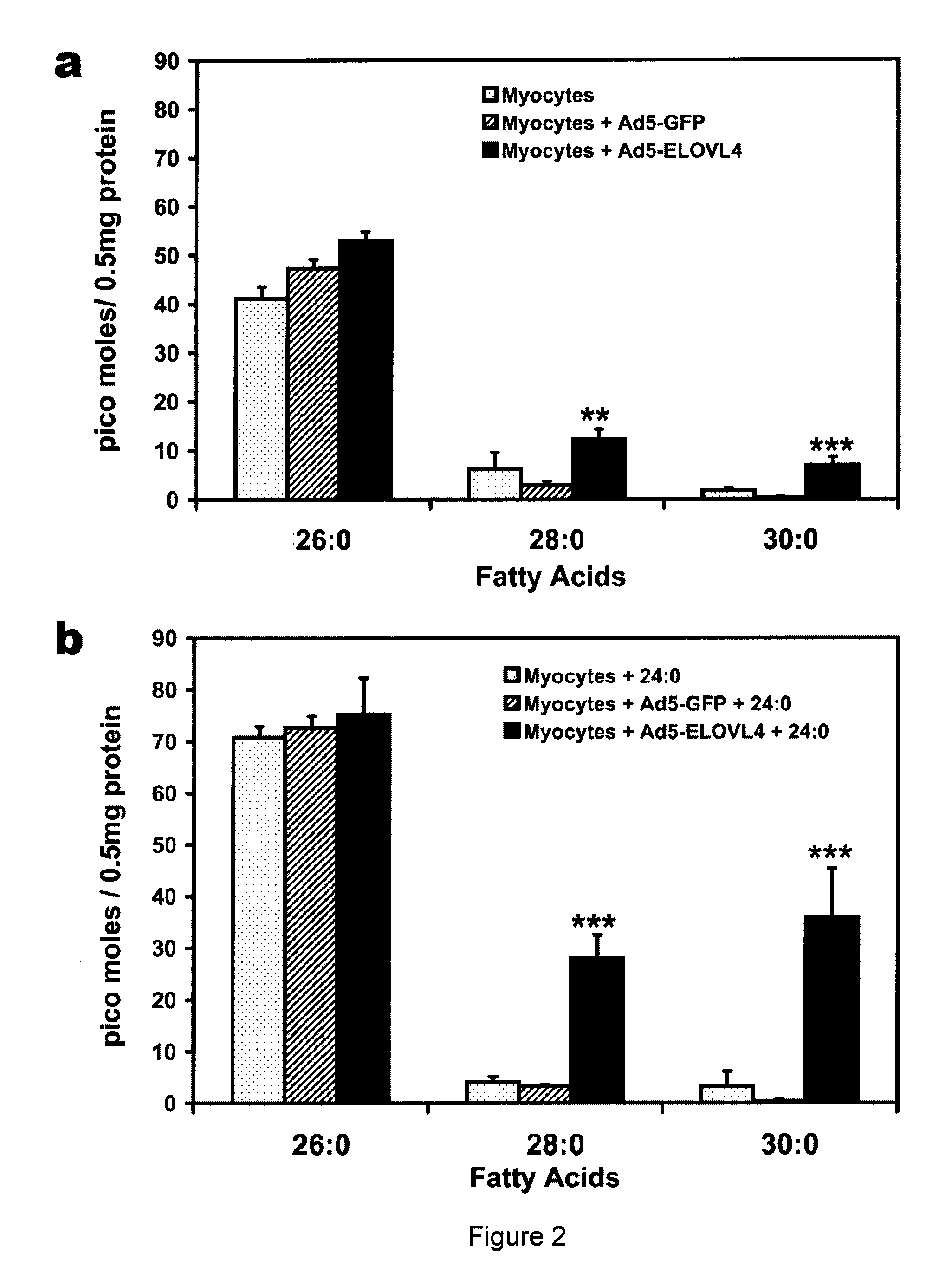
Very long chain polyunsaturated fatty acids, methods of production, and uses
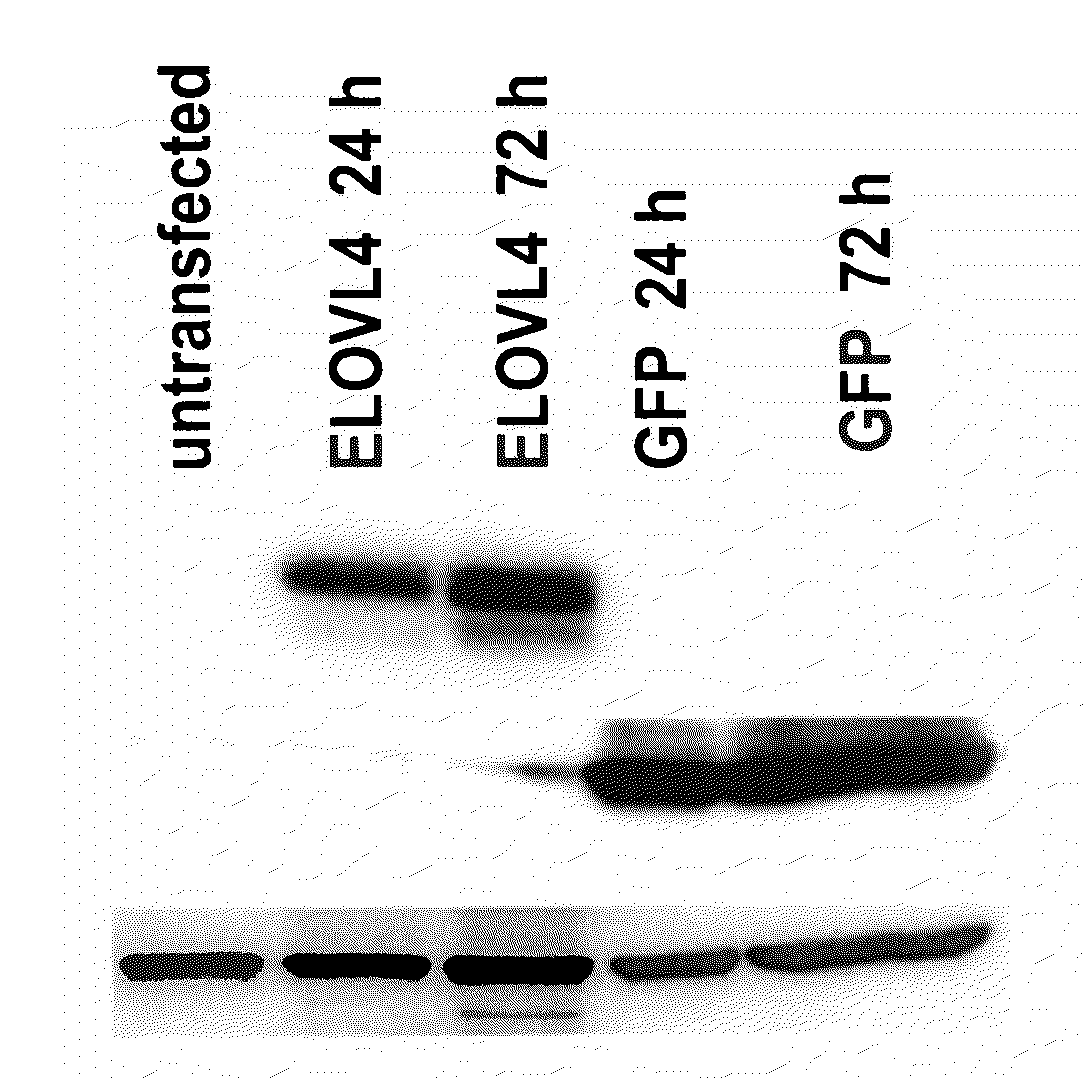
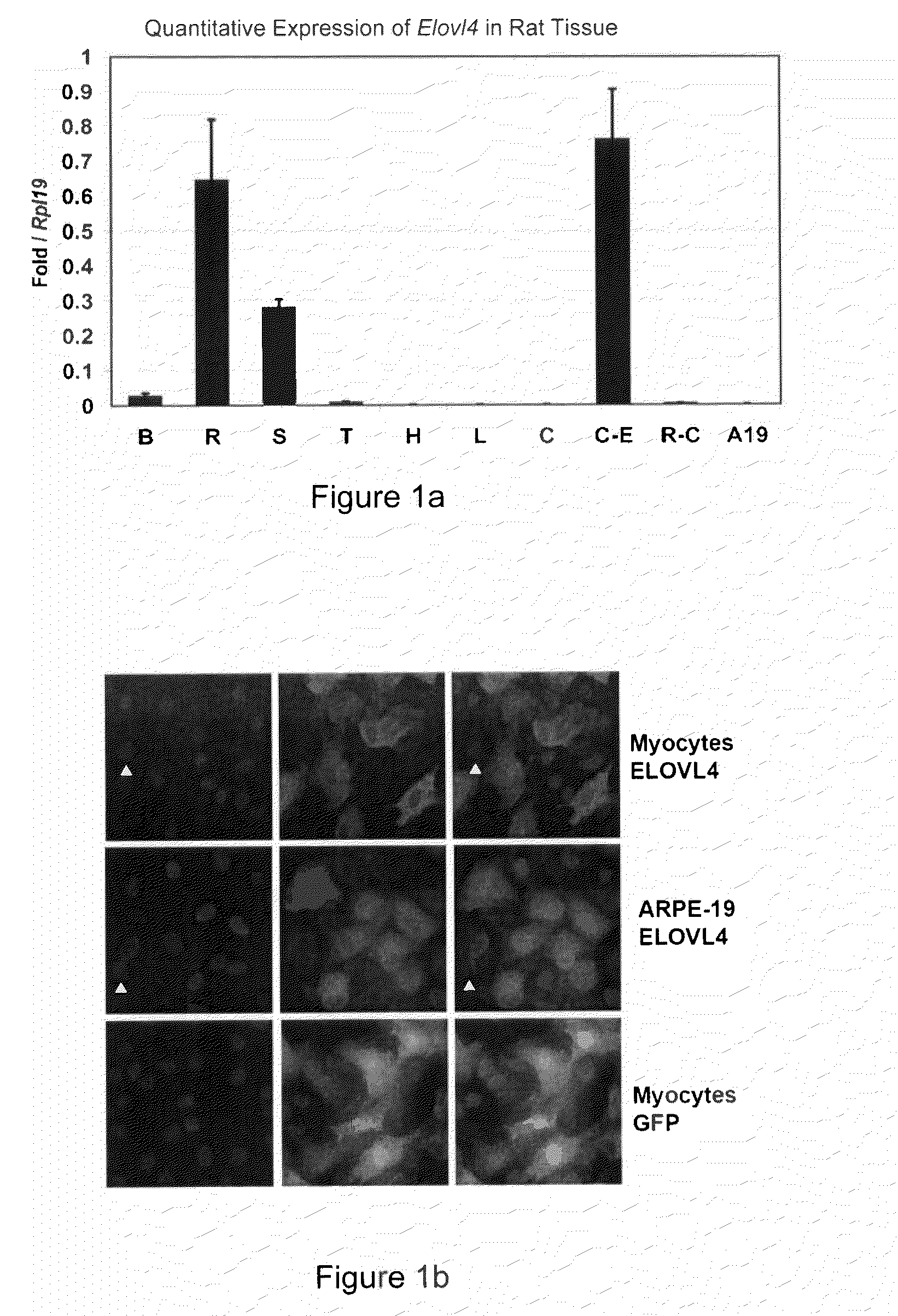
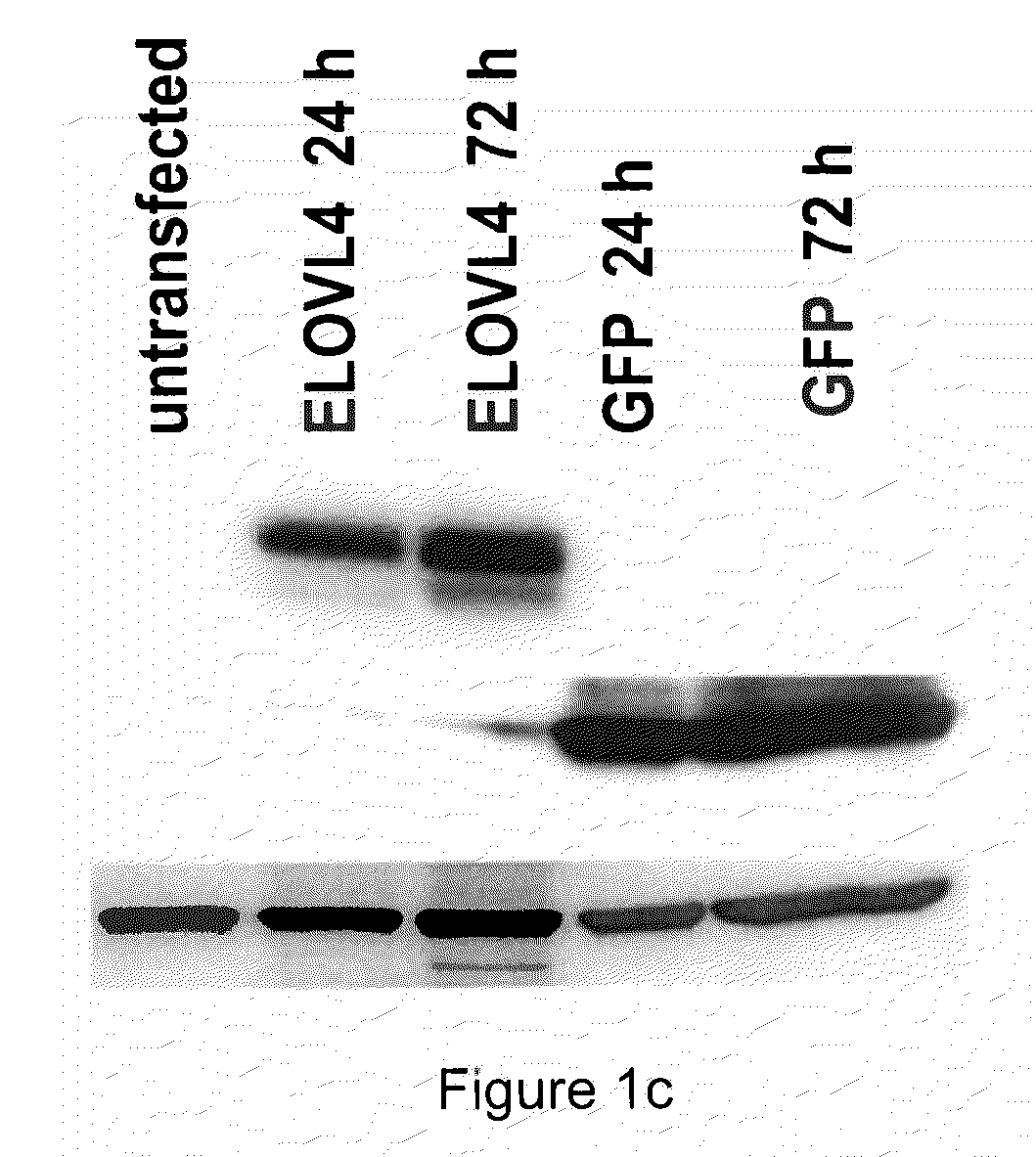
The present invention relates to processes for production of Very Long Chain Polyunsaturated Fatty Acids (VLC-PUFAs). The present invention also relates to compositions (e.g., nutritional supplements and food products) containing such VLC-PUFAs. In one embodiment, the present invention is directed to methods for biosynthesis and production of the VLC-PUFAs described herein (particularly C28-C38 PUFAs, also referred to herein as supraenes or supraenoics) by the expression, in a production host cell, of the full or partial sequence(s) of Elovl4 DNA / mRNA nucleic acids or ELOVL4 protein sequences encoded thereby, from any species (prokaryotic or eukaryotic) for use in the biosynthesis, production, purification and utilization of VLC-PUFAs in particular by the elongation of C18-C26 saturated fatty acids and PUFAs. The composition of the invention comprises, in various embodiments, a dietary supplement, a food product, a pharmaceutical formulation, a humanized animal milk, an infant formula, a cosmetic item and a biodiesel fuel for example. A pharmaceutical formulation can include, but is not limited to: a drug for treatment of neurodegenerative disease, a retinal disorder, age related maculopathy, a fertility disorder, particularly regarding sperm or testes, or a skin disorder.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Prophylactic or therapeutic agent for retinal disease and method for prophylaxis or therapy of retinal disease using jnk (c-jun amino-terminal kinase) - inhibitory peptide, and use of the peptide
Intravitreal administration of a JNK-inhibitory peptide less than 150 amino acids in length, containing at least one D-amino acid, and having (a) a JNK-inhibitory sequence of at least any of SEQ ID NO: 1 and SEQ ID NO: 2, and (b) a transport sequence of at least any of SEQ ID NO: 3 and SEQ ID NO: 4 suppressed spermidine-induced retinal pigment epithelial damage, tunicamycin-induced photoreceptor cell damage, and laser-induced choroidal neovascularization. Thus, the JNK-inhibitory peptide of the present invention is useful for prophylaxis or therapy of a retinal disease. By the use of this JNK-inhibitory peptide, a drug and a method are provided which are capable of preventing or treating a retinal disease even by topical administration to the eye, and use of the JKN-inhibitory peptide for manufacturing the drug is also provided.
Owner:SANTEN PHARMA CO LTD
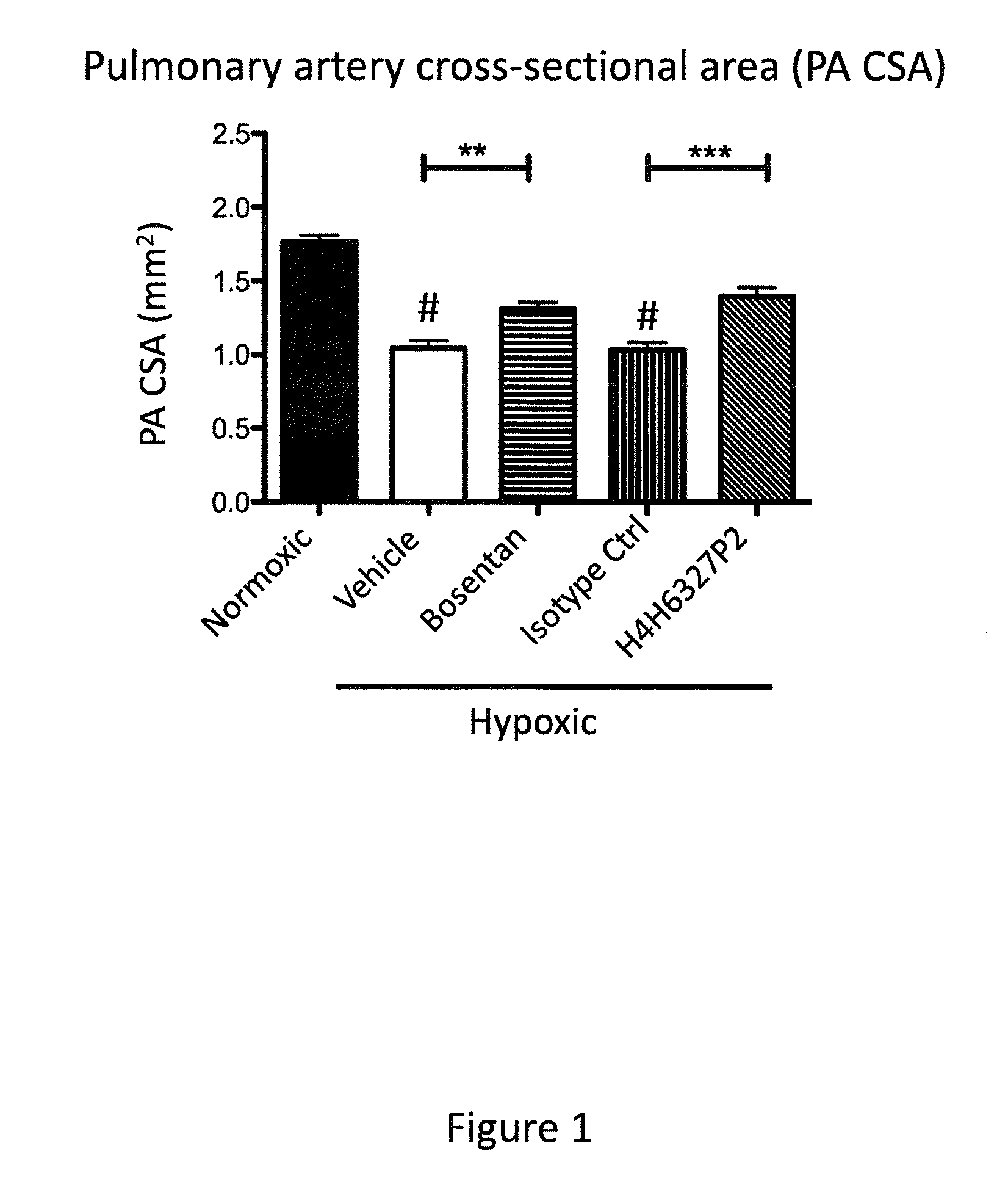
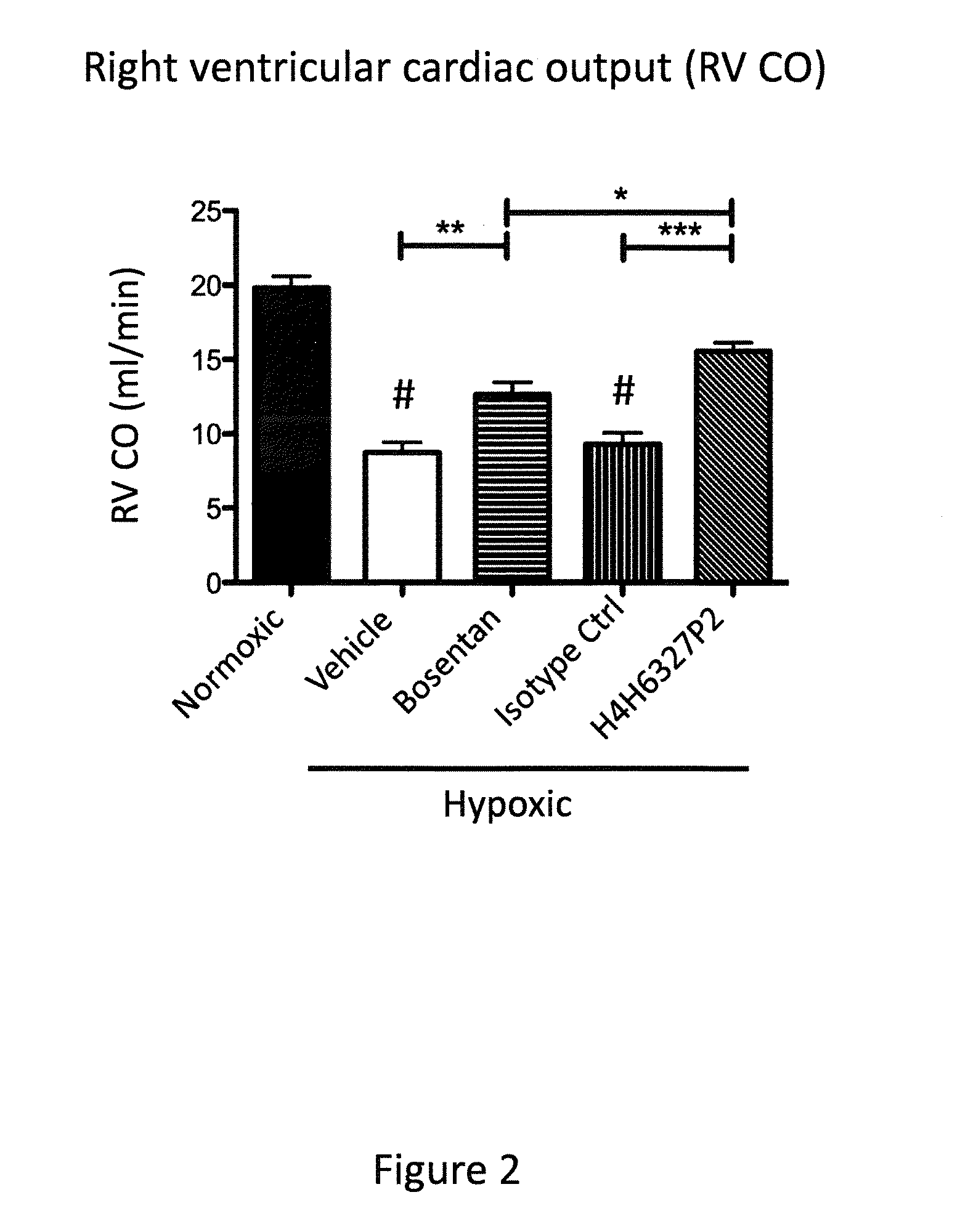
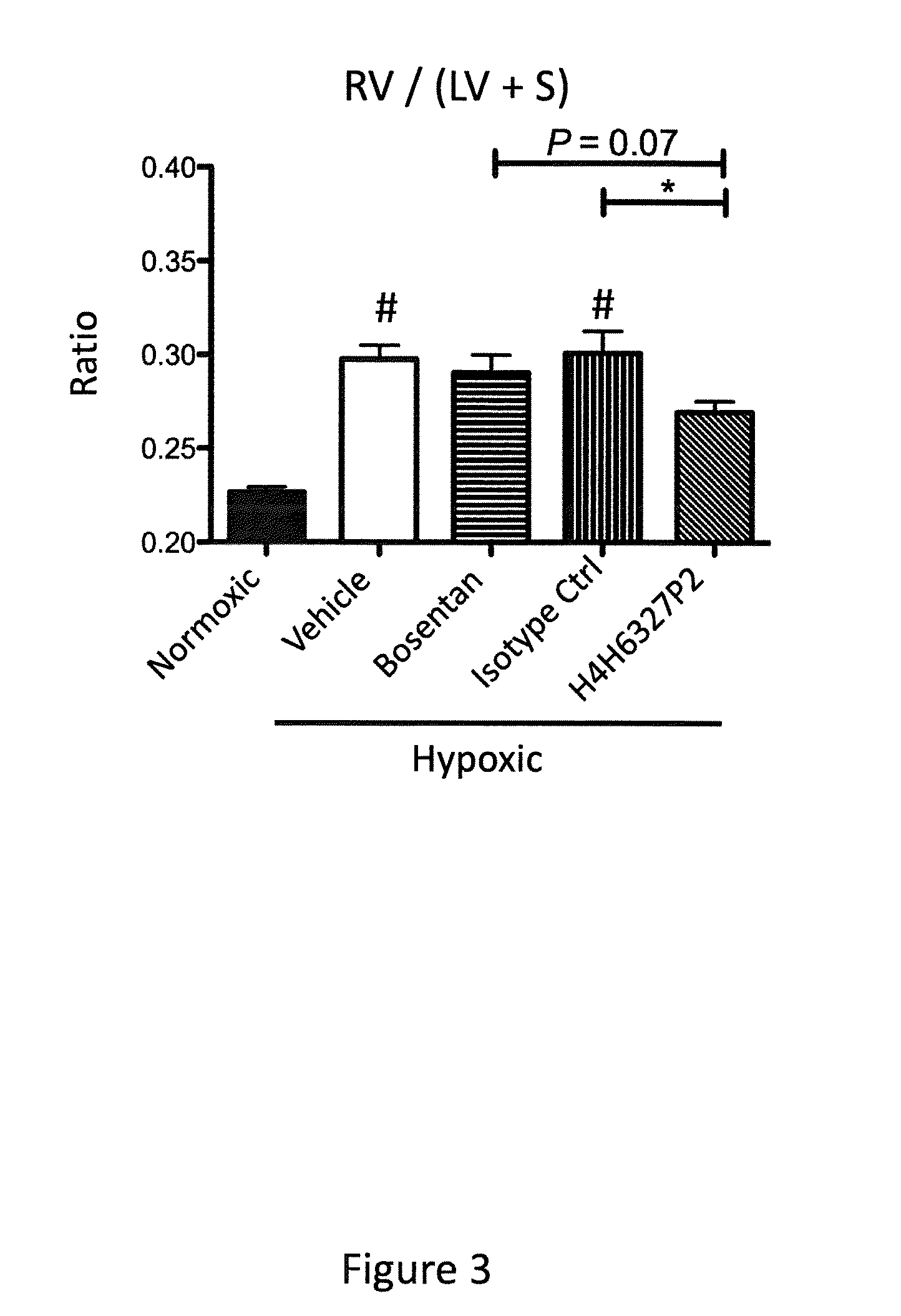
Anti-big-endothelin-1 (big-et-1) antibodies and uses thereof
The present invention provides antibodies that bind big-endothelin-1 (“big-ET-1”), and methods of using same. According to certain embodiments of the invention, the antibodies specifically bind human big-ET-1 but do not bind human small-ET-1 (i.e., the active form of endothelin-1 that results from proteolytic cleavage of big-ET-1 by endothelin-converting enzyme-1 [ECE-1]). According to certain embodiments of the invention, the anti-big-ET-1 antibodies are capable of blocking cleavage of big-ET-1 by ECE-1. The antibodies of the invention are useful for the treatment of big-ET-1-related disorders, including hypertension disorders, fibrotic disorders, neurodegenerative disorders, retinal disorders, pain and cancers.
Owner:REGENERON PHARM INC
Vascular endothelial growth factor-2
InactiveUS20050181979A1Inhibit and prevent growthSenses disorderVirusesVascular endothelial growth factor CRetinal Disorder
The present invention is directed to VEGF-2 polynucleotides and polypeptides and methods of using such polynucleotides and polypeptides. In particular, provided are methods of treating retinal disorders with VEGF-2 polynucleotides and polypeptides.
Owner:HUMAN GENOME SCI INC
Intra-Ocular Implant
InactiveUS20090048671A1FocusReduce the differenceIntraocular lensOptical partsDiseaseRetinal Disorder
An intra ocular implant consisting of optical elements, adapted to forming images on the retina, especially useful in treating presbyopic patients, patients with AMD, patients with other diseases of the retina, and patients in which future development of retinal disorders may occur. An intra ocular implant wherein at least one mirror is operationally connected to the action of the ciliary muscle or its effect on the zonules and capsule of the natural lens; and wherein the mirror is adapted to change curvature, position or optical properties, such that bringing a plurality of objects at different distances into focus is facilitated in presbyopic patients. A lens assembly comprising an intra ocular lens and, an adjustable external or contact lens comprising at least in part of light polarizing material; the assembly at least partially polarizes at least part of the central visual field, at least part of the peripheral visual field or both in any angle.
Owner:LIPSHITZ ISAAC +1
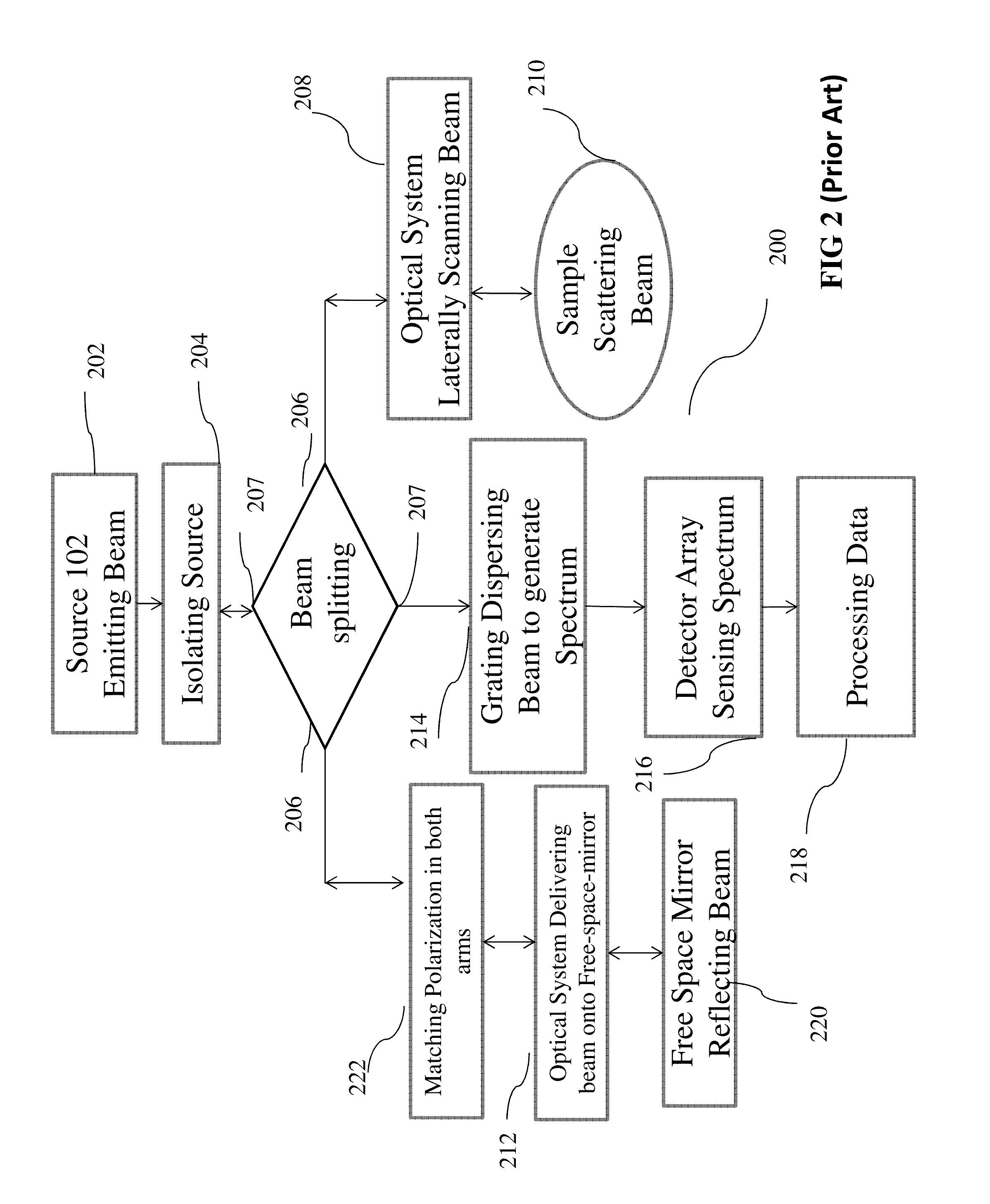
Method and System for Low Coherence Interferometry
ActiveUS20160178346A1Overcome the drawbacksInterferometersScattering properties measurementsDiseaseGlaucoma
Optical Coherence Tomography (OCT) system and apparatus of this instant application is very useful for diagnosis and management of ophthalmic diseases such as retinal diseases and glaucoma etc. Instant innovative OCT diagnostic system leverages advancements in cross technological platforms. The Michelson interferometric system presented in this application could be used for the OCT imaging, which includes biological OCT imaging, medical OCT imaging, ophthalmic OCT imaging, corneal OCT imaging, retinal OCT imaging, and the like. A tunable filter is placed in front of the detector to make the interferometer more sensitive and accurate for examining various samples for diagnosis.
Owner:NETRA SYST
Compositions of very long chain polyunsaturated fatty acids and methods of use
The present invention relates to processes for production of Very Long Chain Polyunsaturated Fatty Acids (VLC-PUFAs). The present invention also relates to compositions (e.g., nutritional supplements, food products, and pharmaceutic compositions) containing such VLC-PUFAs. In one embodiment, the present invention is directed to methods for biosynthesis and production of the VLC-PUFAs described herein (particularly C28-C40 PUFAs, also referred to herein as supraenes or supraenoics) by the expression, in a production host cell, of the full or partial sequence(s) of Elovl4 DNA / mRNA nucleic acids or ELOVL4 protein sequences encoded thereby, from any species (prokaryotic or eukaryotic) for use in the biosynthesis, production, purification and utilization of VLC-PUFAs in particular by the elongation of C18-C26 saturated fatty acids and PUFAs. The composition of the invention comprises, in various embodiments, a dietary supplement, a food product, a nutritional formulation, a pharmaceutical formulation, a humanized animal milk, an infant formula, and a cosmetic item and for example. A pharmaceutical formulation can include, but is not limited to: a composition for enhancing neural development and function, a drug for treatment of neurodegenerative disease, an ocular disorder, a retinal disorder, age related maculopathy, a fertility disorder, particularly regarding sperm or testes, or a skin disorder.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Methods and compositions for treating and inhibiting retinal neovascularization
Methods and compositions for the prophylactic and therapeutic treatment of retinal disorders associated with neovascularization using topical ophthalmic compositions comprising hydroxamic acid matrix metalloproteinase inhibitors such as batimastat.
Owner:SUN PHARMA GLOBAL FZE
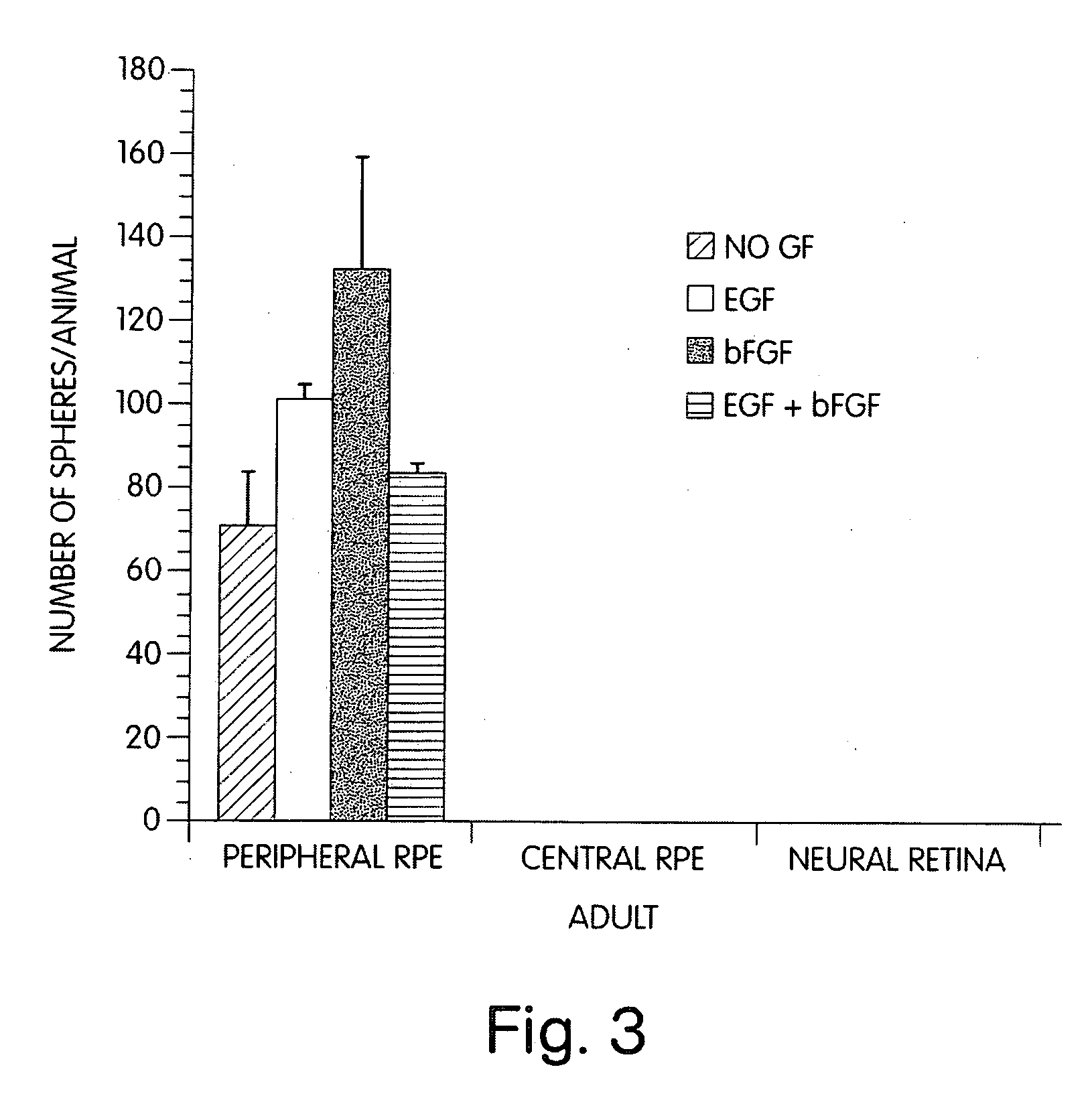
Pharmaceuticals containing retinal stem cells
InactiveUS20050031599A1Effectively lead to differentiationBiocidePeptide/protein ingredientsMammalIn vivo
The invention relates to stem cells isolated from the retina of mammals and retinal cells differentiated from these stem cells. The invention also relates to a method of isolating retinal stem cells and inducing retinal stem cells to produce retinal cells. Retinal stem cells may also be induced in vivo to produce retinal cells. The invention also includes pharmaceuticals made with retinal stem cells or retinal cells which may be used to restore vision lost due to diseases, disorders or abnormal physical states of the retina. The invention includes retinal stem cell and retinal cell culture systems for toxicological assays, for isolating genes involved in retinal differentiation or for developing tumour cell lines.
Owner:KOOY DEREK VAN DER +3
Method of treating edematous retinal disorders
The present invention provides a method of treating edematous retinal disorders. The method comprises administration of a pharmaceutical formulation comprising a hydrolysis-resistant P2Y receptor agonist to stimulate the removal of pathological extraneous fluid from the subretinal and retinal spaces and thereby reduce the accumulation of said fluid associated with retinal detachment and retinal edema. The P2Y receptor agonist can be administered with therapeutic and adjuvant agents commonly used to treat edematous retinal disorders. The pharmaceutical formulation useful in this invention comprises a P2Y receptor agonist with enhanced resistance to extracellular hydrolysis, such as dinucleoside polyphosphate compounds, or hydrolysis-resistant mononucleoside triphosphates.
Owner:MERCK SHARP & DOHME LLC
Vision test for determining retinal disease progression
The present invention provides a reading test to measure vision loss. In one embodiment, the vision loss is due to disease progression. The tests are useful in evaluating the effects of intervention in vision deterioration. The tests are non-invasive, simple, quick, sensitive, reproducible, and easy to administer. The tests measure the subject's reading speed and accuracy under defined conditions of illumination and contrast. The results of these tests may be used to determine if treatment of a disease should be initiated, terminated, altered, or remain unchanged.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Ophthalmic NSAIDS as Adjuvants
InactiveUS20110054031A1Reduce riskMaximize visual acuityOrganic active ingredientsBiocideVeinAdjuvant
The disclosure provides methods and ophthalmic NSAIDs as adjuvants to VEGF inhibitors useful for treating retinal disorders, including but not limited to wet AMD, diabetic retinopathy, diabetic macular edema, central retinal vein occlusion, and branch retinal vein occlusion.
Owner:BAUSCH & LOMB PHARMA HLDG +1
Circular preferential hyperacuity perimetry video game to monitor macular and retinal diseases
Systems and methods for providing a video game to map macular visual acuity comprising a test where a fixation point is ensured by brief simultaneous presentation of central and pericentral targets. The game may be implemented on a hardware platform including a video display, a user input device, and a video camera. The camera is used to monitor ambient light level and the distance between the device and the eyes of the test subject. The game serves as a macular acuity perimeter that produces a map of the acuity of an eye that may be compared with normative data. The type of acuity tested is preferably Vernier acuity, but resolution acuity can also be tested. The test results are transmitted to a health care professional by telecommunications means to facilitate the diagnosis or monitoring of age-related macular degeneration or other relevant eye diseases.
Owner:GOBIQUITY INC
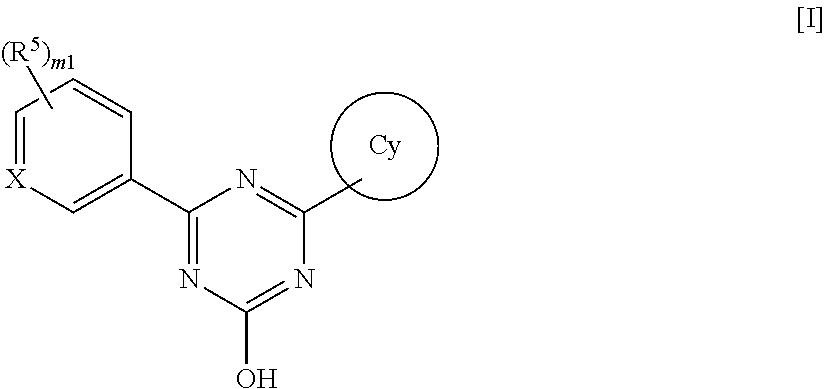
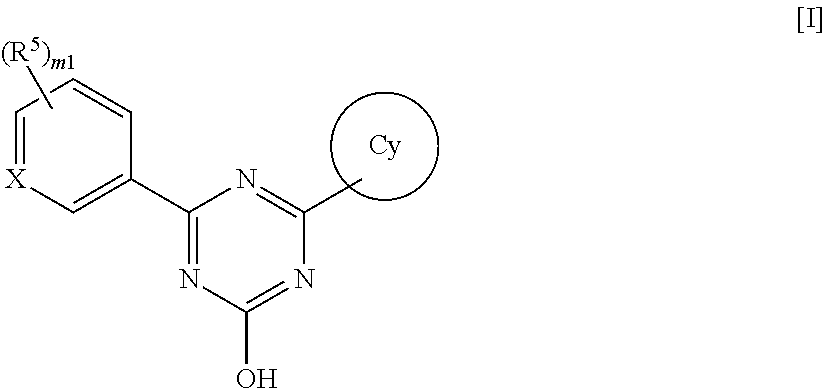
Triazine compounds and pharmaceutical use thereof
InactiveUS20150266834A1Suppression problemInhibition is effectiveSenses disorderNervous disorderIntra ocular pressureDiffuse scleroderma
Owner:JAPAN TOBACCO INC
Glycan binding proteins as therapeutic targets for retinal disorders and treatment methods based thereon
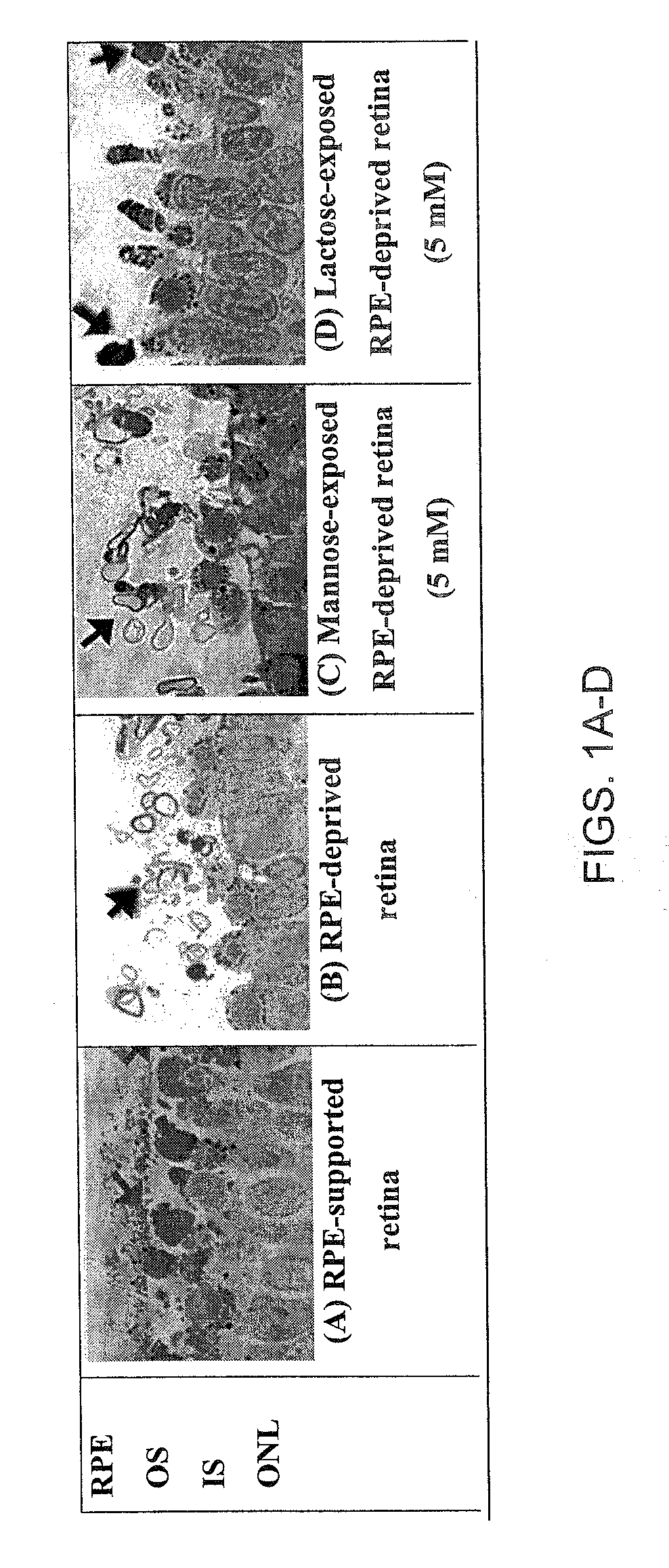
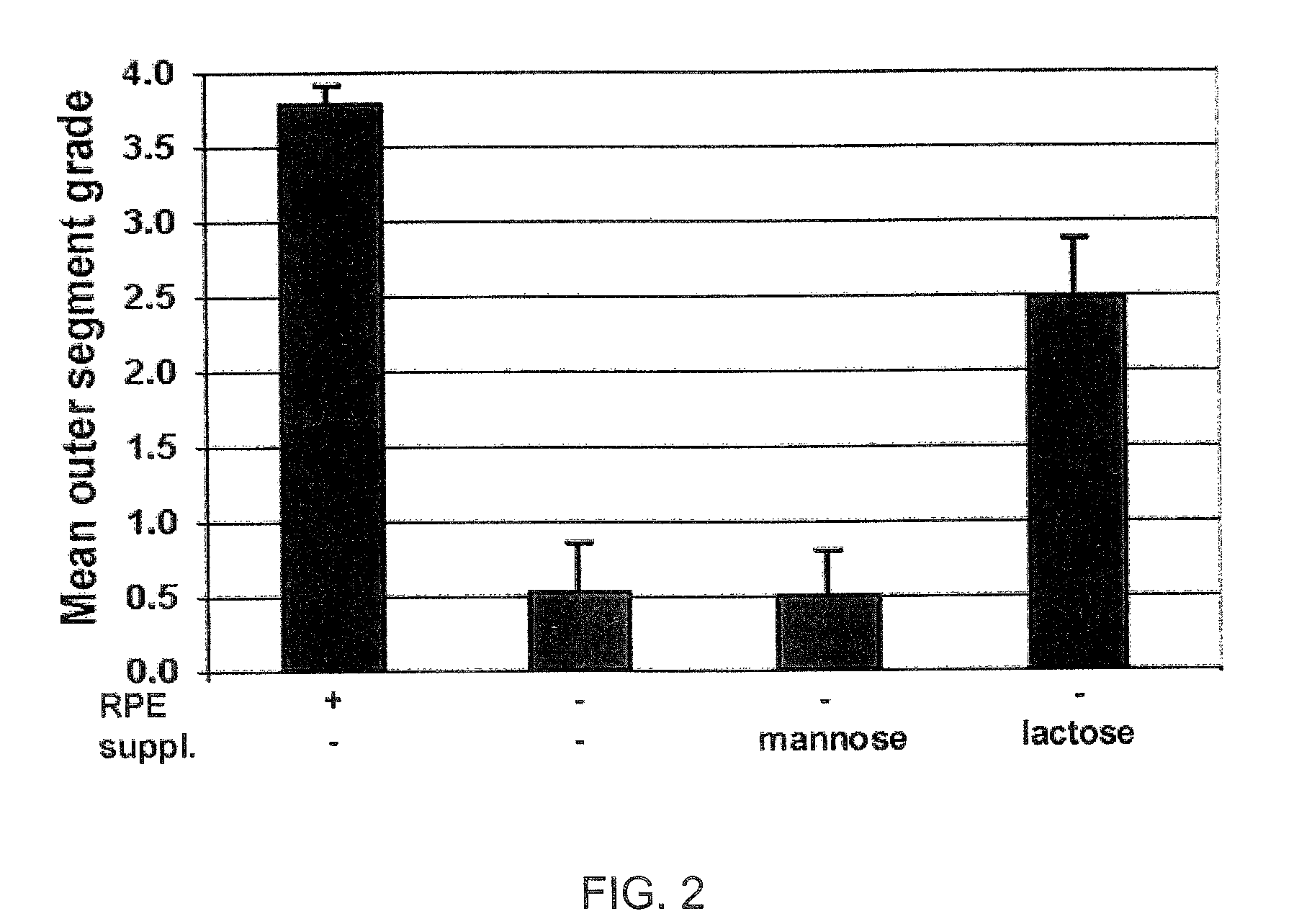
Disclosed are novel methods of treatment for retinal diseases and conditions including age-related macular degeneration, genetic-based retinal degenerations and retinal detachment. A novel glycan binding protein thought to be a cell surface receptor has been discovered in the retina. The retinal glycan binding receptor is shown to play an important role in promoting assembly of outer segment (OS) membranes by the photoreceptor cells of the eye, a process that is essential for vision. Based on the finding that certain sugars can bind with very high affinity to the retinal glycan receptor and stimulate its function, the invention provides novel therapeutic agents for treatment of retinal diseases that are multivalent N-linked glycans. Preferred pharmaceutical compositions in accordance with the present invention comprise active agents having the general formula: (Gal-GlcNAc)n-Man3-GlcNAc2, where n is 1-4. Particularly preferred multivalent glycans are galactosylated, biantennary (NA2), and asialo, galactosylated, triantennary (NA3) oligosaccharides.
Owner:UNIV OF TENNESSEE RES FOUND
Adeno-associated virus variant capsids and use for inhibiting angiogenesis
Provided herein are variant adeno-associated virus (AAV) capsid proteins having one or more modifications in amino acid sequence relative to a parental AAV capsid protein, which, when present in an AAV virion, confer increased infectivity of one or more types of retinal cells as compared to the infectivity of the retinal cells by an AAV virion comprising the unmodified parental AAV capsid protein.Also provided are recombinant AAV virions and pharmaceutical compositions thereof comprising a variant AAV capsid protein as described herein, methods of making these rAAV capsid proteins and virions, and methods for using these rAAV capsid proteins and virions in research and in clinical practice, for example in, e.g., the delivery of nucleic acid sequences to one or more cells of the retina forthe treatment of retinal disorders and diseases.
Owner:4D MOLECULAR THERAPEUTICS INC
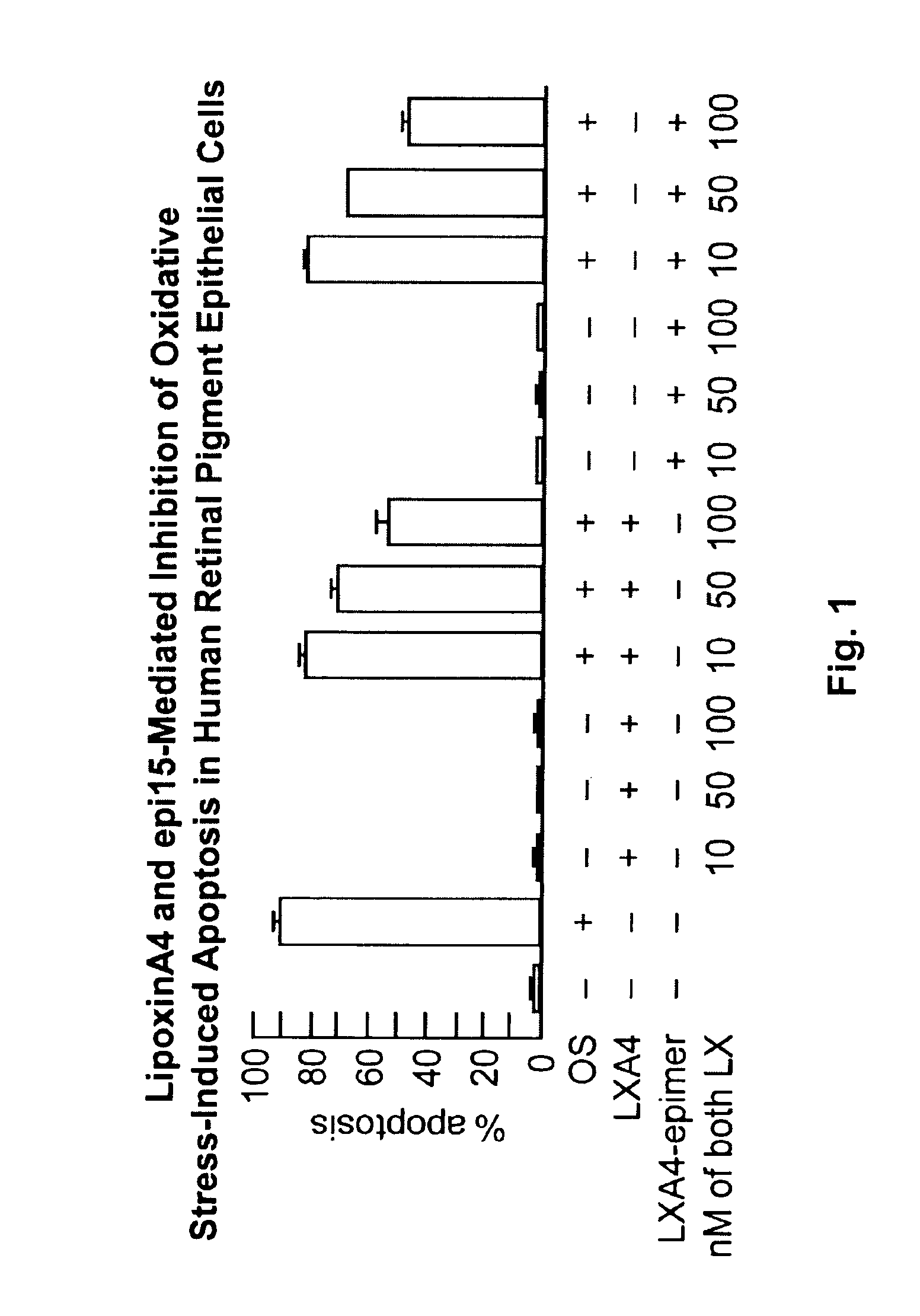
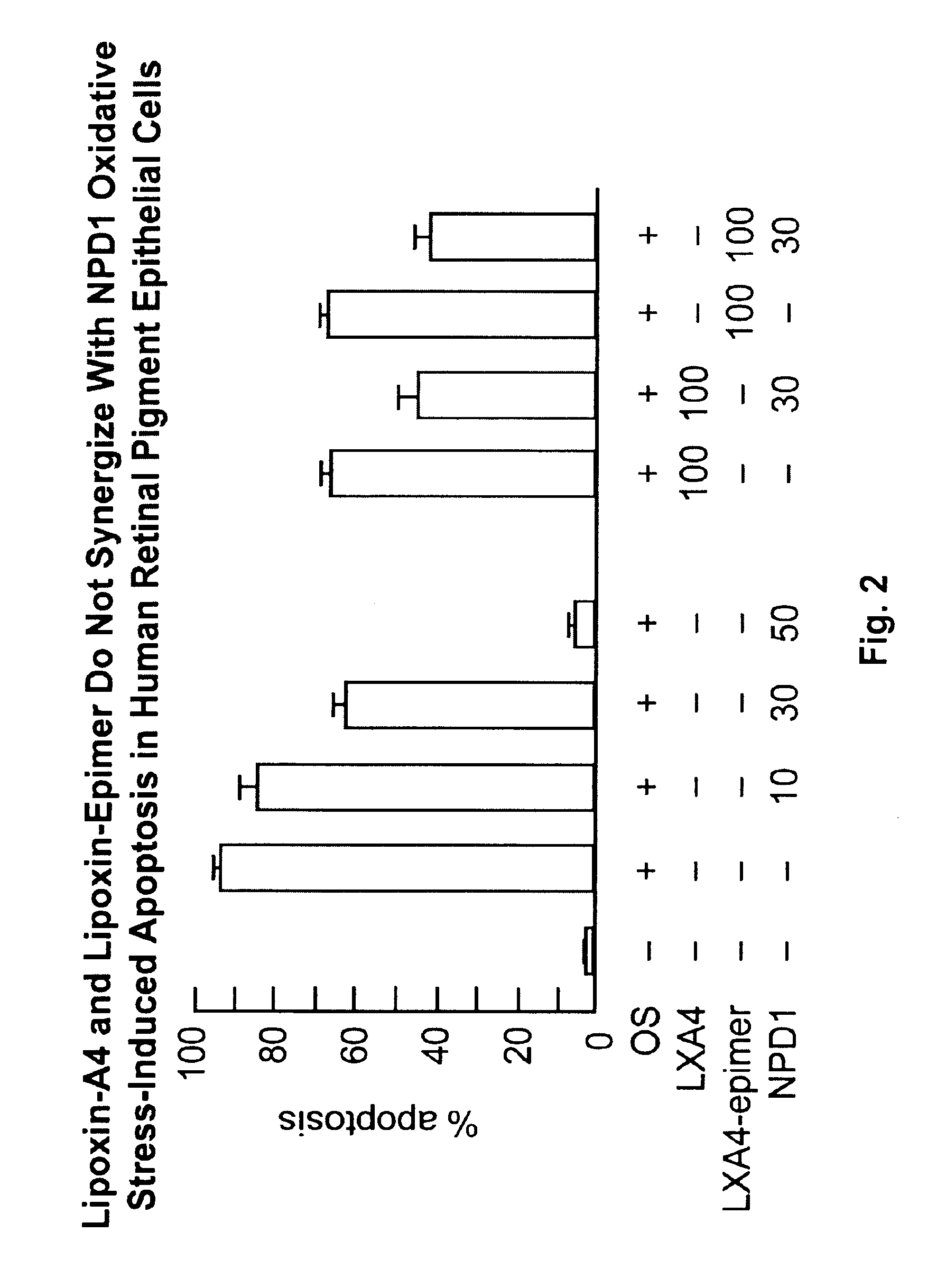
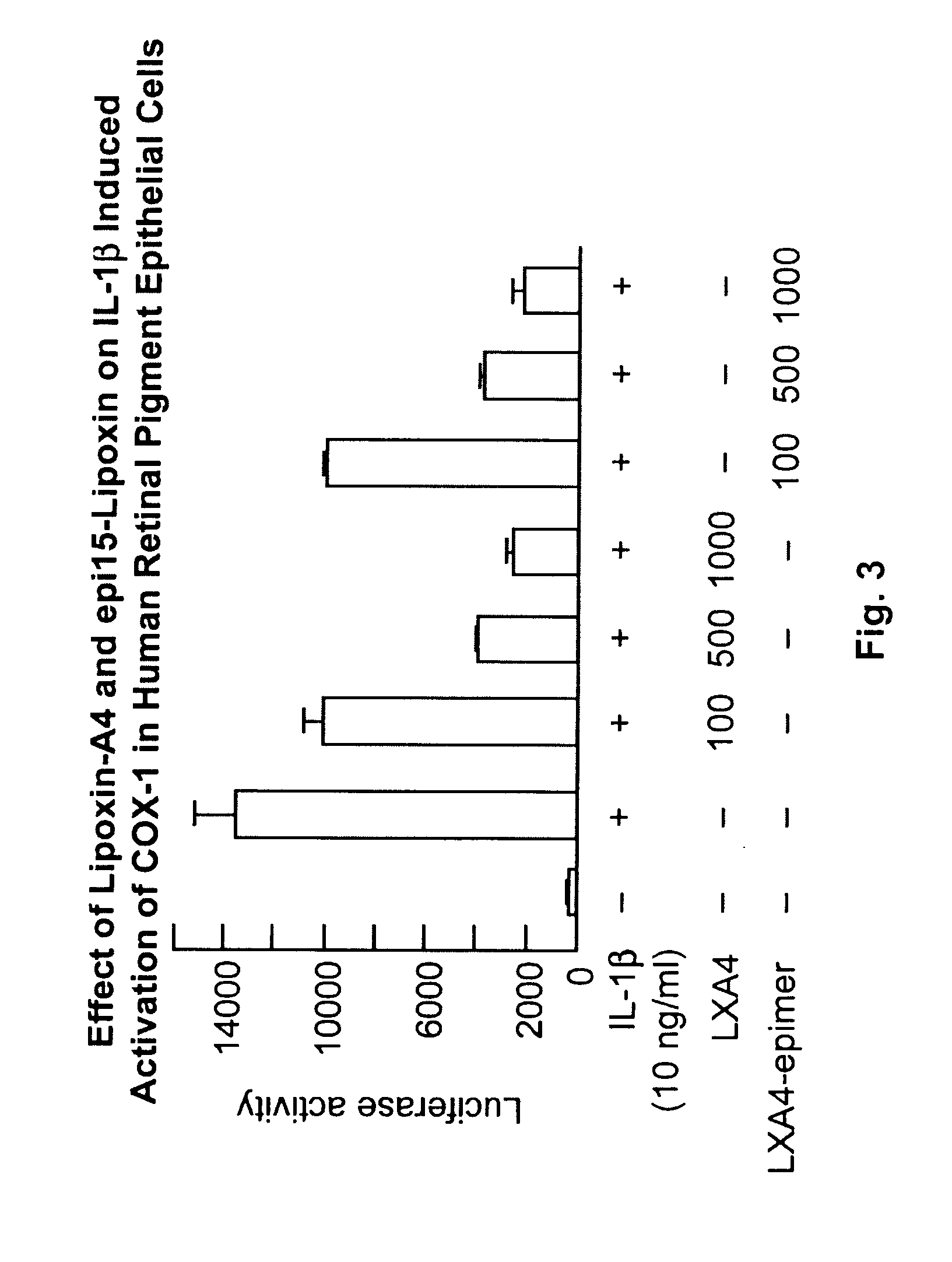
Lipoxin A4 Protection for Retinal Cells
InactiveUS20100324138A1Inhibit apoptosisBiocideSenses disorderNeuroprotective factorsOxidative Stress Induction
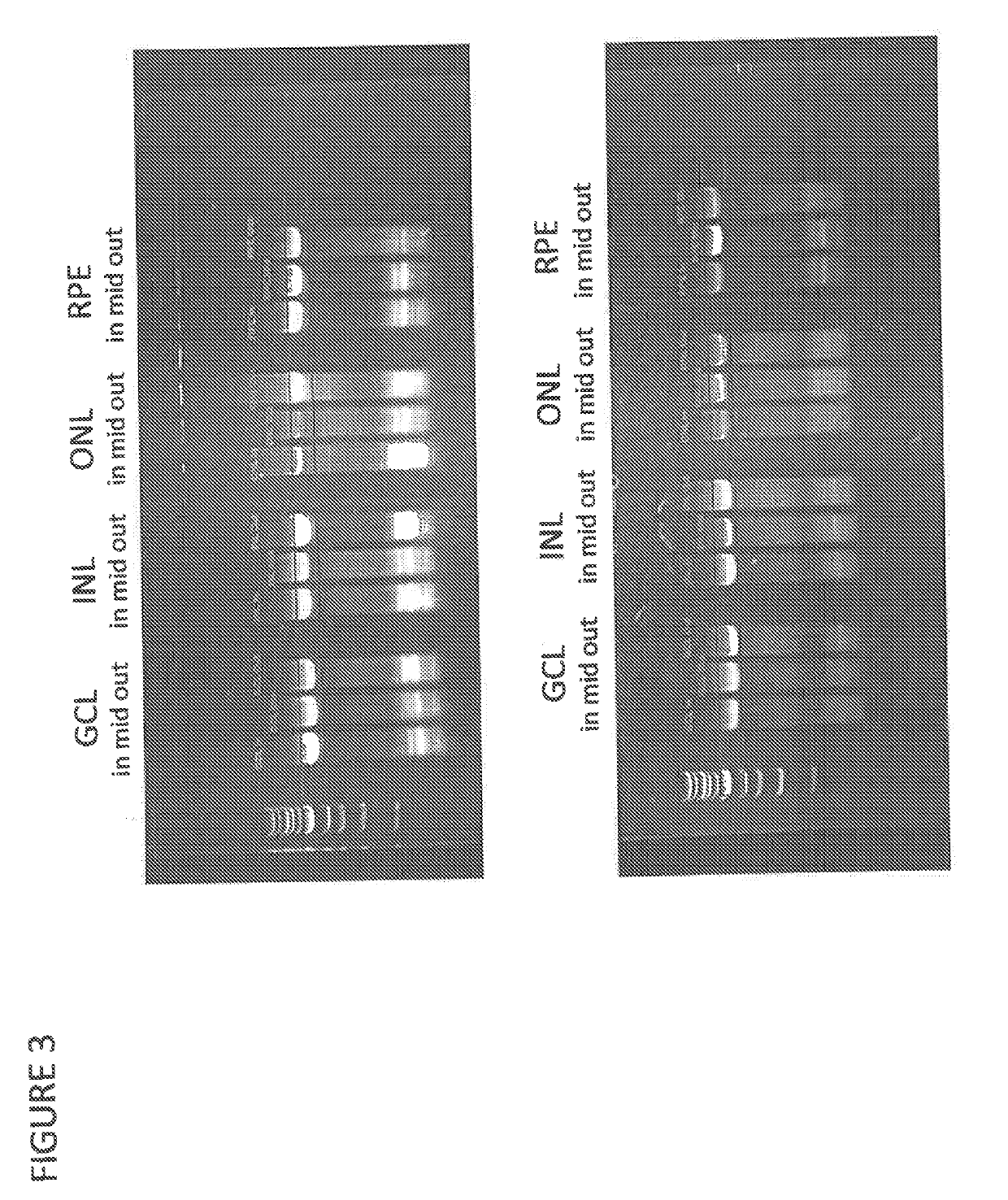
Lipoxin A4 and its analogs have been found to be effective in inhibiting apoptosis of retinal pigment epithelial cells induced by oxidative stress. Thus lipoxin A4 and its analogs, for example, lipoxin A4 epimer 15, can be used to prevent and treat retinal diseases due to the progressive degeneration of photoreceptors and retinal pigment epithelial cells (RPE cells), e.g., the dry form of age-related macula degeneration. They can also be combined with other compounds known to prevent apoptosis in retinal pigment epithelial cells, e.g., docosahexaenoic acid and neuroprotectin D1.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
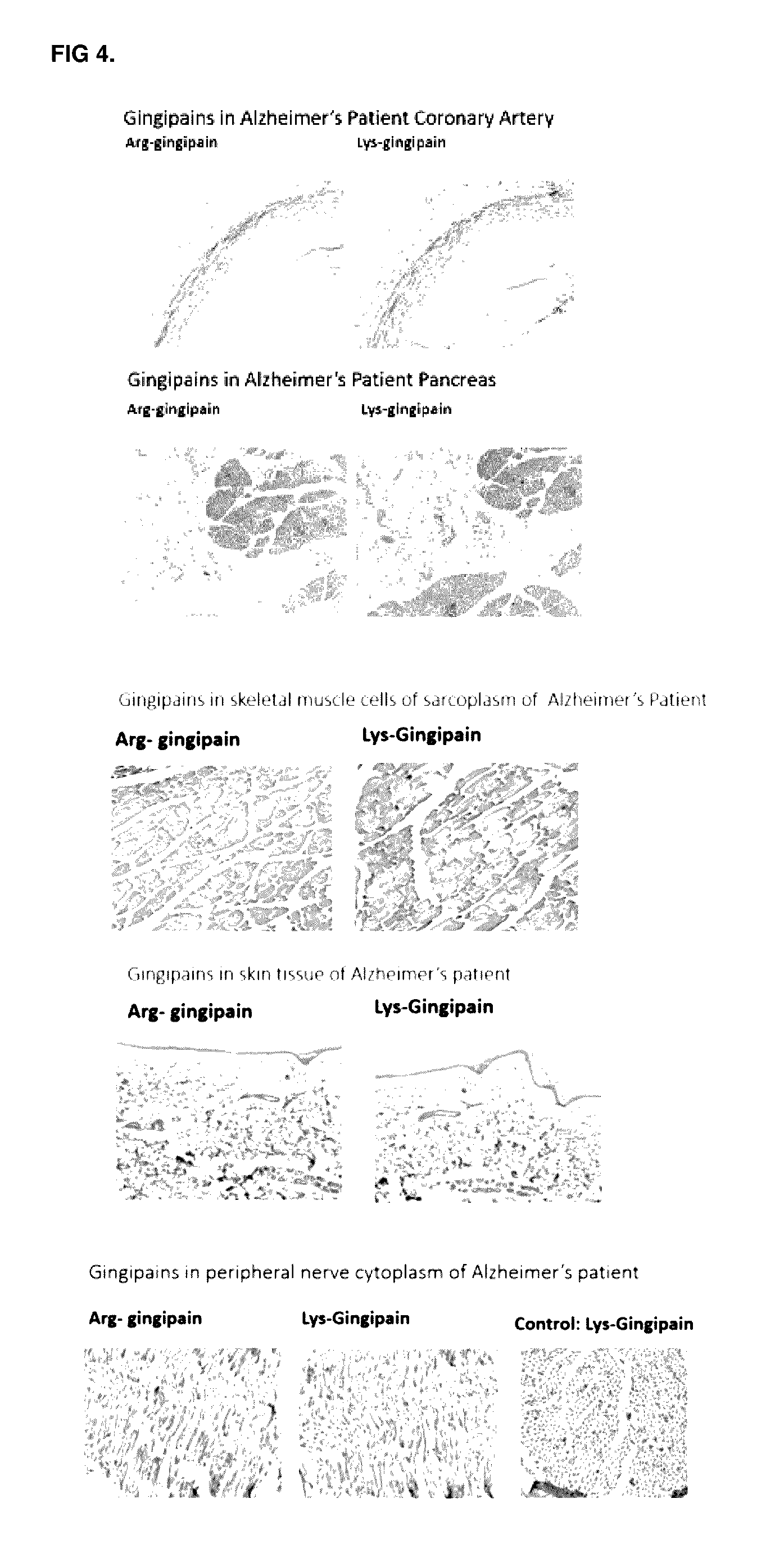
Methods of use for therapeutics targeting the pathogen porphyromonas gingivalis
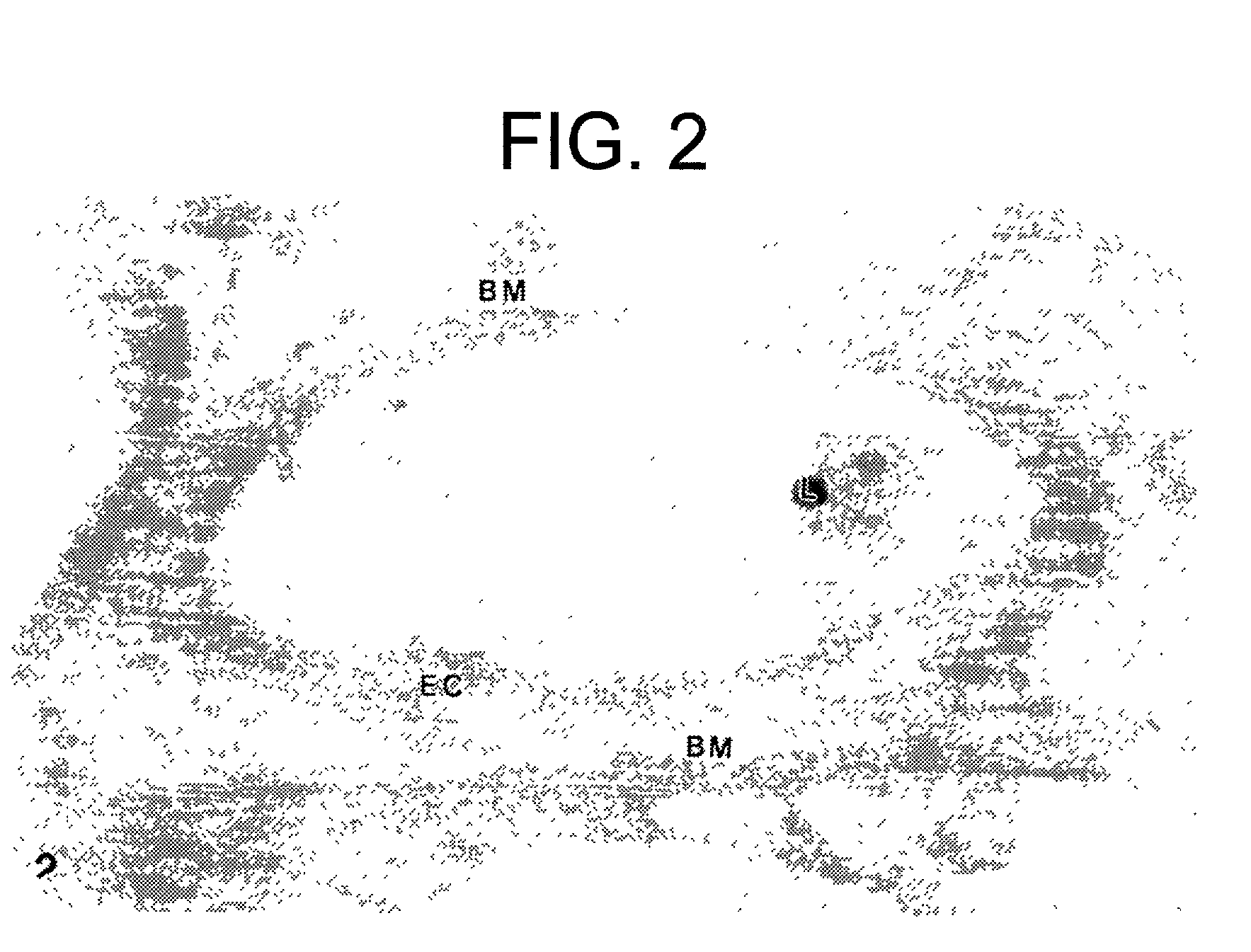
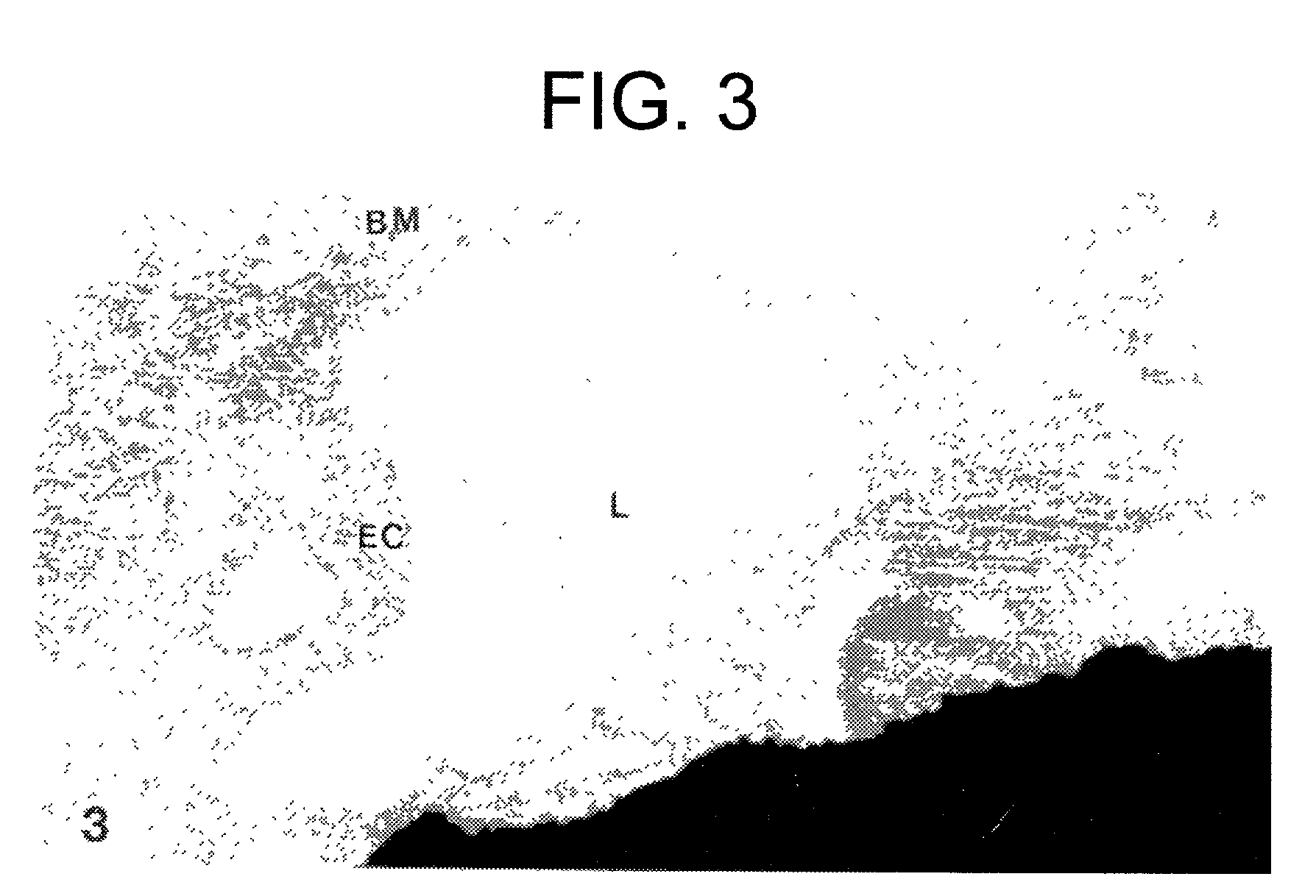
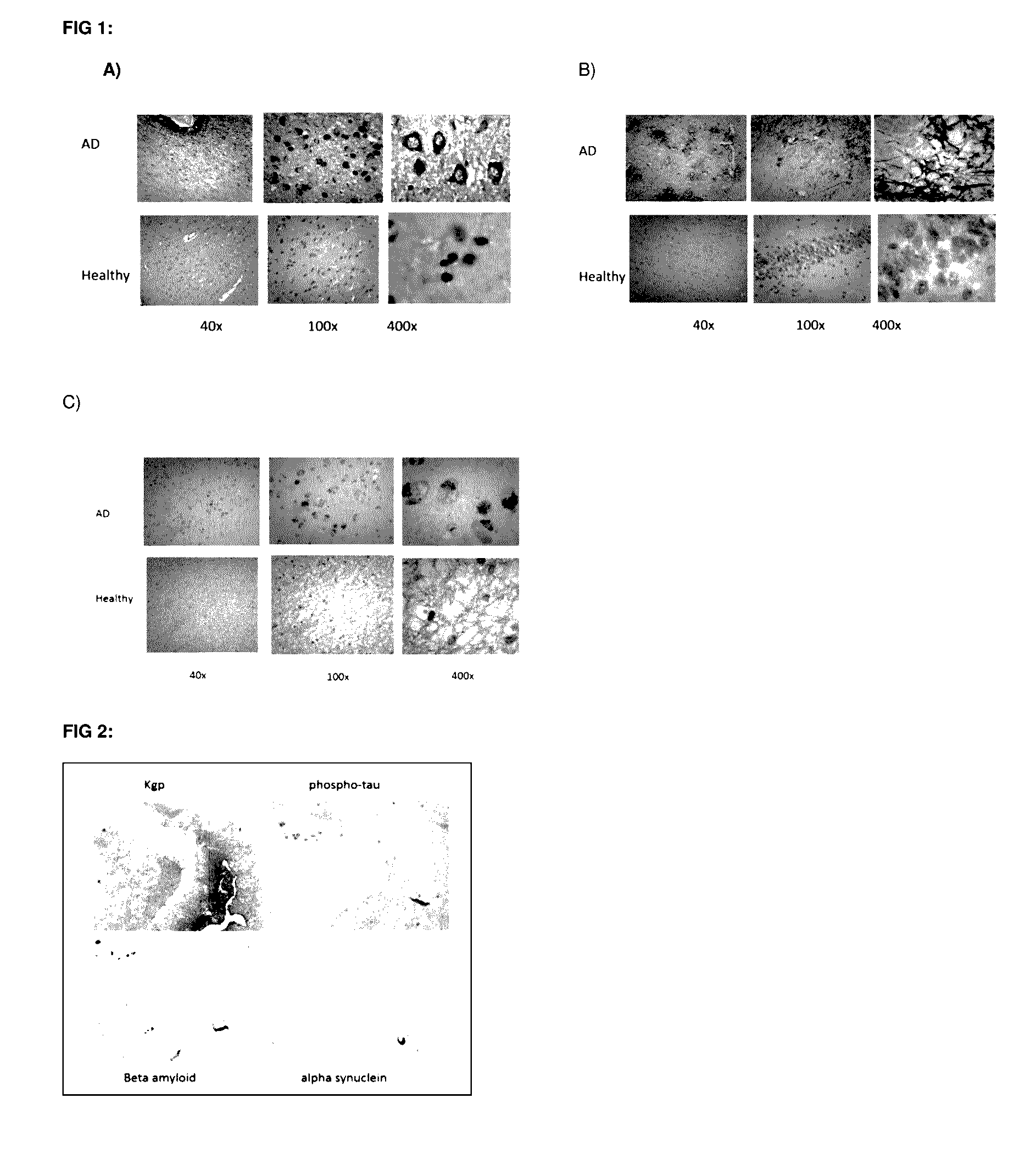
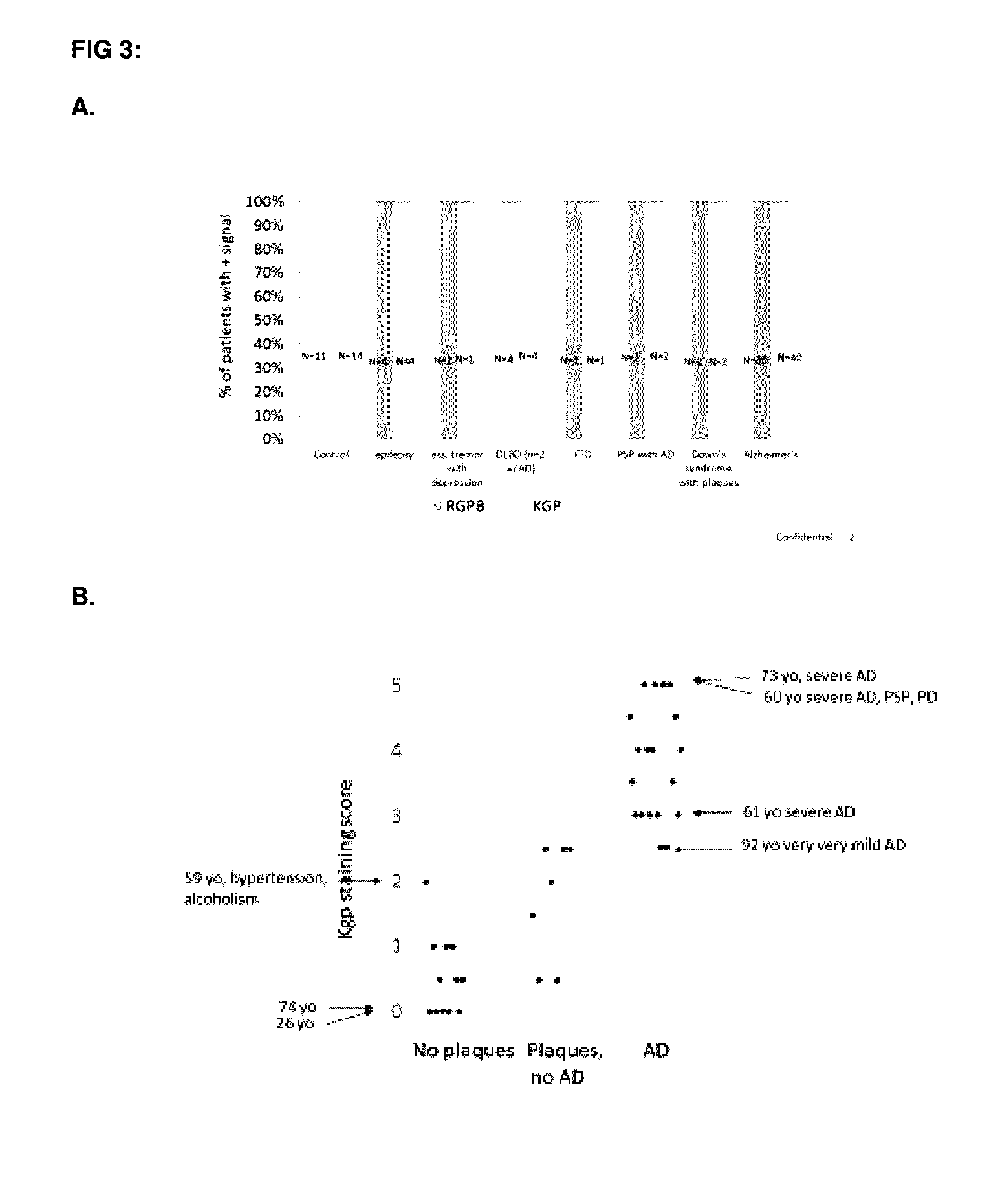
InactiveUS20170014468A1Reducing circulating activityEnhance phagocytosisDipeptide ingredientsAmide active ingredientsPorphyromonas gingivalisArthritis
The invention relates to compositions, formulations, vaccines and antibodies for the prevention and / or treatment of aging and brain disorders, including Alzheimer's disease, diabetes, cardiovascular disease, retinal disorders and arthritis. The invention also provides methods of treatment of aging disorders, brain disorders, including Alzheimer's disease, diabetes, cardiovascular disease, retinal disorders, and arthritis by administering compositions, formulations, vaccines and antibodies described in the specification. The invention further provides methods for diagnosing or assessing risk of development of brain disorders in humans and animals. The invention further provides animal models for testing novel therapeutics for brain disorders.
Owner:CORTEXYME INC
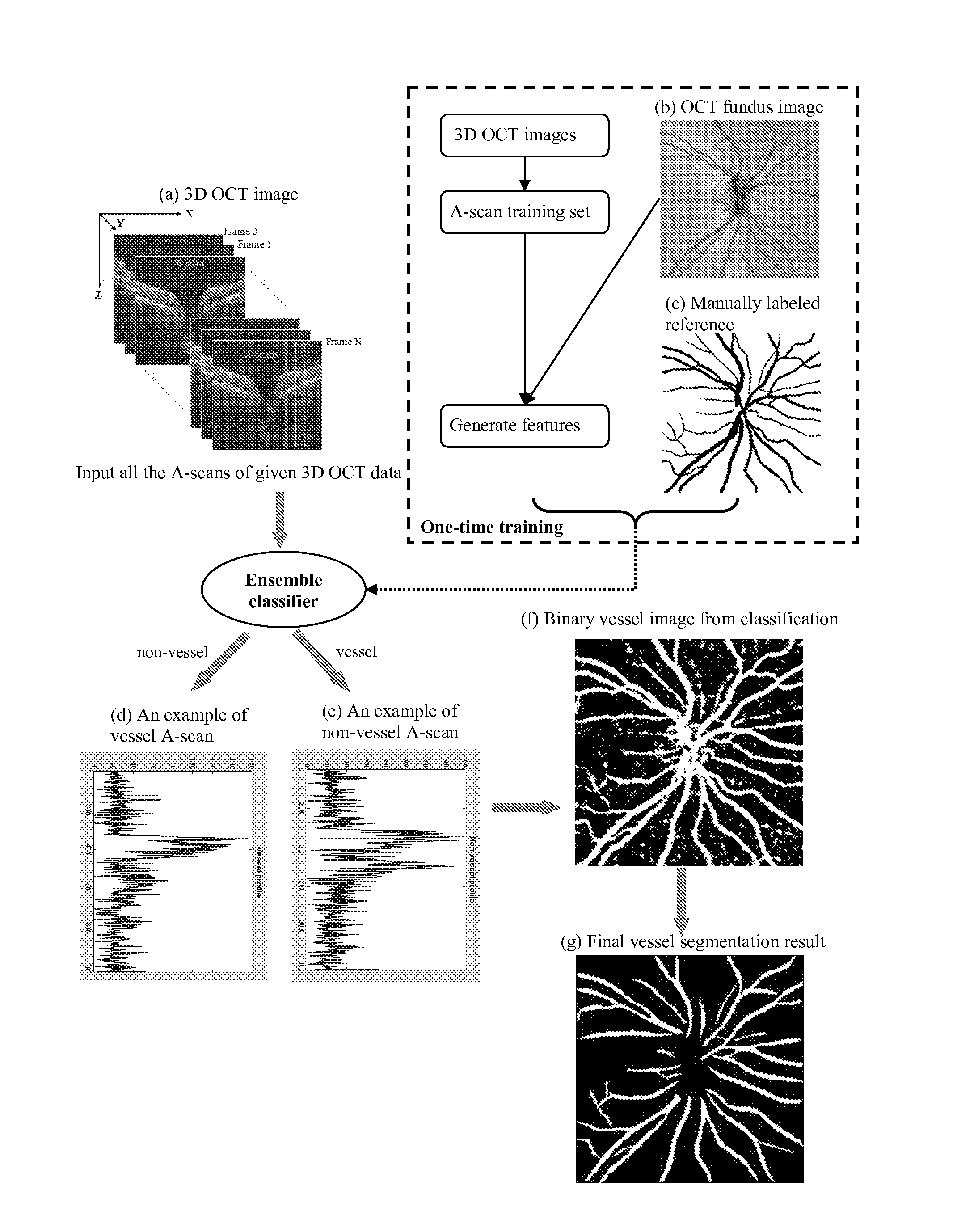
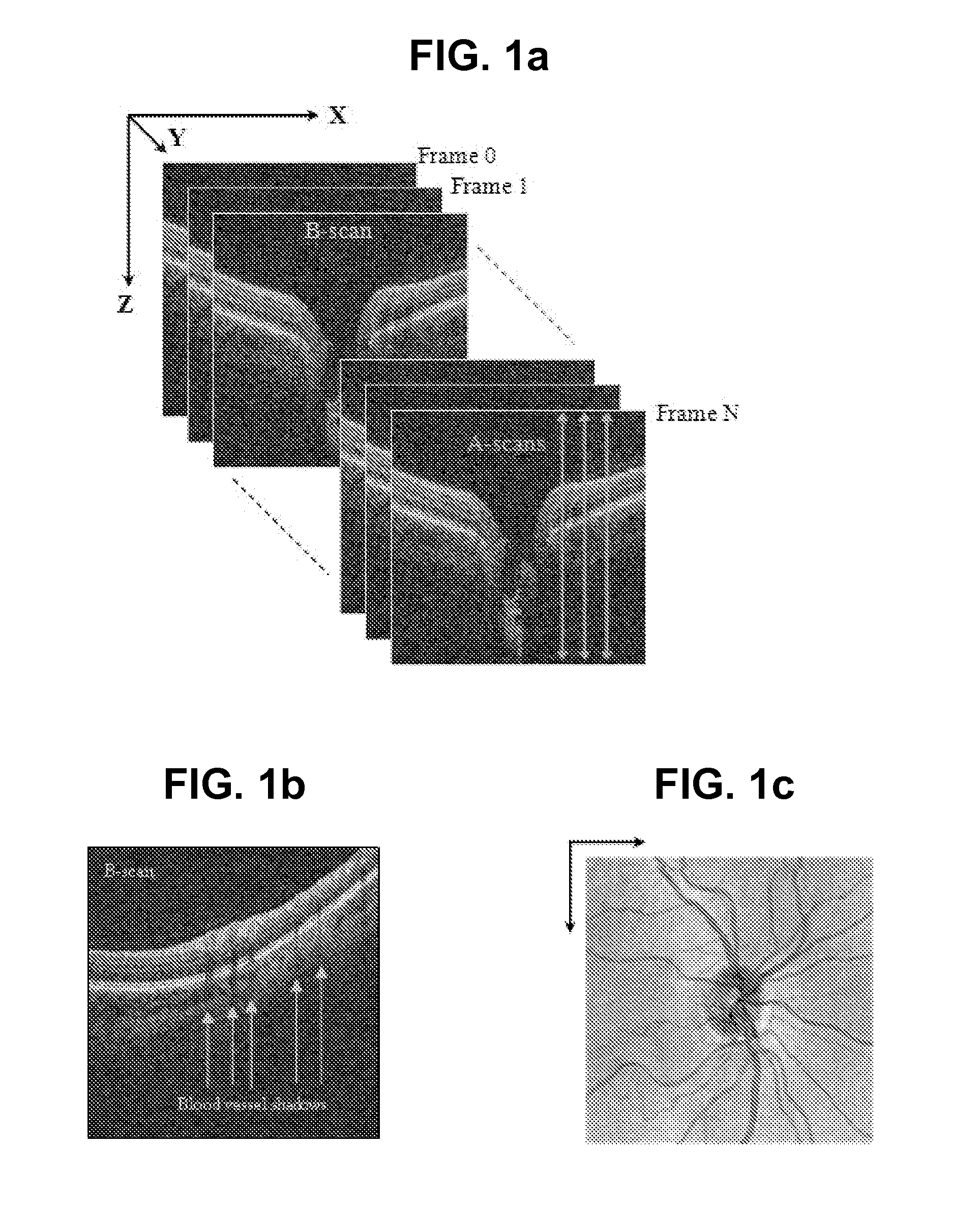
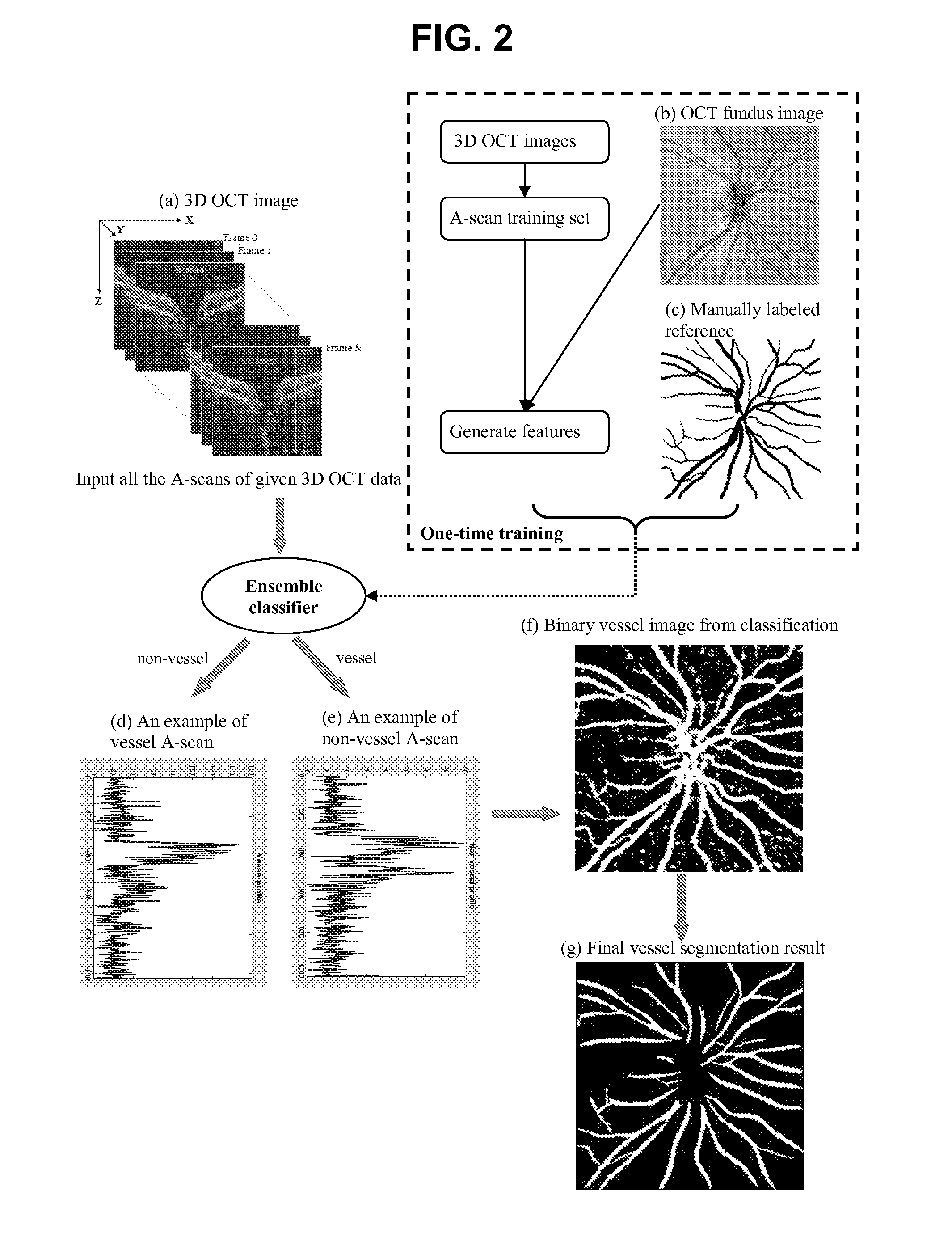
Blood vessel segmentation with three-dimensional spectral domain optical coherence tomography
Owner:UNIVERSITY OF PITTSBURGH
Use of Inhibitors of Acid Sphingomyelinase to Treat Acquired and Inherited Retinal Degenerations
ActiveUS20150366876A1Efficacy of treatmentLack and slowing of disease progressionBiocideSenses disorderMedicineDisease patient
Owner:WISCONSIN ALUMNI RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com