Pharmaceuticals containing retinal stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Dissection of Retina and Retinal Pigment Epithelium (RPE)
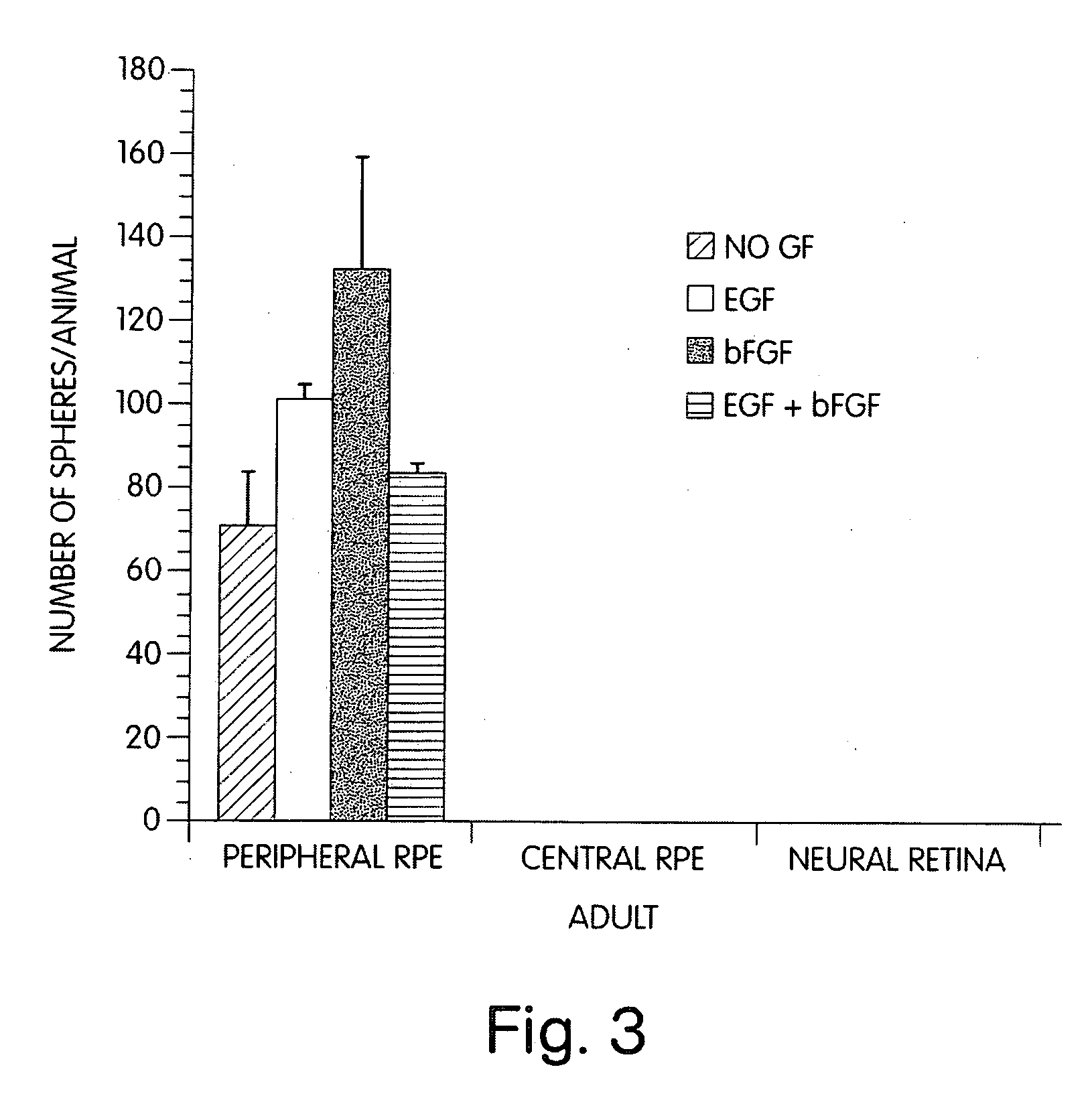
[0079] We considerably narrowed the area from which the retinal stem cell originates in the adult eye. The pigmented cells associated with the ciliary margin is the only area from which retinal spheres can be isolated in the adult animal. Dissections done on younger animals (embryonic or early post-natal) typically include the entire RPE. The cells of this invention may be used with biomaterials in a method of medical treatment of ocular dysfunction or disease. The dissection of the adult and embryonic neural retinal and RPE layers was done similarly to the method described for dissecting chick embryo retina in Opas et al., Development Biology, 161:440-454, (1993) in artificial cerebrospinal fluid (aCSF) containing 124 mM NaCl, 5 mM KCl, 1.3 mM MgCl2, 2 mM CaCl2, 26 mM NaHCO3, and 10 mM D-glucose (pH 7.4) previously aerated (15 min) with 95% O2-5% CO2 at room temperature. RPE cultures were prepared by enucleating mouse embryo...
example 2
Isolation and Culturing of Retinal Stem Cells in the Presence of Growth Factors
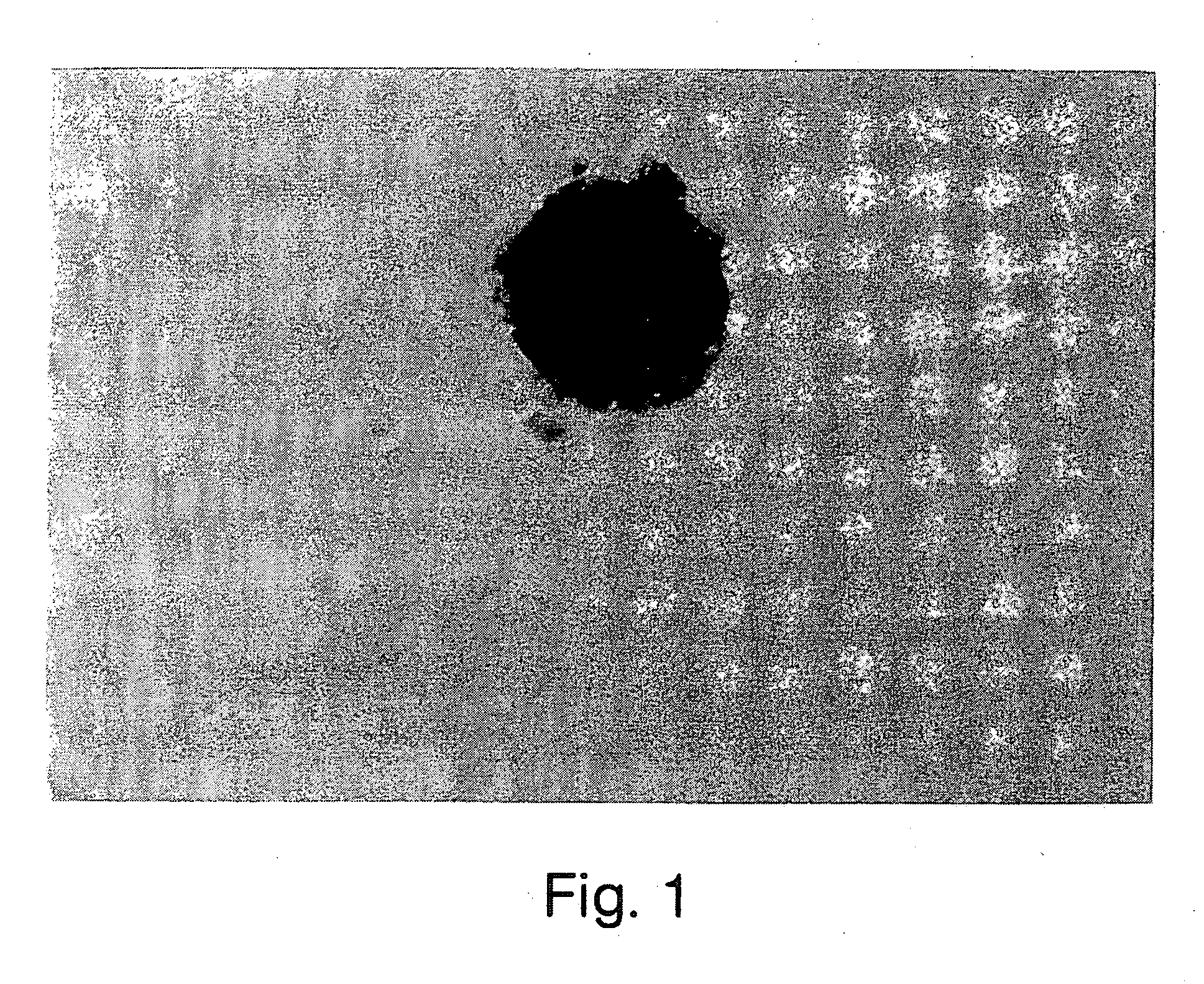
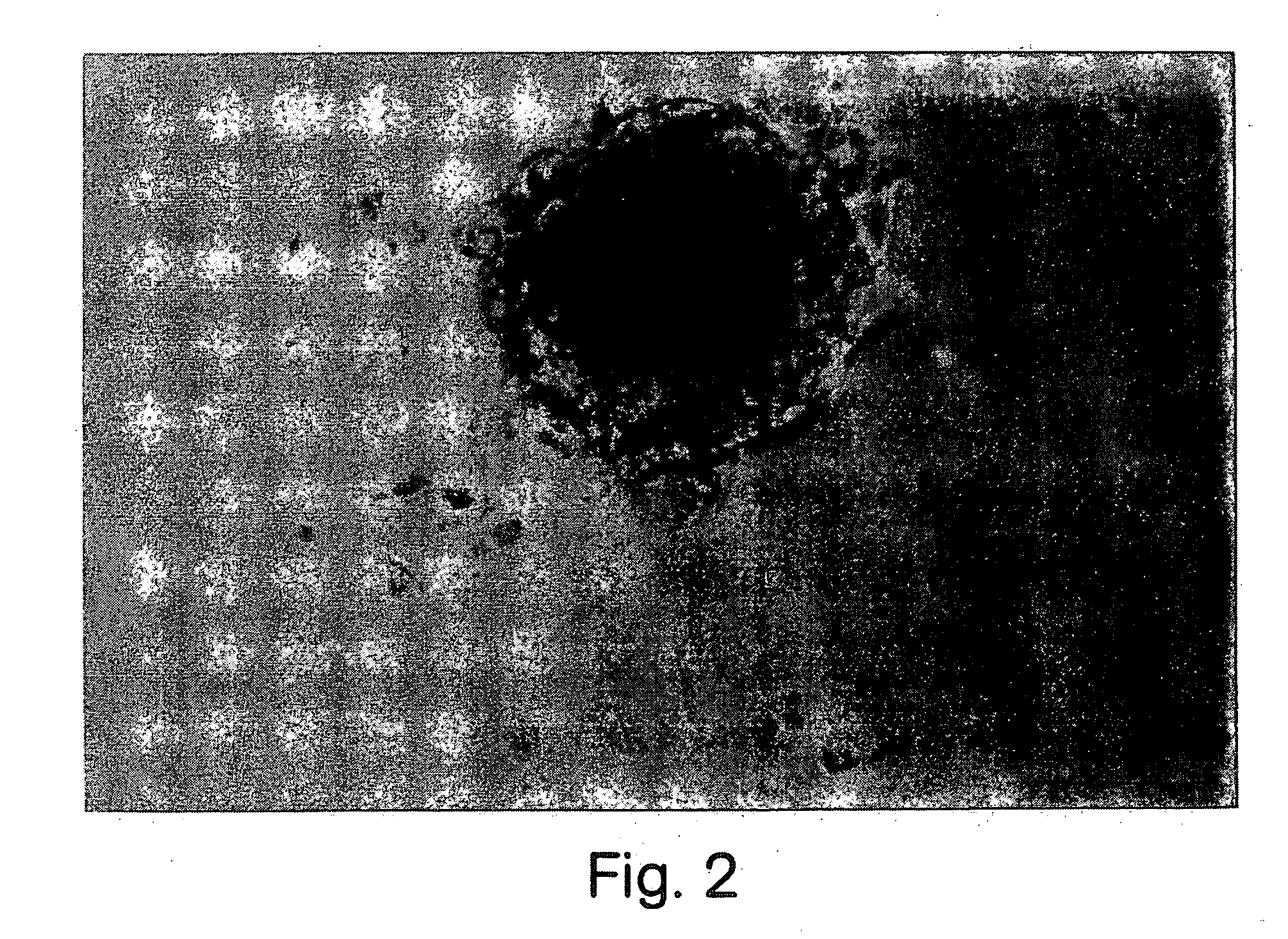
[0080] Retinal cells were dissociated and cultured in the presence of growth factors according to the methods described in Morshead et al., Neuron, Vol. 13:1071-1082 (1994) and Reynolds et al., 1993. After dissection and enzymatic treatment, neural retina and RPE tissues were cut into 1 mm sections and transferred into serum-free culture medium (described below) containing 0.7 mg / mL trypsin inhibitor (Boehringer-Mannheim) to stop the enzymatic reaction and mechanically dissociated (trituration) with a fire-polished Pasteur pipette. The cell suspension was then centrifuged at 150×g for 5 min, the media was aspirated and the pellet was resuspended in fresh serum-free media only.
[0081] The dissociated cells were plated in noncoated 35 mm culture dishes (NUNC 96 well plates) at desired densities (determined by Trypan blue exclusion) with serum-free medium containing 20 ng / mL EGF (UBI; purified from mouse su...
example 3
ChX 10 Marker Occurs in Retinal Stem Cells
[0085] EGF, FGF2 and heparin together induced the proliferation of retinal stem cells in serum-free medium, which produced a sphere of undifferentiated precursor cells. EGF alone in serum-free medium also produced undifferentiated precursor cells. These spheres were not immunoreactive for glial fibrillary acidic protein (GFAP) (an intermediate filament protein specific for astrocytes), neuron-specific enolase (a neuron-specified enzyme), or myelin basic protein (MBP) (a cell surface protein specific to oligodendrocytes). The spheres were, however, immunoreactive for nestin (characterized by Lehndahl et al., Cell 60:585 (1990)) which is an intermediate filament protein found in undifferentiated CNS cells. The precursor cells were also immunoreactive for the Chx 10 protein marker (characterized by Liu et al., Neuron, Vol. 13:377-393 (1994); Burmeister et al. Nature Genetics 12:376-384, 1996). Chx 10 marker is a homeobox gene that is normally ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com