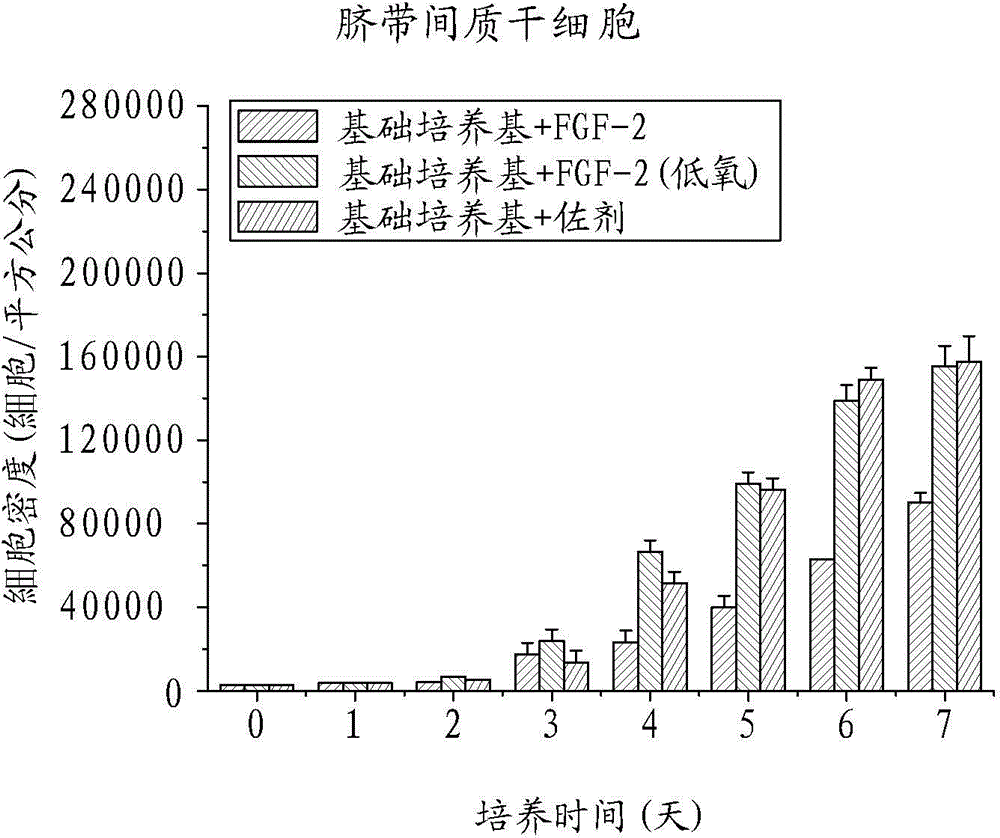
Patents
Literature
74 results about "Mesenchymal stem cell proliferation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The multiplication or reproduction of mesenchymal stem cells, resulting in the expansion of a stem cell population. A mesenchymal stem cell, or MSC, is a cell that retains the ability to divide and proliferate throughout life to provide progenitor cells that can differentiate into specialized mesenchymal cells. [CL:0000134, GOC:yaf, PMID:20626275]
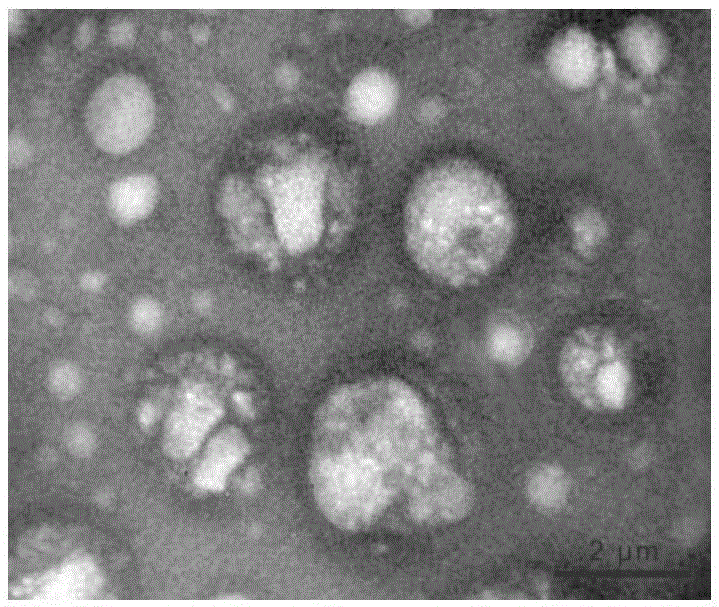
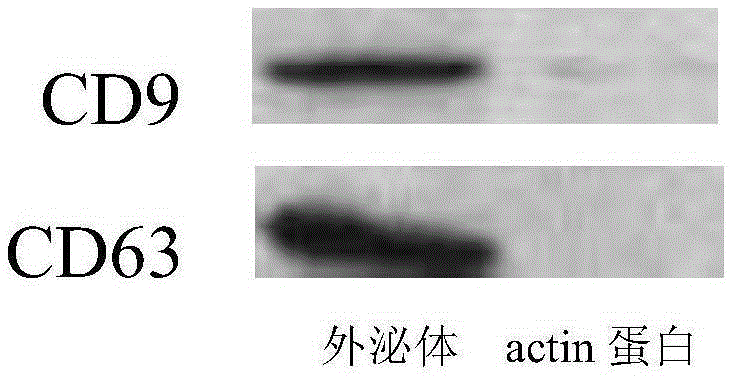
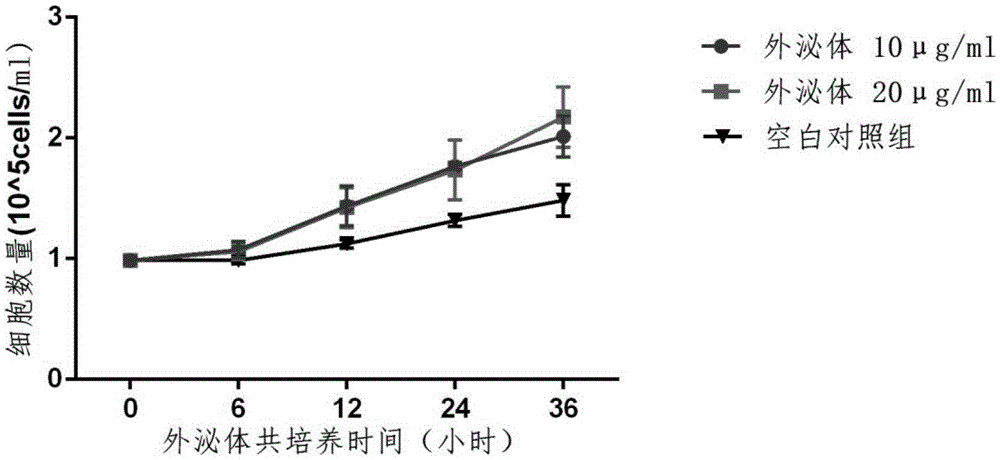
Method for promoting human bone mesenchymal stem cell proliferation based on exosome
ActiveCN105349487AStable in natureComposition determinedUnknown materialsSkeletal/connective tissue cellsEarly generationMesenchymal stem cell proliferation
The invention relates to the field of biotechnology and mainly relates to a use of an early-generation human umbilical cord mesenchymal stem cell exosome in promotion of human bone mesenchymal stem cell growth. The use method is a method for promoting bone mesenchymal stem cell growth. The invention also relates to an extraction method of a human umbilical cord mesenchymal stem cell exosome. The early-generation human umbilical cord mesenchymal stem cell exosome and human bone mesenchymal stem cells are co-cultured so that human bone mesenchymal stem cell growth is obviously promoted, cell doubling time is shortened and a ratio of an S period in a cell period is increased.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
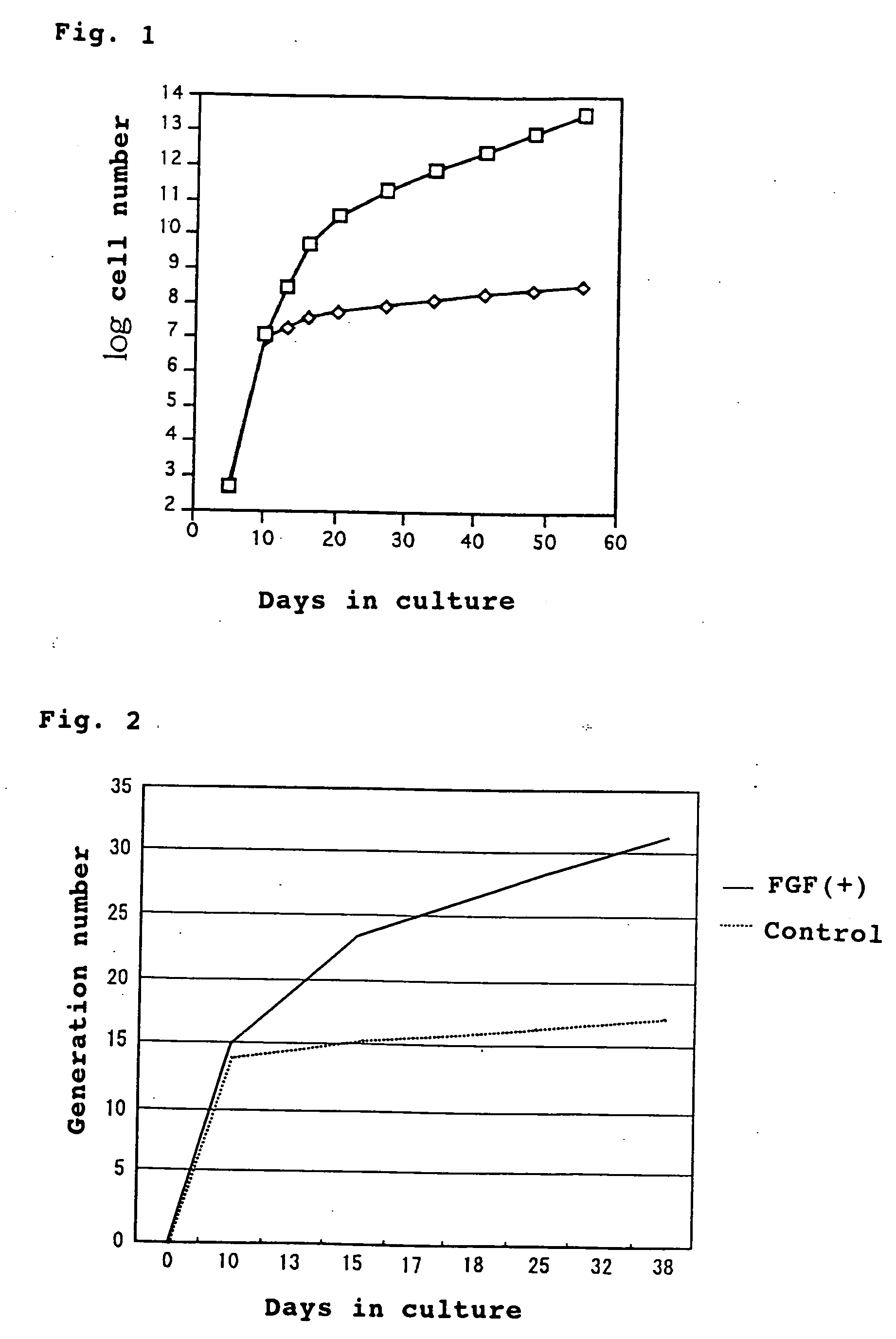
Method of culturing mesenchymal stem cells
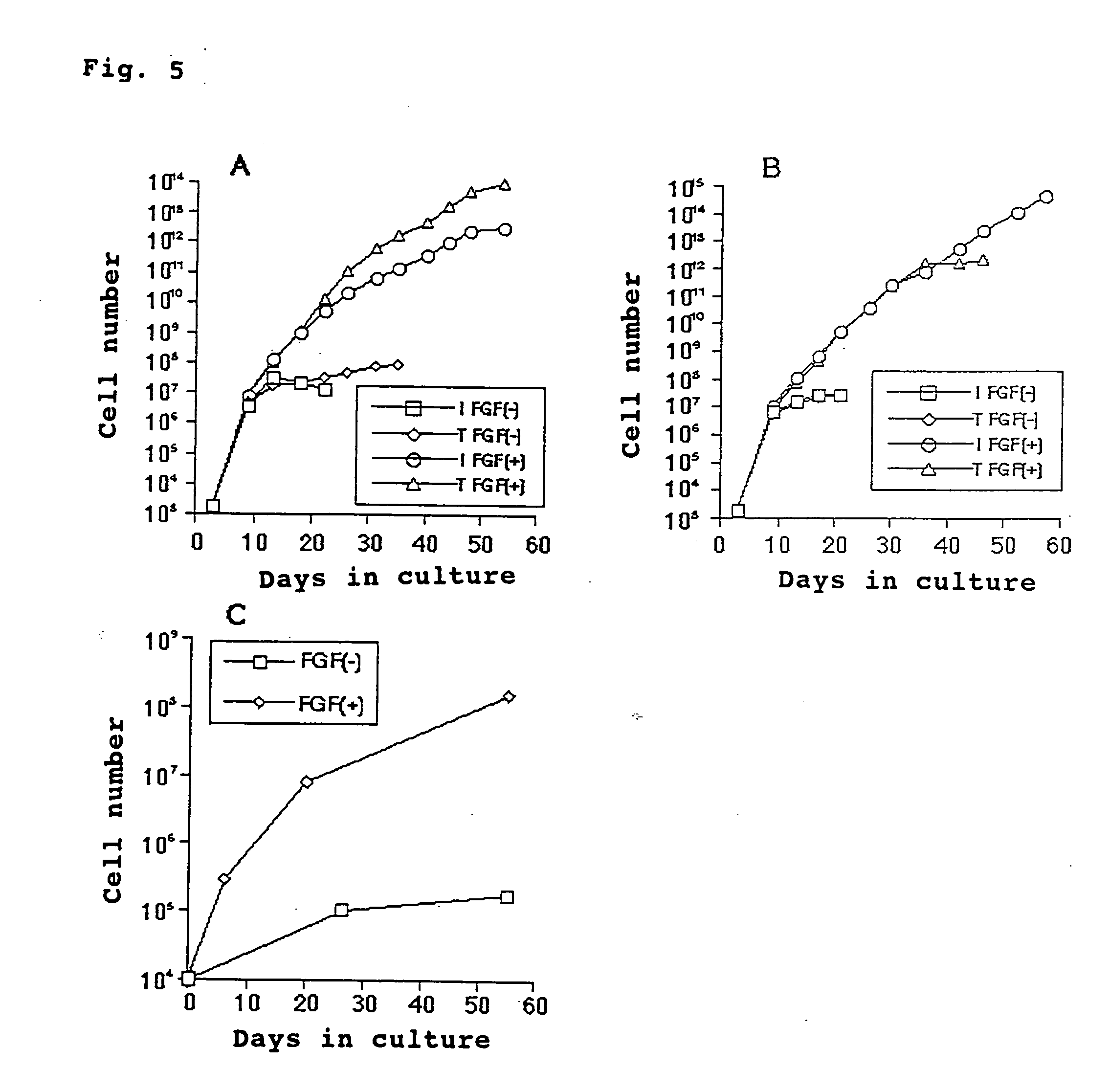
InactiveUS20050013804A1Readily availableRetaining pluripotencyBiocideArtificial cell constructsMesenchymal stem cell proliferationFibroblast growth factor
This invention provides a novel method of culturing mesenchymal stem cells whereby a remarkably larger number of mesenchymal stem cells can be obtained compared with the conventional culture methods. This culture method is characterized by adding to a medium a fibroblast growth factor (FGF) as a substance which stimulates the proliferation potency of the mesenchymal stem cells while retaining the pluripotency thereof and prolongs the life. According to the method, mesenchymal stem cells can be cultured at least over 30 generations and, moreover, about 10<5 >to 10<6 >times as much as cell count in the conventional culture methods can be obtained.
Owner:YUKIO KATO
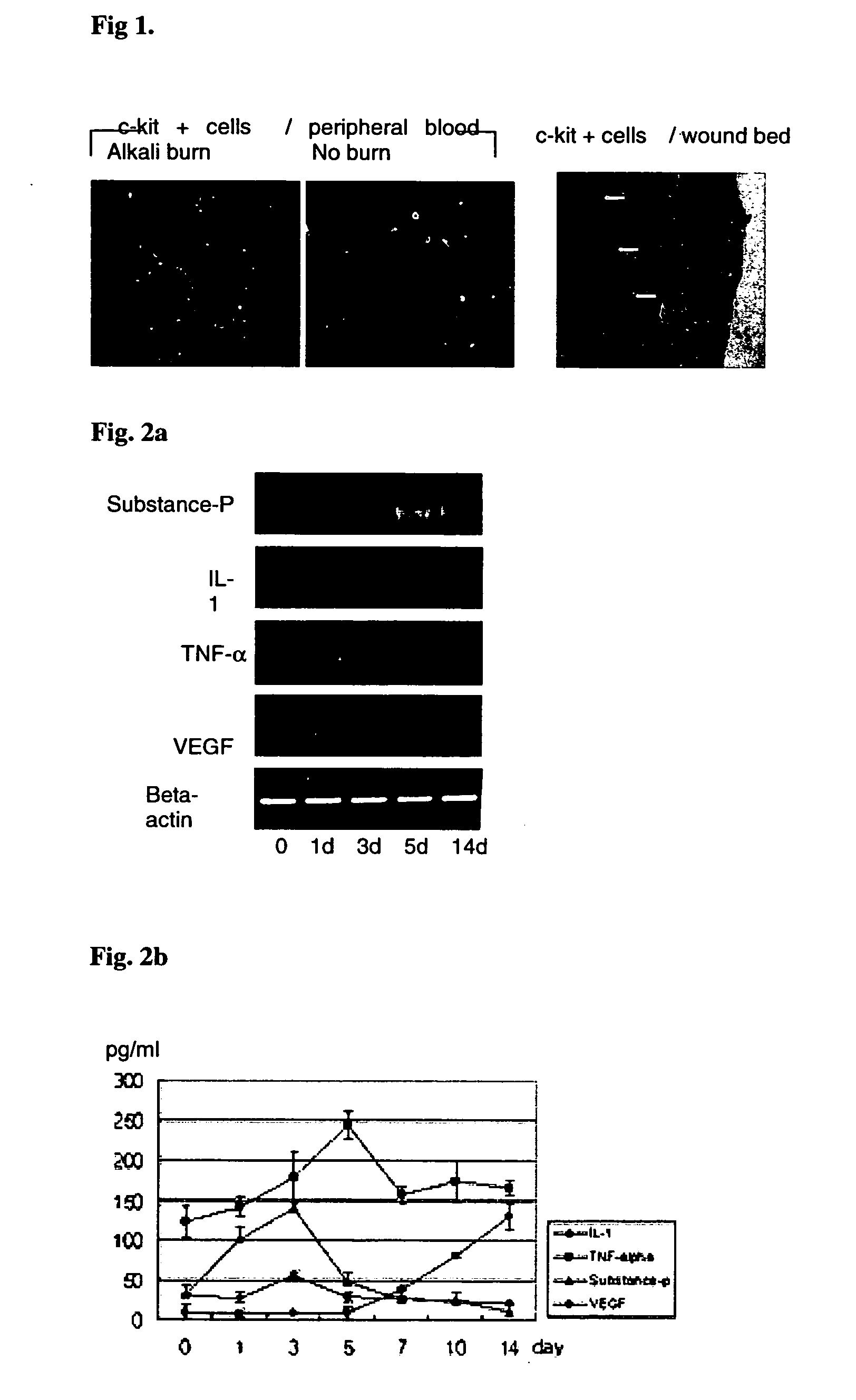
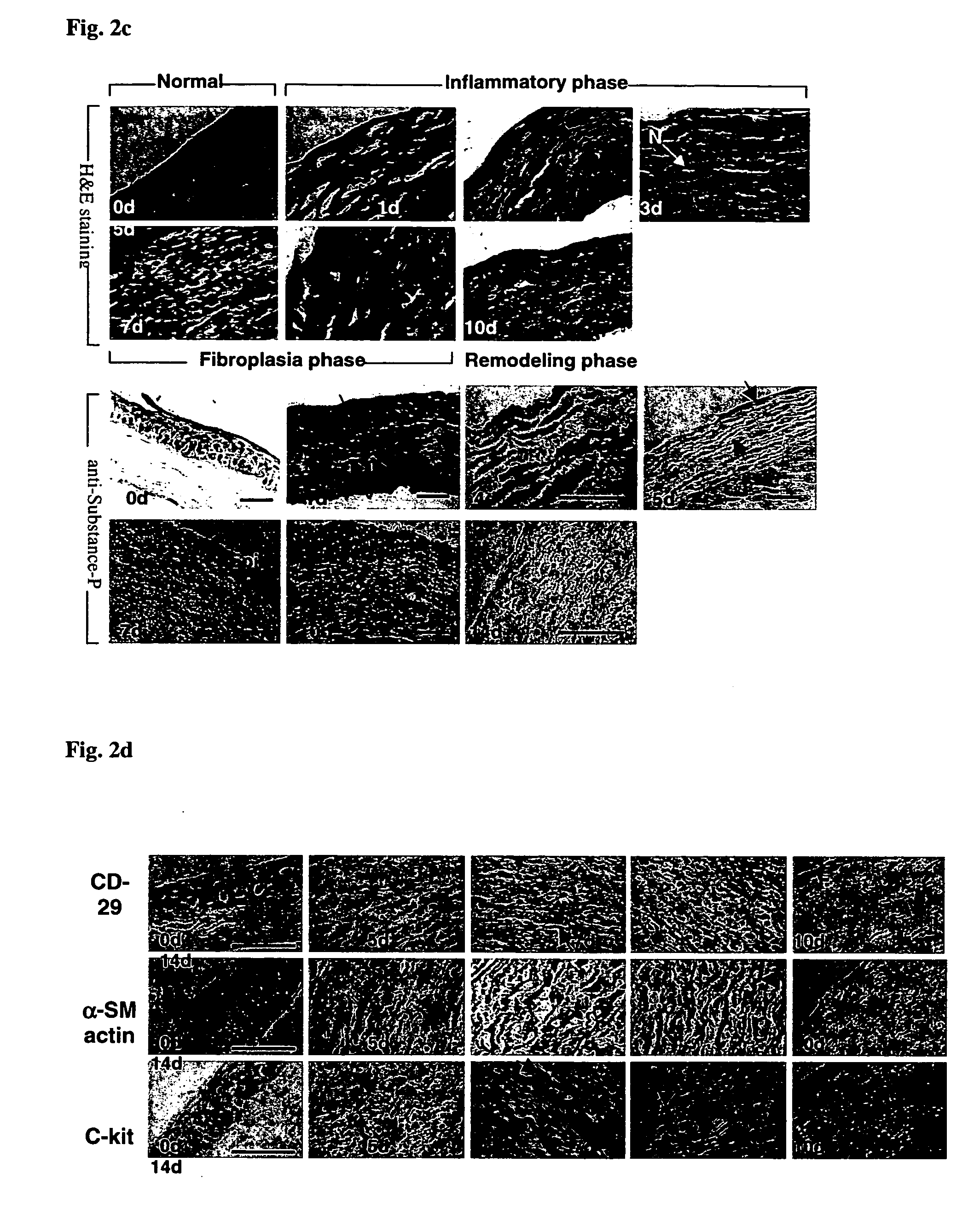
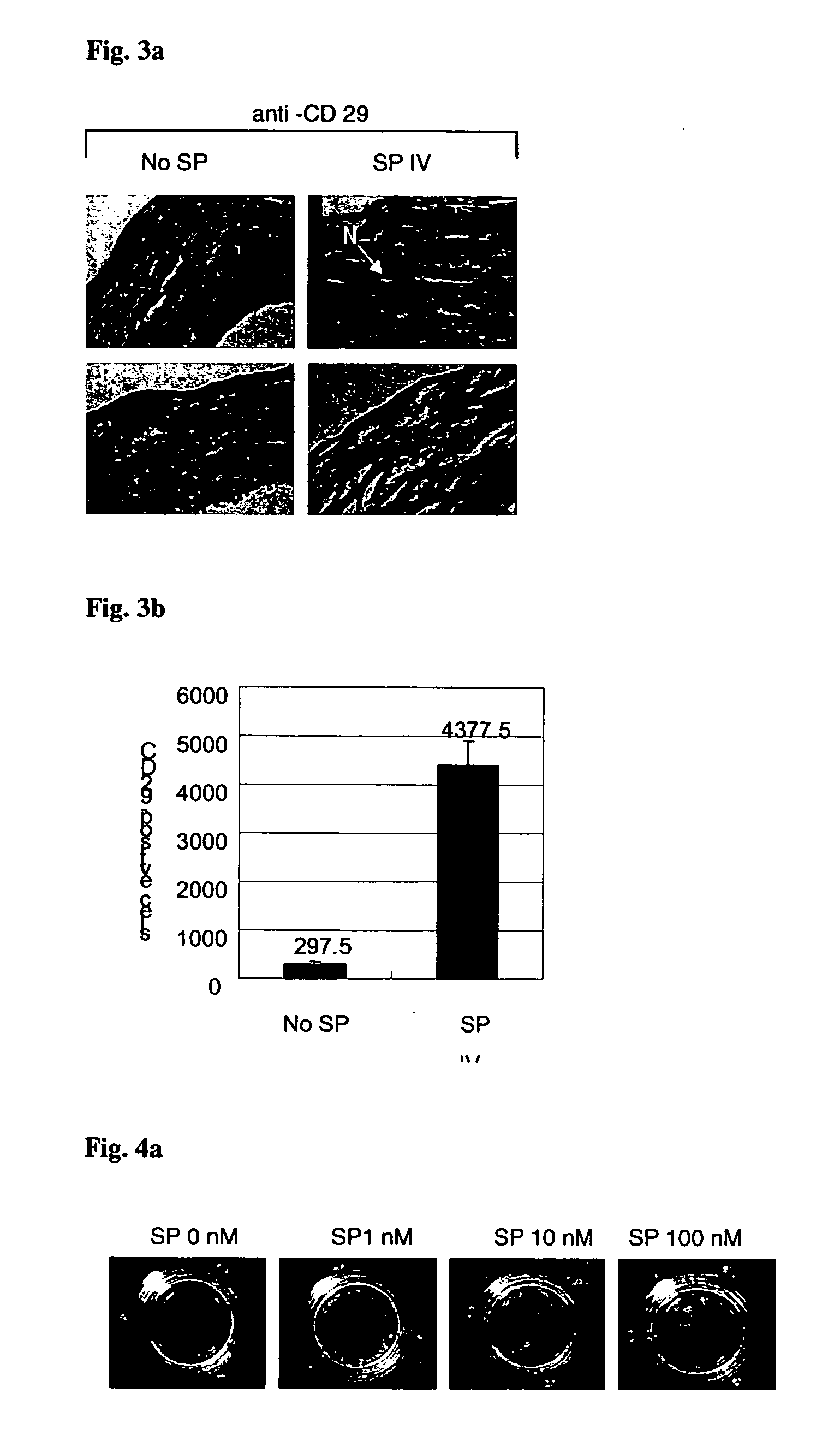
Use of substance P for mobilization of Mesenchymal stem cells or proliferation of Mesenchymal stem cells and for wound healing or facilitating wound healing
ActiveUS20060127373A1Promote migrationFacilitates cornea wound healingBiocideTachykinin ingredientsWound healingInjury mouth
The present invention relates to a use of Substance-P for the manufacture of a medicament for mobilization or proliferation of Mesenchymal stem cells (MSCs) from the bone marrow, or facilitating said mobilization or proliferation, and use of Substance-P for the manufacture of a medicament for wound-healing or facilitating wound-healing.
Owner:KOREA INST OF RADIOLOGICAL & MEDICAL SCI +1
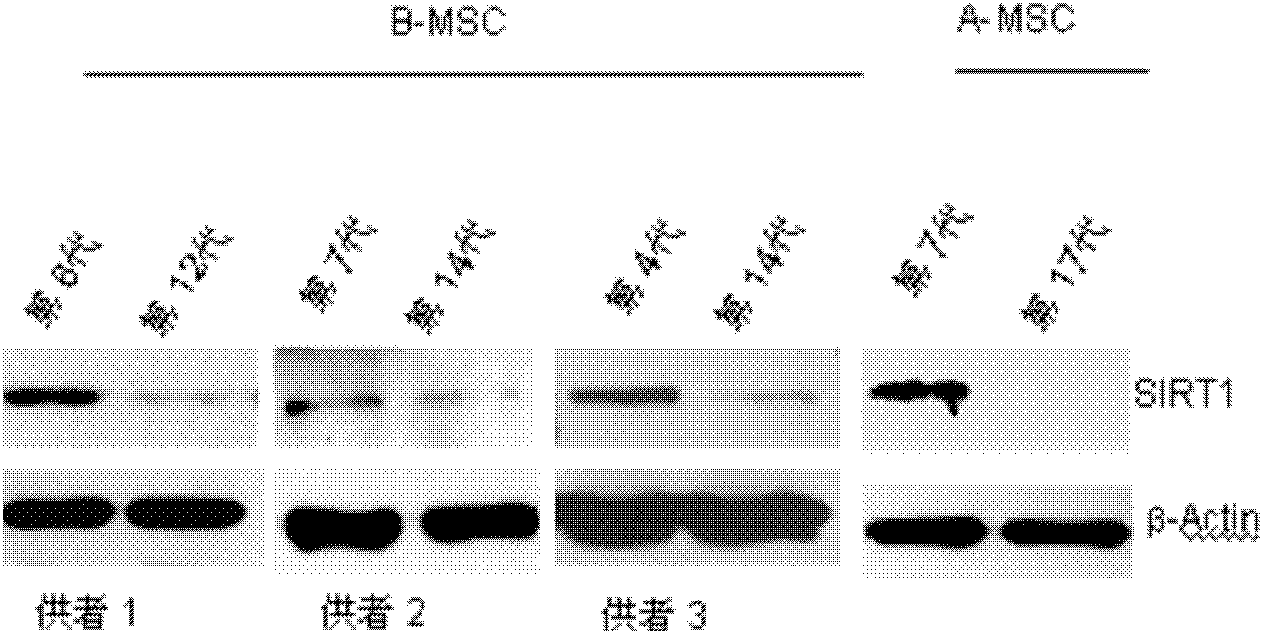
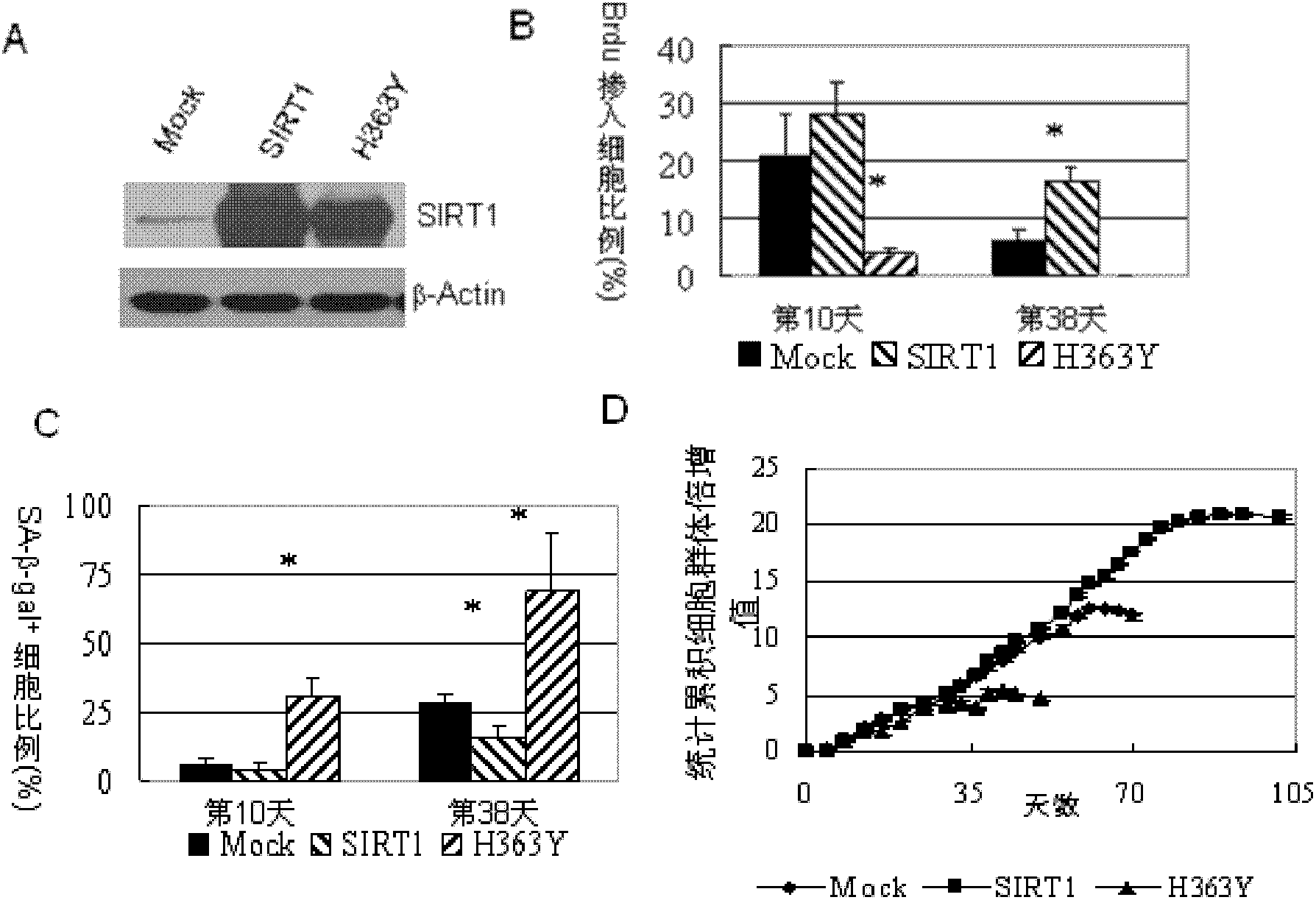
Application of protein acetylation enzyme SIRT1 (Silent Mating type Information Regulation 2Homolog1) in promoting proliferation and delaying senility of mesenchymal stem cells
ActiveCN102382801APromote proliferationReduce proliferationSkeletal/connective tissue cellsGenetic engineeringIn vitro proliferationMesenchymal stem cell proliferation
The invention discloses an application of protein acetylation enzyme SIRT1 (Silent Mating type Information Regulation 2Homolog1), namely promoting proliferation and delaying senility of mesenchymal stem cells. The experimental result shows that the SIRT1 is needed by the in vitro proliferation of the mescenchymal stem cells, and the condition is verified by MSC (mesenchymal stem cells from marrow) and MSC from fat, and the proliferation and the senility of the MSC can be regulated by regulating the expression level of SIRT1 in the MSC. The invention has very important significances on the determination of the regulation mechanism of the proliferation and the senility of the MSC, the prolonging of the service life of the MSC, and the histocyte substitution and repairing treatment based on the MSC, and is broad in application prospect.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Human fat mesenchymal stem cell bank and construction method thereof
ActiveCN103074298APromote rapid proliferationImprove separation efficiencySkeletal/connective tissue cellsRed blood cellCryopreservation
The invention discloses a human fat mesenchymal stem cell bank and a construction method thereof. The human fat mesenhymal stem cell bank is constructed by the following steps: (1), aseptically acquiring human fat; (2) removing oil and fat; (3), digesting collagenase; (4) performing schizolysis on red blood cells; (5) expanding culture; and (6), cryopreservation. A human fat mesenchymal stem cell is a type of pluripotent stem cell of fat tissue, can induce, differentiate and form cells of tissue types such as fat, bone, cartilage, muscle and the like. Fat tissue is wide in sources and easy for material obtaining, does not relate to ethical issues, is convenient for autograft, and brings less pain to patients; and in addition, compared with the mesenchymal stem cells of sources such as marrow and the like, the fat mesenchymal stem cell is fast in multiplication and high in separation efficiency.
Owner:冯文峰
Method for culturing mesenchymal stem cell and method for producing biological tissue prosthesis
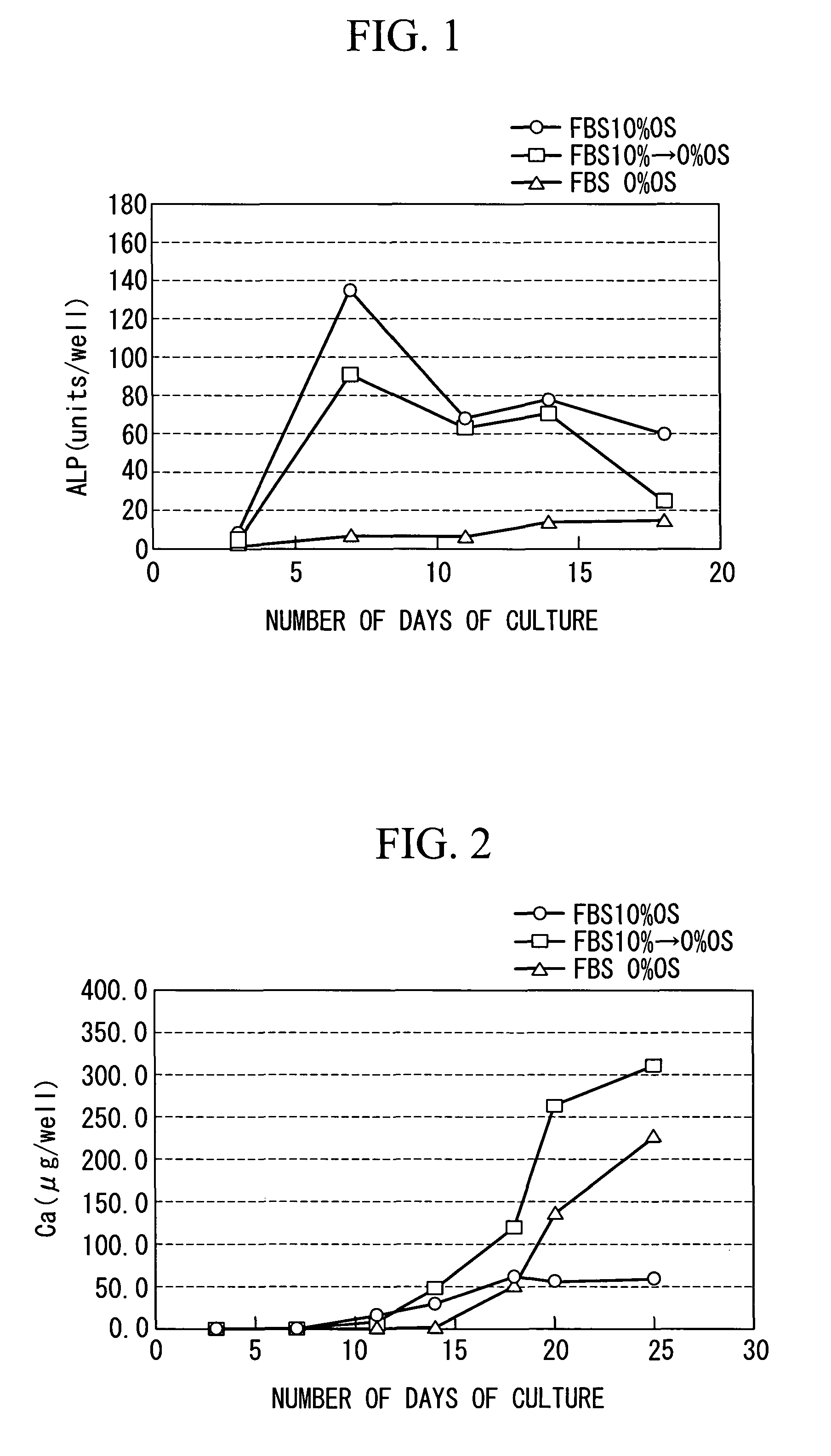
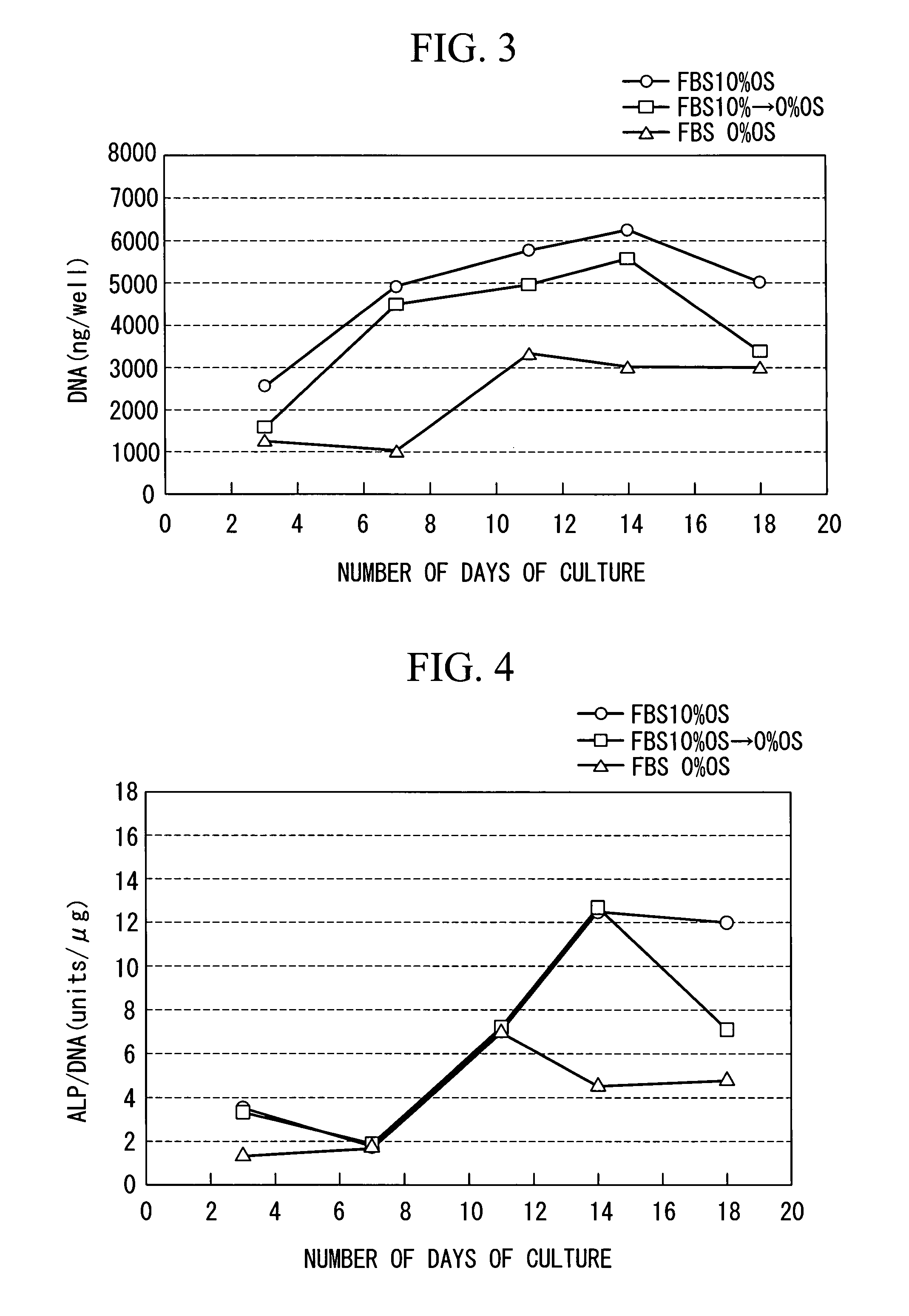
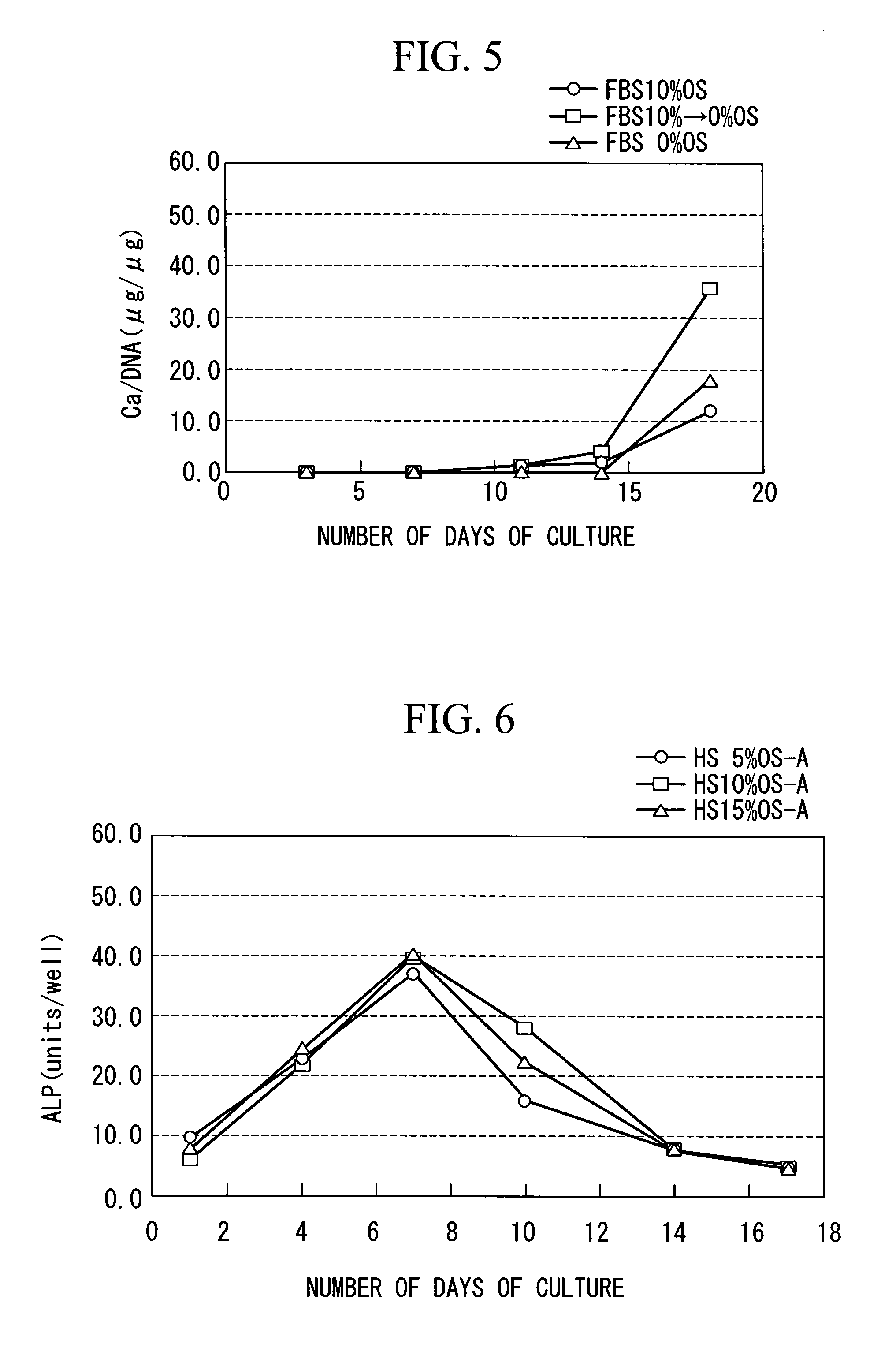
InactiveUS20100028997A1Reduce concentrationReduce harmCulture processArtificial cell constructsProsthesisMesenchymal stem cell proliferation
The purpose is to proliferate a mesenchymal stem cell to a sufficient degree while reducing the amount of blood serum contained in a biological tissue progenitor cell to be grafted, and to efficiently differentiate the mesenchymal stem cell into the biological tissue progenitor cell. There is provided a method for culturing a mesenchymal stem cell, comprising: a first culture step of proliferating a mesenchymal stem cell in a medium containing blood serum; and a second culture step of differentiating the mesenchymal stem cell into a biological tissue progenitor cell in a medium containing blood serum at a lower concentration than that in the medium used in the first culture step.
Owner:OLYMPUS CORP
Mesenchymal stem cells which express human hepatic growth factor,manufacturing method thereof, and use thereof as therapeutic agent for liver diseases
InactiveUS20110274670A1Suppress apoptosisSuppress cirrhosisOrganic active ingredientsBiocideApoptosisUmbilical cord
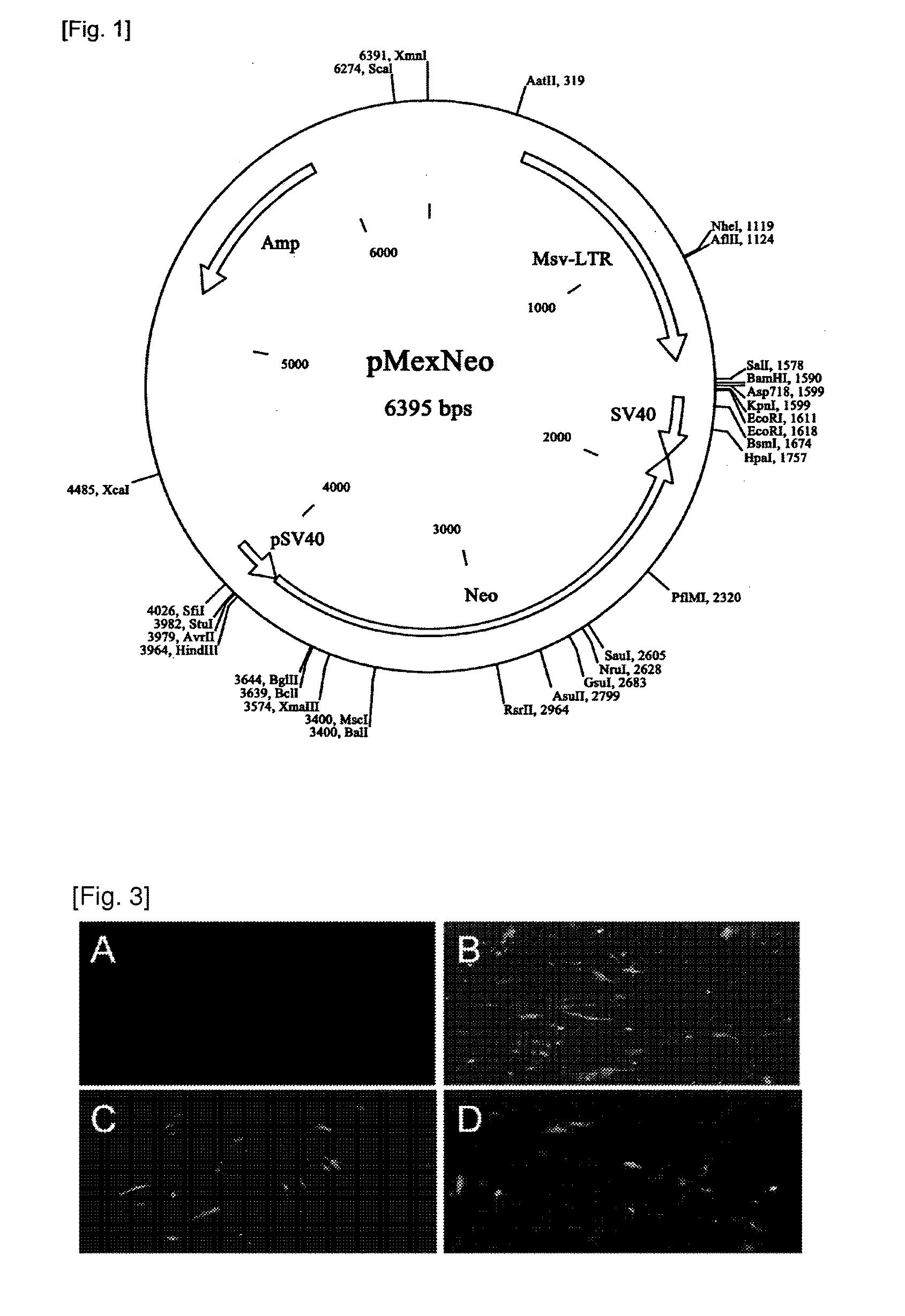
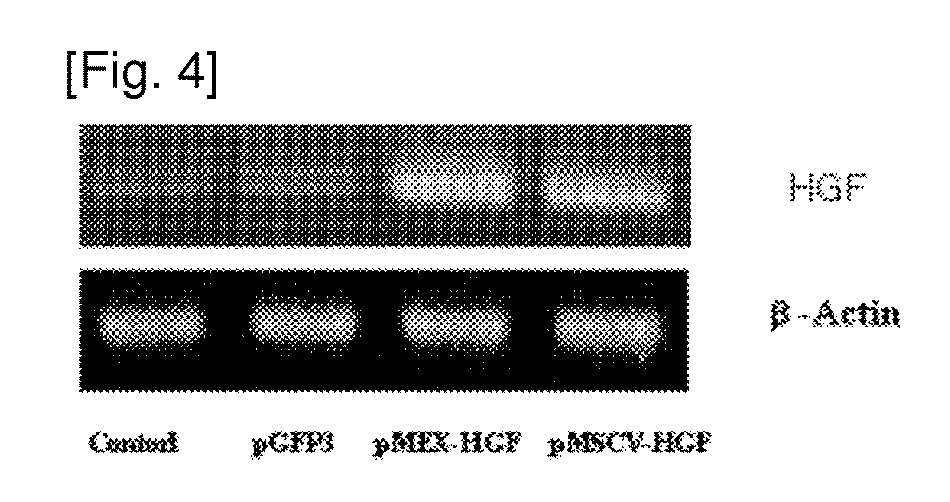
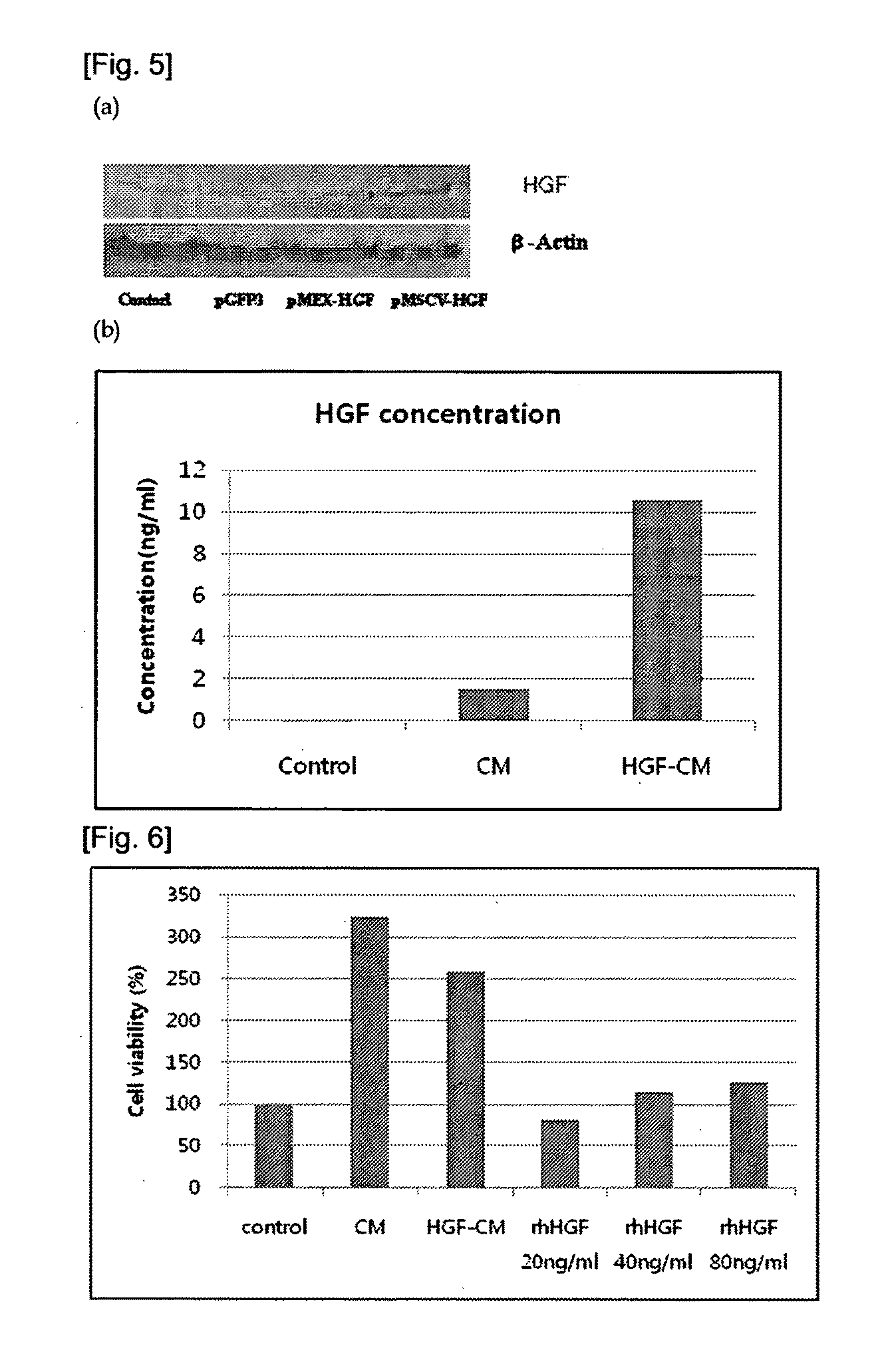
The present invention relates to adult stem cells and a manufacturing method thereof. More specifically, the present invention relates to a recombinant expression vector containing a human hepatic growth factor (hHGF) gene, mesenchymal stem cells which are transformed thereby and express the hHGF, a manufacturing method of the mesenchymal stem cells, conditioned media (CM) which is obtained from the transformed cells and proliferates hepatocytes, a culture method of the mesenchymal stem cells producing the same, and the use of the transformed mesenchymal stem cells and their culture media as an agent for preventing and treating liver diseases. The manufacturing method of the mesenchymal stem cells comprises the steps of: isolating and culturing umbilical cord blood-derived mesenchymal stem cells; transforming the mesenchymal stem cells with the recombinant expression vector; and selecting the mesenchymal stem cells. The mesenchymal stem cells, which produce the hHGF in the present invention effectively, proliferate hepatocytes, suppress apoptosis and effectively suppress liver cirrhosis. Therefore, the mesenchymal stem cell can be widely used in preventing and treating various liver diseases.
Owner:NAM MYEONG JIN +1
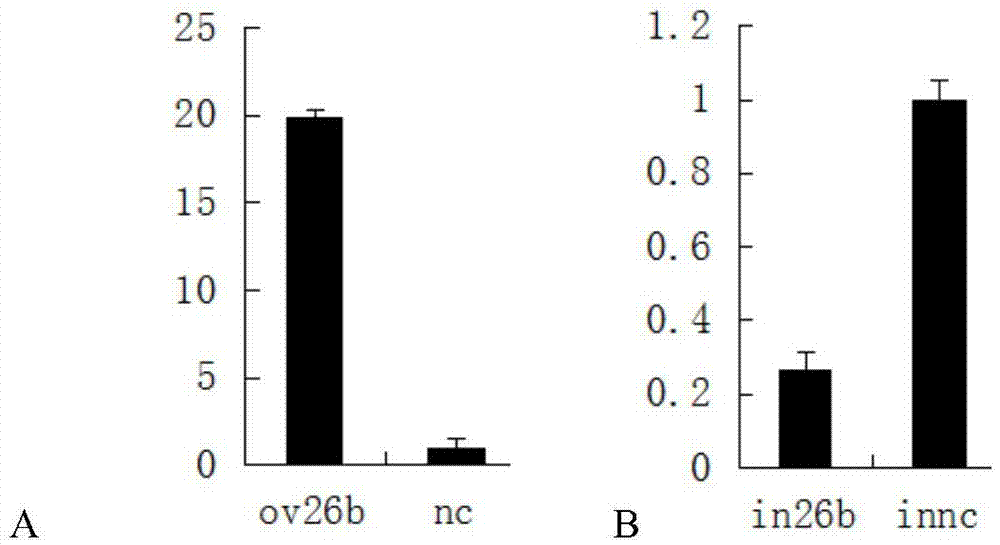
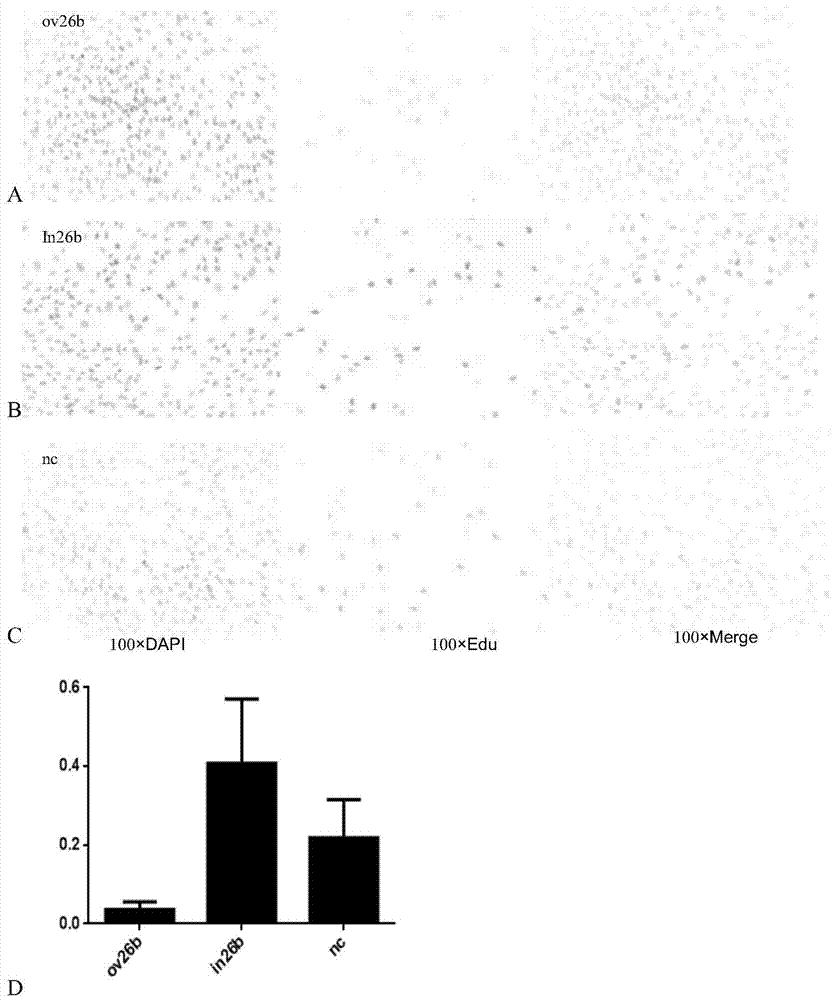
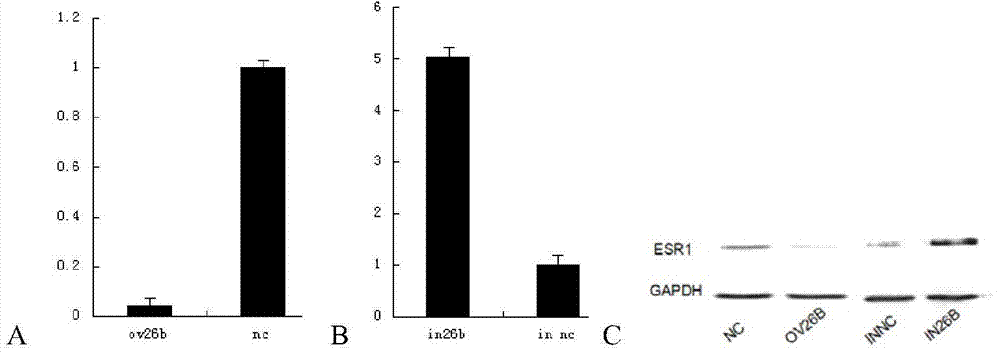
Application of MicroRNA26b-3p inhibitor in preparation of human umbilical cord derived mesenchymal stem cell
ActiveCN104726500APromote proliferationOvercome the defect of slow proliferation in in vitro cultureMicroencapsulation basedVector-based foreign material introductionEstrogen receptorMesenchymal stem cell proliferation
The invention relates to the technical field of biology and provides application of a MicroRNA26b-3p inhibitor in preparation of a reagent for promoting proliferation of a human umbilical cord derived mesenchymal stem cell. The reagent comprises but is not limited to: (1) a MicroRNA26b-3p inhibitor, (2) a recombinant vector containing a MicroRNA26b-3p inhibitor coding gene, (3) recombinant virus containing the MicroRNA26b-3p inhibitor coding gene, and (4) a recombinant virus vector containing the MicroRNA26b-3p inhibitor coding gene. Experiments prove that the MicroRNA26b-3p inhibitor can be used for improving the expression of an estrogen receptor and remarkably increasing the proliferation speed of the human umbilical cord derived mesenchymal stem cell. The application disclosed by the invention provides new conception and method for rapidly proliferating the human umbilical cord derived mesenchymal stem cell and obtaining regenerative medical mesenchymal stem cell for clinical application more quickly.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
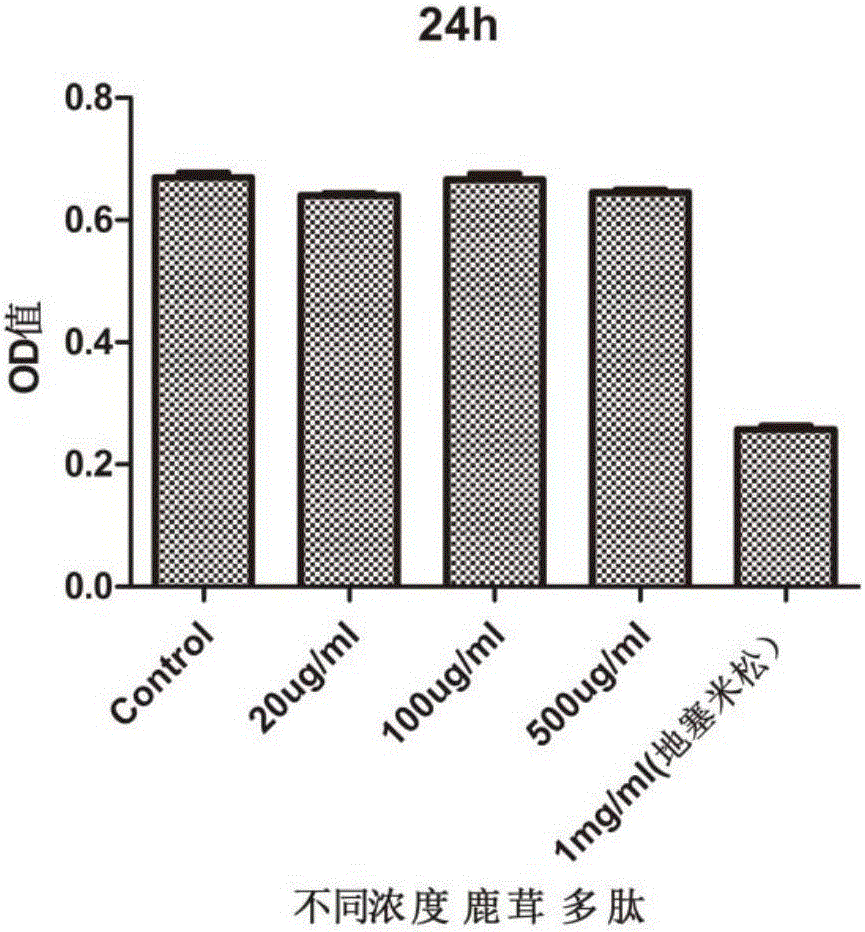
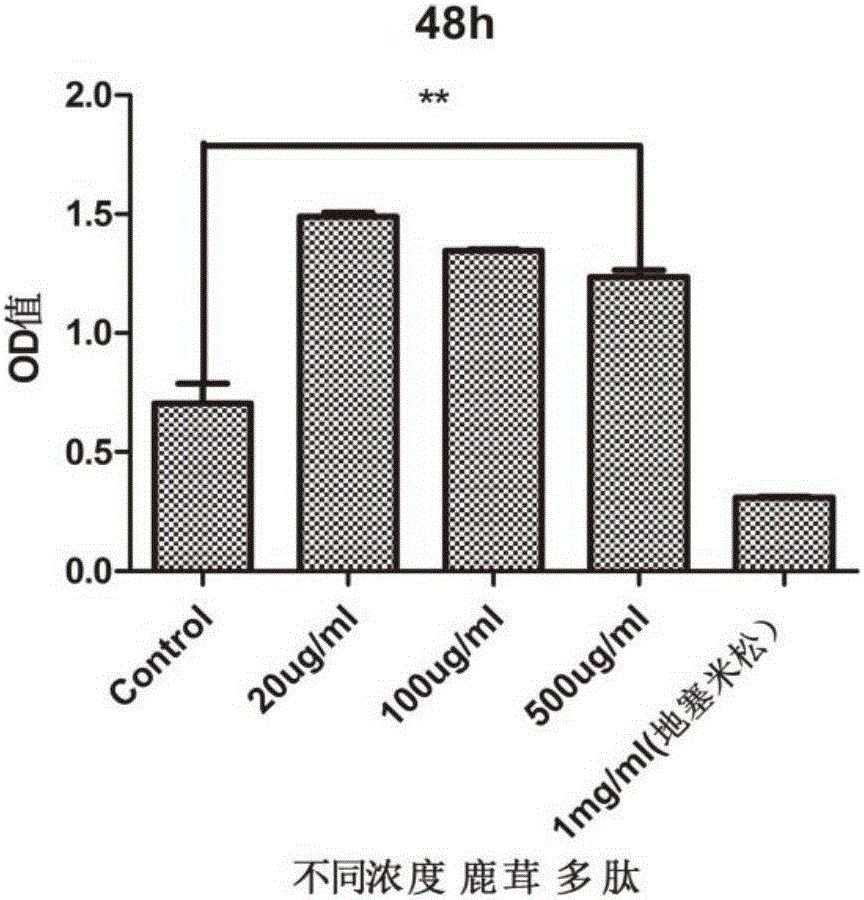
Application of pilose antler peptide in promoting proliferation of mesenchymal stem cells
InactiveCN106367384APromote value-addedPromote wound healingSkeletal/connective tissue cellsCell culture active agentsStromal cellDamage repair
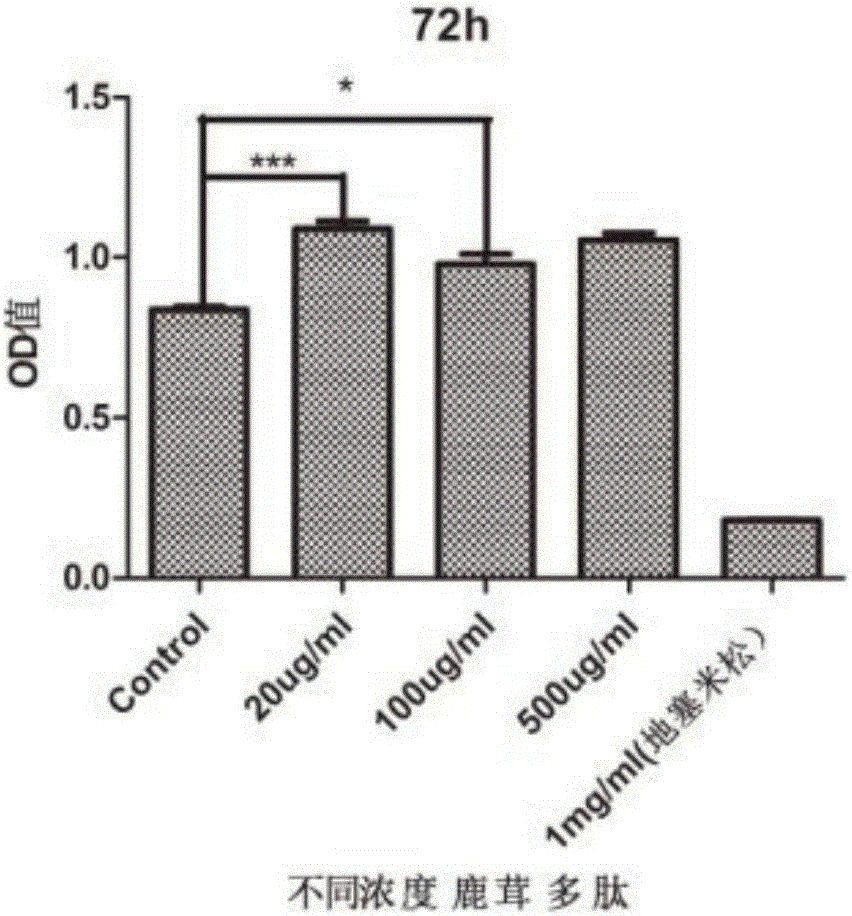
The invention belongs to the technical field of biological medicines, and discloses application of pilose antler peptide in promoting proliferation of mesenchymal stem cells. The pilose antler peptide which contains 32 amino acids obtained in the invention has an effect on proliferation of the ADSCs (Adipose Tissue-Derived Stromal Cells). The amino acid sequence of the pilose antler peptide is VLSATDKTNVLAAWGKVGGNAPAFGAEALERM. The pilose antler peptide has a most remarkable effect when the concentration of the pilose antler peptide is 20 ug / ml and the acting time is 48 hours, and is beneficial to promoting kidney damage repair and promoting skin wound healing and bone repair.
Owner:DALIAN UNIV OF TECH
In-situ photocuring antibacterial bone defect repair gel and preparation method thereof
ActiveCN112891618AAvoid infectionActivate the photothermal effectTissue regenerationProsthesisAntibacterial activityBiocompatibility
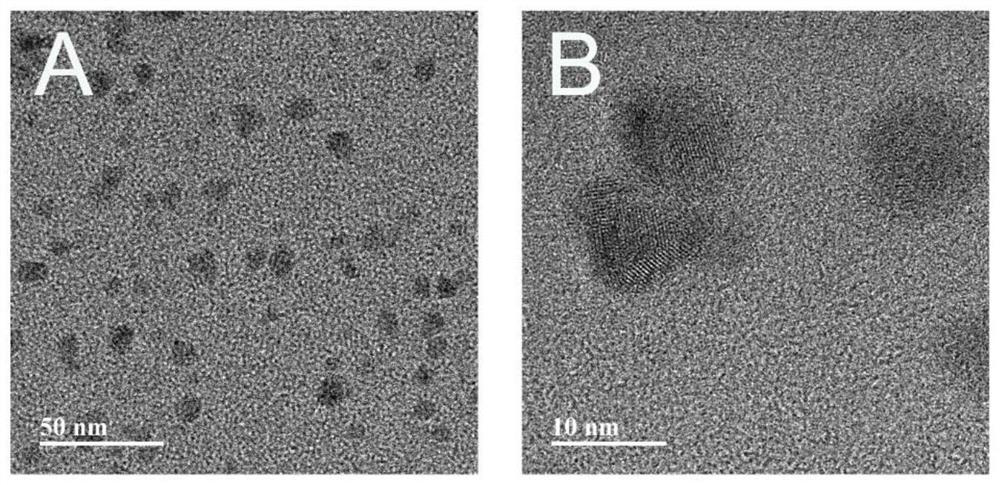
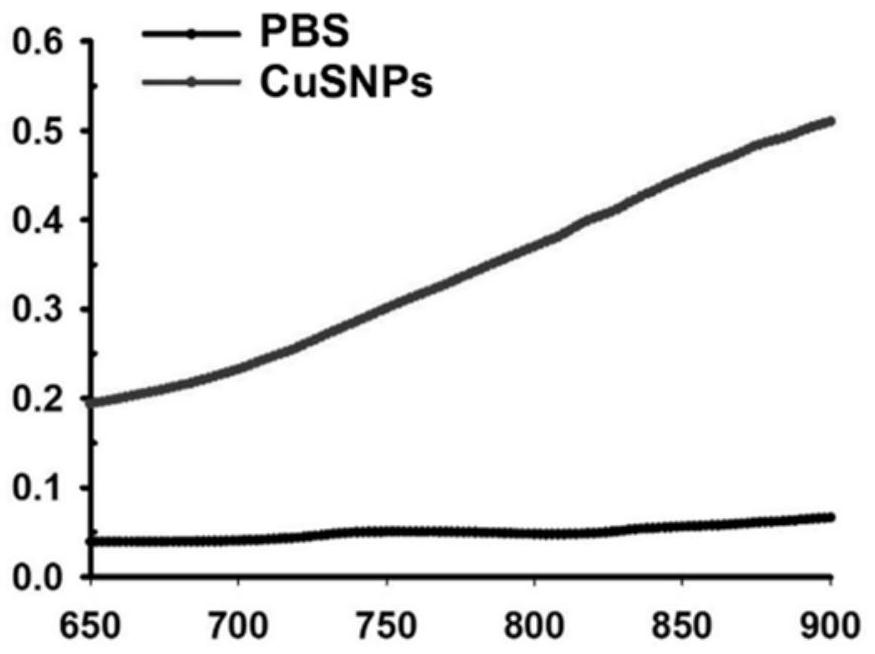
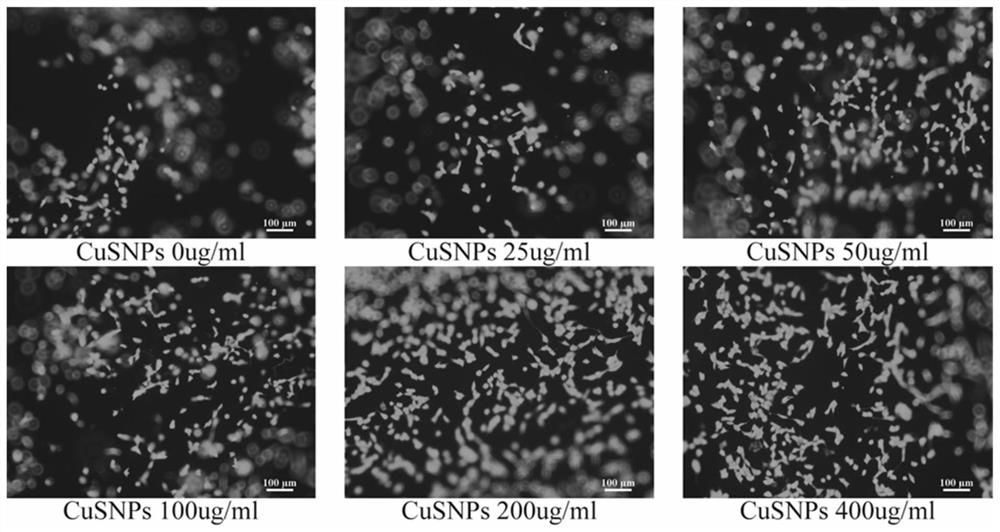
The invention provides in-situ photocuring antibacterial bone defect repair gel and a preparation method thereof. The gel is prepared by photo-crosslinking methylacryloyl copper sulfide nanoparticles and methylacryloyl gelatin. Modified CuSNPs is introduced into GelMA gel, and after the gel is added, CuSNPs-GelMA composite hydrogel can be obtained through in-situ photocuring shaping. Near-infrared light irradiates the composite material, the photothermal effect of CuSNPs in the CuSNPs-GelMA composite hydrogel can be activated, and mesenchymal stem cell proliferation and osteogenic differentiation of a periodontal defect area are promoted; and Cu< 2+ >slowly released by the composite material better endows a bone defect repair scaffold system with continuous antibacterial activity, and periodontal postoperative infection can be prevented to a great extent. The composite material is reasonable and convenient to prepare, low in price, high in application operability, good in biocompatibility and good in osteogenic effect, widens periodontal regeneration operation indications and is also suitable for repairing other bone defects.
Owner:ZHEJIANG UNIV
Method for culturing mesenchymal stem cell and method for producing biological tissue prosthesis
InactiveUS7972767B2Effectively lead to differentiationReduce the amount requiredCulture processDead animal preservationProsthesisMesenchymal stem cell proliferation
The purpose is to proliferate a mesenchymal stem cell to a sufficient degree while reducing the amount of blood serum contained in a biological tissue progenitor cell to be grafted, and to efficiently differentiate the mesenchymal stem cell into the biological tissue progenitor cell. There is provided a method for culturing a mesenchymal stem cell, comprising: a first culture step of proliferating a mesenchymal stem cell in a medium containing blood serum; and a second culture step of differentiating the mesenchymal stem cell into a biological tissue progenitor cell in a medium containing blood serum at a lower concentration than that in the medium used in the first culture step.
Owner:OLYMPUS CORP
Preparation method of high-concentration human-source mesenchymal stem cell lysis solution
ActiveCN110804607AOvercome purityOvercome yieldCell dissociation methodsSkeletal/connective tissue cellsHigh concentrationLysis
The invention provides a preparation method of a high-concentration human-source mesenchymal stem cell lysis solution. The preparation method comprises the following steps of step of breaking: performing ultrasonic breaking on a human-source mesenchymal stem cell lysis solution of which the fusion degree is 80-90%, which is obtained by collection by using 60-100W ultrasonic waves for 10-30min to obtain broken cell liquid; step of centrifugation: performing centrifugation on the broken cell liquid under the condition that the temperature is 2-8 DEG C and centrifugal force is 2000-5000g for 10-30min, and after centrifugation, taking supernatant; step of purification: performing filtering and concentrating on the supernatant by a 3K ultra filtration membrane to obtain a concentrated solutionof which the volume is 1 / 5-1 / 10 of the volume of the supernatant; and performing further concentrating on the concentrated solution by a hollow fiber ultrafiltration column to obtain the high-concentration human-source mesenchymal stem cell lysis solution. The method solves the problems that in the prior art, the extracted stem cell factors are low in purity, low in yield, low in activity, low inefficiency, low in yield and the like, and the obtained stem cell lysis solution has favorable capacity for promoting proliferation of mesenchymal stem cells and promoting collagen synthesis.
Owner:深圳市润科生物科技有限公司
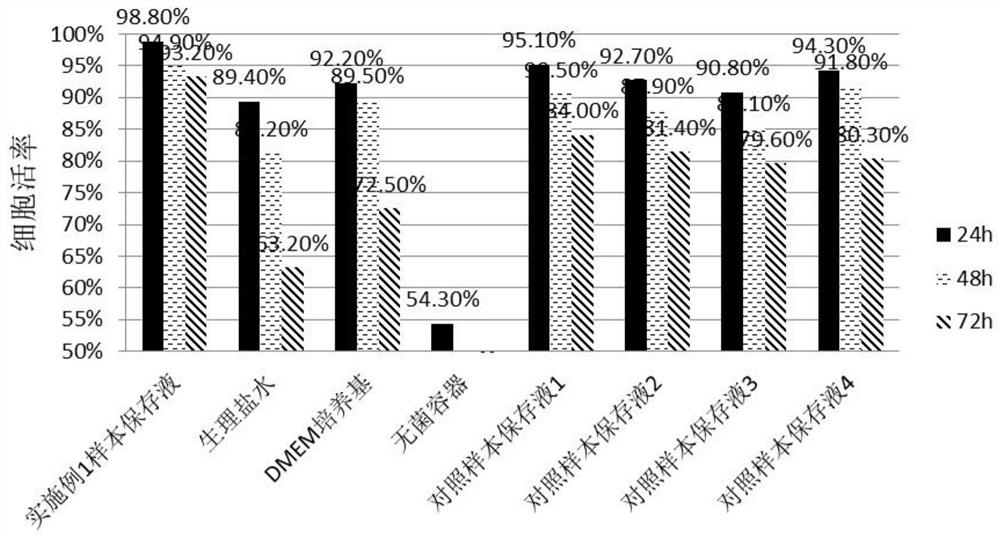
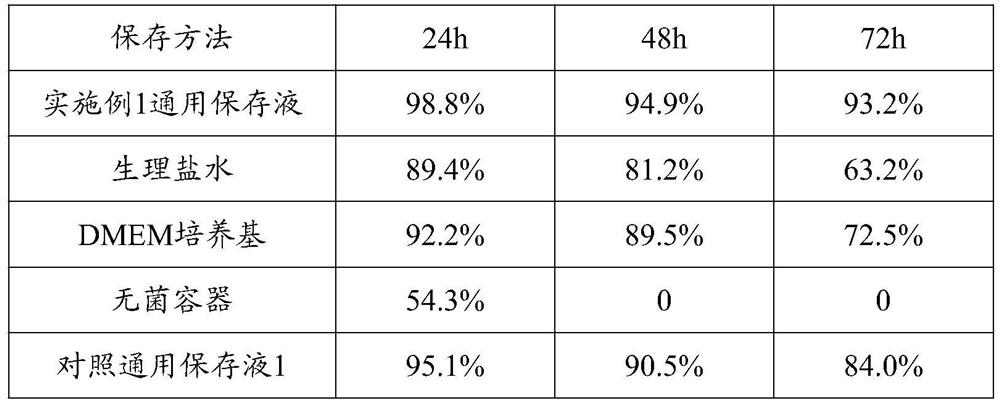
General preserving fluid for human umbilical cord, amniotic membrane and placenta samples and preparation method thereof
The invention belongs to the technical field of stem cells, and particularly relates to a universal preserving fluid for human umbilical cord, amniotic membrane and placenta samples and a preparationmethod thereof. In the universal preservation solution, the DMEM basal culture medium provides the most basic nutrition for the mesenchymal stem cells of the sample; insulin can promote the sample mesenchymal stem cells to absorb and utilize sugar in the universal preservation solution, and the cell activity is maintained; the human serum albumin provides dynamic balance for the mesenchymal stem cells and maintains cell osmotic pressure; vitamin C can prevent free radicals generated by long-time oxidation from damaging the mesenchymal stem cells; the EGF can promote the proliferation of the mesenchymal stem cells and maintain the original form of the cells. Experimental results show that the preserving fluid can be used for preserving umbilical cords, amnions and placentas for a long time,the number of obtained mesenchymal stem cells is large, the survival rate is high, and human albumin, insulin, vitamin C and EGF have a synergistic effect on increasing the number of preserved umbilical cord mesenchymal stem cells and increasing the survival rate of the mesenchymal stem cells.
Owner:杜德(江门)生物科技有限公司
Human fat mesenchymal stem cell transportation fluid and preparation method thereof
The invention discloses a human fat mesenchymal stem cell transportation fluid and a preparation method thereof. The transportation fluid is prepared from the following components in percentage by mass: 30-50 percent of fetal calf serum, 10-20 percent of DMSO, 5-10 percent of 0.2% heparin calcium solution and 20-40 percent of 0.2% trehalose solution. The human fat mesenchymal stem cells are storedby adopting the transportation fluid, the cell death speed can be slowed, and the proliferation and differentiation activity of the fat mesenchymal stem cells can be maintained.
Owner:JIANGSU RE STEM BIOTECH
Culture method for promoting proliferation and differentiation of mesenchymal stem cells and serum-free culture medium
ActiveCN113249314AReduce clinical riskImprove securityCulture processSkeletal/connective tissue cellsHuman plateletVitamin C
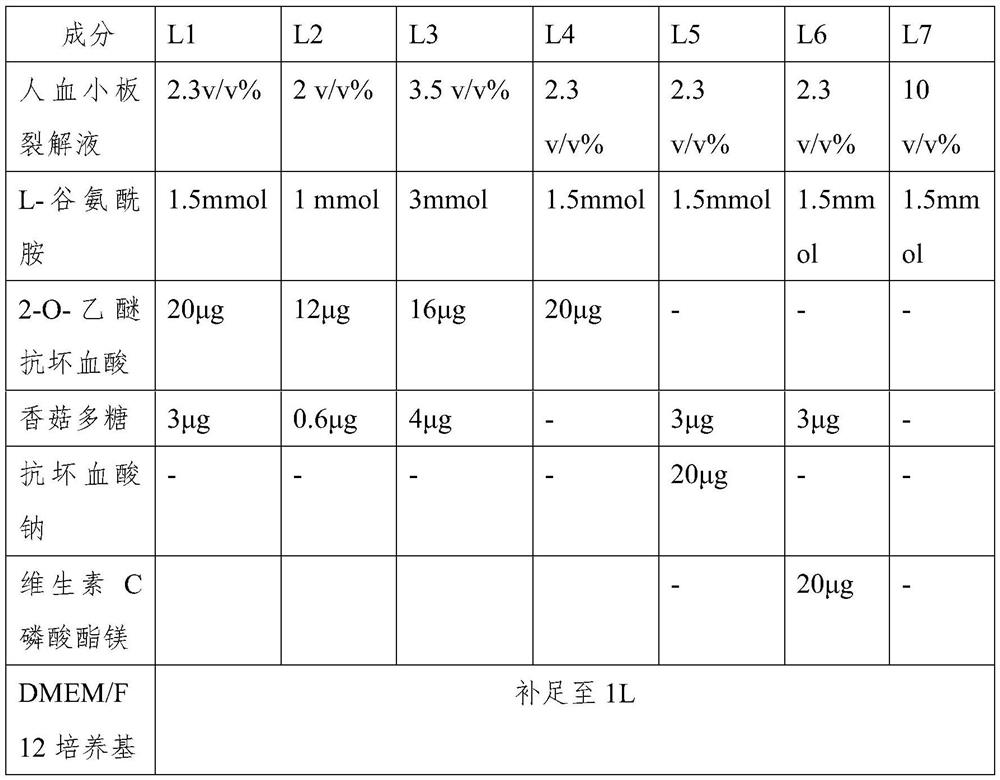
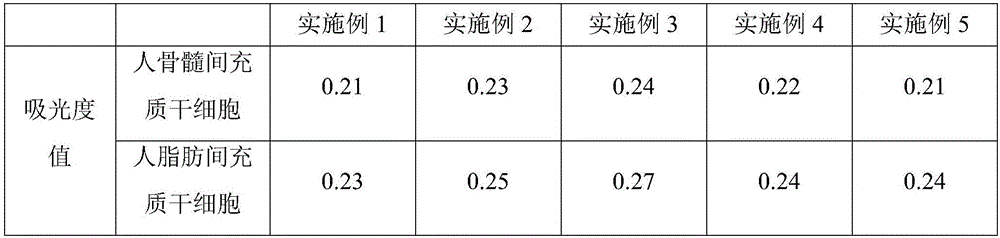
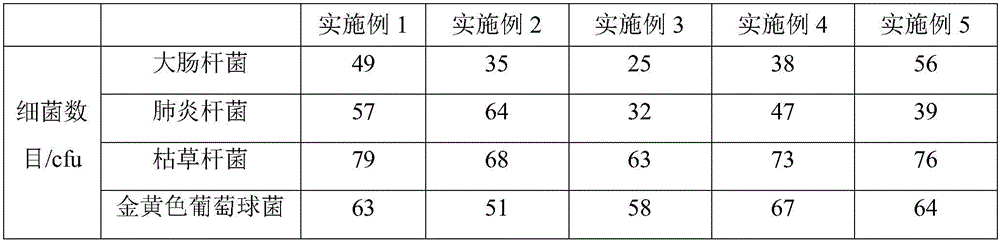
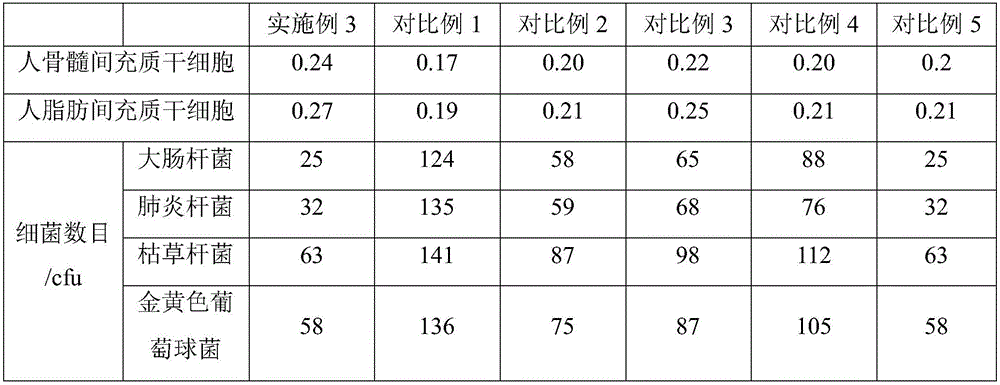
The invention belongs to the technical field of stem cell culture, and particularly relates to a culture method for promoting proliferation and differentiation of mesenchymal stem cells and a serum-free culture medium. The serum-free culture medium is prepared from the following components: 0.1%-3.5% of human platelet lysate, 1-3mmol / L of L-glutamine, 5-30[mu]g / mL of vitamin C derivative and the balance of a basic culture medium, wherein the total volume is 1L. According to the serum-free culture medium, lentinan and 2-O-diethyl ether ascorbic acid are added, so that the requirement of in-vitro continuous multiplication culture of the mesenchymal stem cells can be met by only adopting a platelet lysis solution with relatively low concentration, the proliferation and high differentiation performance of the mesenchymal stem cells can be well maintained, so that the culture cost is greatly reduced, and meanwhile, the clinical application risk is reduced.
Owner:徐飞
Traditional Chinese medicinal complex and preparation method thereof and human mesenchymal stem cell medium
InactiveCN106566805APromote proliferationImprove antibacterial propertiesCulture processSkeletal/connective tissue cellsStem cell cultureCholesteryl myristate
Owner:浙江译美生物科技有限公司
Rapid induced differentiation method of mesenchymal stem cells, kit and application of rapid induced differentiation method
InactiveCN113462642AImprove proliferative abilityEnergeticNervous disorderSkeletal disorderCell activityMesenchymal stem cell proliferation
The invention discloses a rapid induced differentiation method of mesenchymal stem cells, a kit and application thereof. The rapid induced differentiation method of mesenchymal stem cells provided by the invention can be used for preparing enough number of mesenchymal stem cells capable of meeting clinical requirements in a short time at high yield, because there is no step of forming an embryoid body, the preparation process is simple, and the method has the advantages of high differentiation efficiency, convenience in purification and separation and the like. In addition, animal serum and other non-human additives are not involved in the differentiation process, the mesenchymal stem cells are more suitable for clinical requirements, and the mesenchymal stem cells obtained through differentiation are high in multiplication capacity and vigorous in cell activity.
Owner:CHENGNUO REGENERATIVE MEDICINE TECH (ZHUHAI HENGQIN NEW AREA) CO LTD
Novel inducer of chondrocyte proliferation and differentiation
ActiveUS20110177069A1Acceleration of prechondrocyteIncrease differentiationCompound screeningApoptosis detectionRANKLMesenchymal stem cell proliferation
This invention provides a method for administration of an effective amount of RANKL-binding molecules that act on prechondrocytes and / or mesenchymal stem cells, accelerate cartilage differentiation, proliferation, and maturation of such cells, enhance chondrocyte differentiation, and induce chondrocyte proliferation to induce chondrocyte proliferation and differentiation or increase cartilage matrix production and a pharmaceutical composition used for inducing chondrocyte proliferation and differentiation or increasing cartilage matrix production. The pharmaceutical composition used for treatment or prevention of a chondropathies comprises, as an active ingredient, a compound that acts on prechondrocytes and / or mesenchymal stem cells and induces at least one of the following: (a) acceleration of prechondrocyte and / or mesenchymal stem cell differentiation; (b) acceleration of prechondrocyte and / or mesenchymal stem cell proliferation; (c) acceleration of prechondrocyte and / or mesenchymal stem cell maturation; (d) enhancement of chondrocyte differentiation; (e) chondrocyte proliferation; and (f) increased production of the cartilage matrix.
Owner:ORIENTAL YEAST
Vitamin C-containing culture medium for promoting umbilical cord mesenchymal stem cell proliferation
ActiveCN110734893APromote proliferationIncrease the number ofCulture processSkeletal/connective tissue cellsVitamin CCell activity
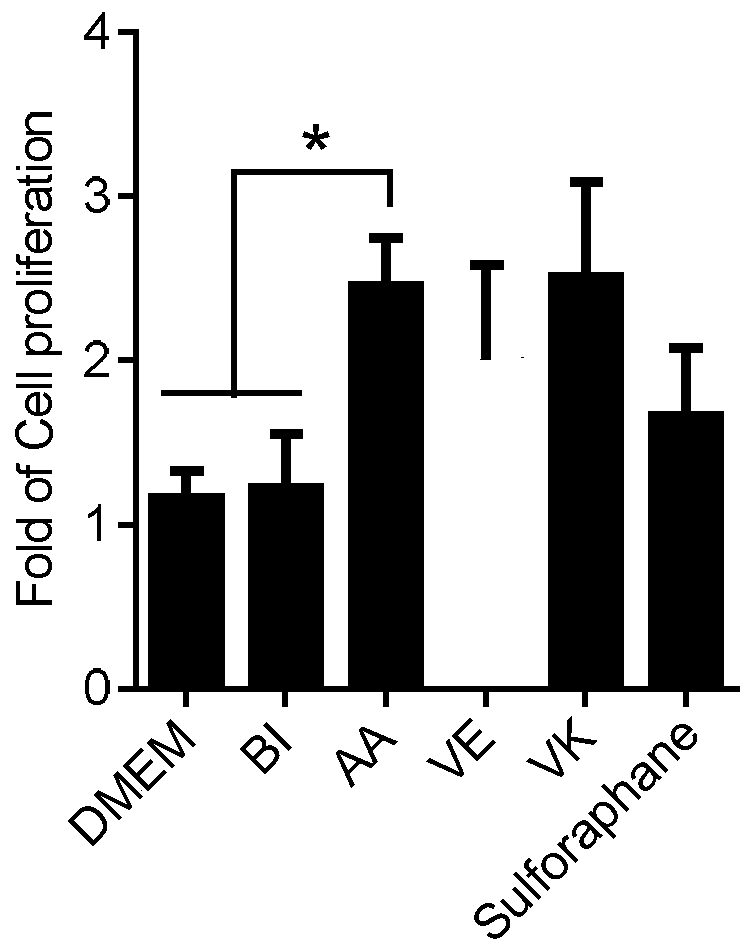
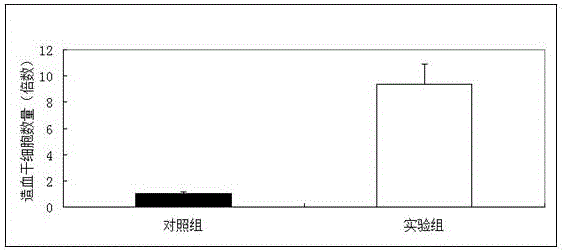
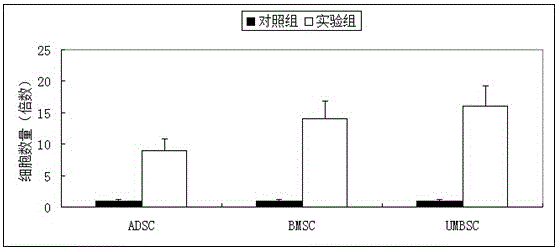
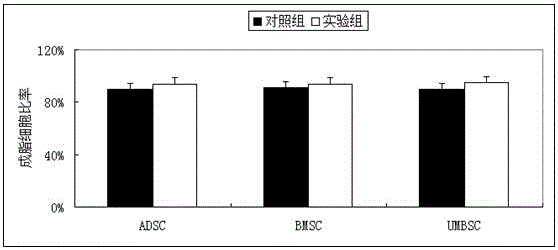
The invention discloses application of vitamin C in promoting umbilical cord mesenchymal stem cell proliferation without influencing stem cell activity, viability, differentiation and senescence, andapplication of vitamin C in preparing a culture medium for promoting umbilical cord mesenchymal stem cell proliferation without influencing stem cell activity, viability, differentiation and senescence. A phenomenon is found that after a current stem cell culture medium is adopted to achieve stem cell adherence, AA is added (without other complex components being added), and then proliferation ofstem cells can be promoted. Through data, a result is proven that although AA can significantly increase the quantity of the stem cells within a short period of time, the activity, viability and cellcycle of the stem cells are not different from those of stem cells cultured in a control culture medium without AA. Therefore, a fact is illustrated that AA supplementation can simply promote cell proliferation, not reduce the activity and viability of the stem cells, and not promote stem cell senescence.
Owner:华辰未来(北京)生物医学技术有限公司
Microelement composition having activating function on stem cells and application thereof
The invention discloses a microelement composition having activating function on stem cells and application thereof. The composition is prepared from 0.1-2 mmol of iron, 1-10 micromoles of copper, 1-20 micromoles of zinc, 5-100 micromoles of cobalt, 5-50 micromoles of manganese, 1-10 micromoles of chromium, 1-10 nmol of selenium, 0.1-50 micromoles of iodine, 0.1-10 micromoles of nickel, 0.1-10 micromoles of molybdenum, 0.1-5 micromoles of vanadium, 1-10 nmol of tin, 1-30 mmol of silicon, 5-50 micromoles of strontium, 0.1-10 micromoles of boron, 5-100 micromoles of rubidium, 1-50 micromoles of lithium, 10-100 nmol of germanium, 1-10 mmol of titanium and 1-300 micromoles of tungsten. The microelement composition having the activating function on the stem cells is provided and applied to activation culture of the stem cells. Experiment results find that the microelement composition has the functions of promoting proliferation of hematopoietic stem cells and mesenchymal stem cells in vitro and does not influence the differentiation potential of the mesenchymal stem cells, and besides microelements have the functions of promoting activation and proliferation of stem cells in mammal bodies like mice.
Owner:苏州博棠再生医学科技有限公司
Method for promoting proliferation of human mesenchymal stem cells
InactiveCN108949683APromote proliferationDoes not affect multilineage differentiation abilityCulture processSkeletal/connective tissue cellsMesenchymal stem cell proliferationOsteocyte
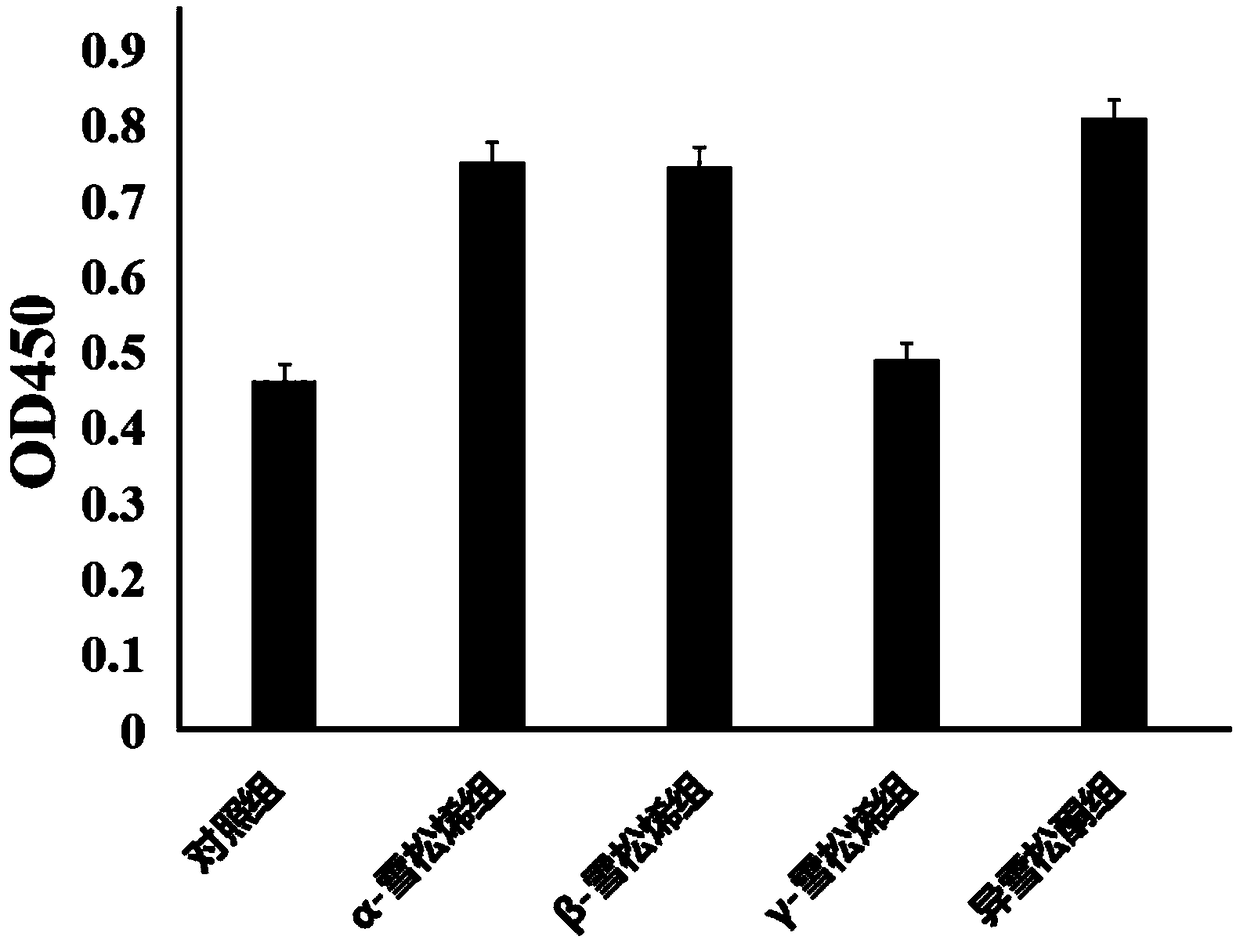
The invention discloses a method for promoting proliferation of human mesenchymal stem cells. The human mesenchymal stem cell is a progenitor cell of osteoblast in bone marrow, research on the osteogenic differentiative capacity of the human mesenchymal stem cell is a hotspot in the orthopedic field, and the method for promoting the stem cell to differentiate toward osteogenesis is a main method for preventing and treating osteoporosis. Currently, application of the mesenchymal stem cell is difficult in massively acquiring mesenchymal stem cells. The method discovers that alpha-cedrene, beta-cedrene and isocedrone can be used for effectively promoting reproduction of human mesenchymal stem cells without influencing the multi-directional differentiation, can be used as additives to preparea culture medium for promoting reproduction of the human mesenchymal stem cells, and can be used for preparing medicines for promoting reproduction of the human mesenchymal stem cells.
Owner:淮安康宜莱干细胞生物科技有限公司
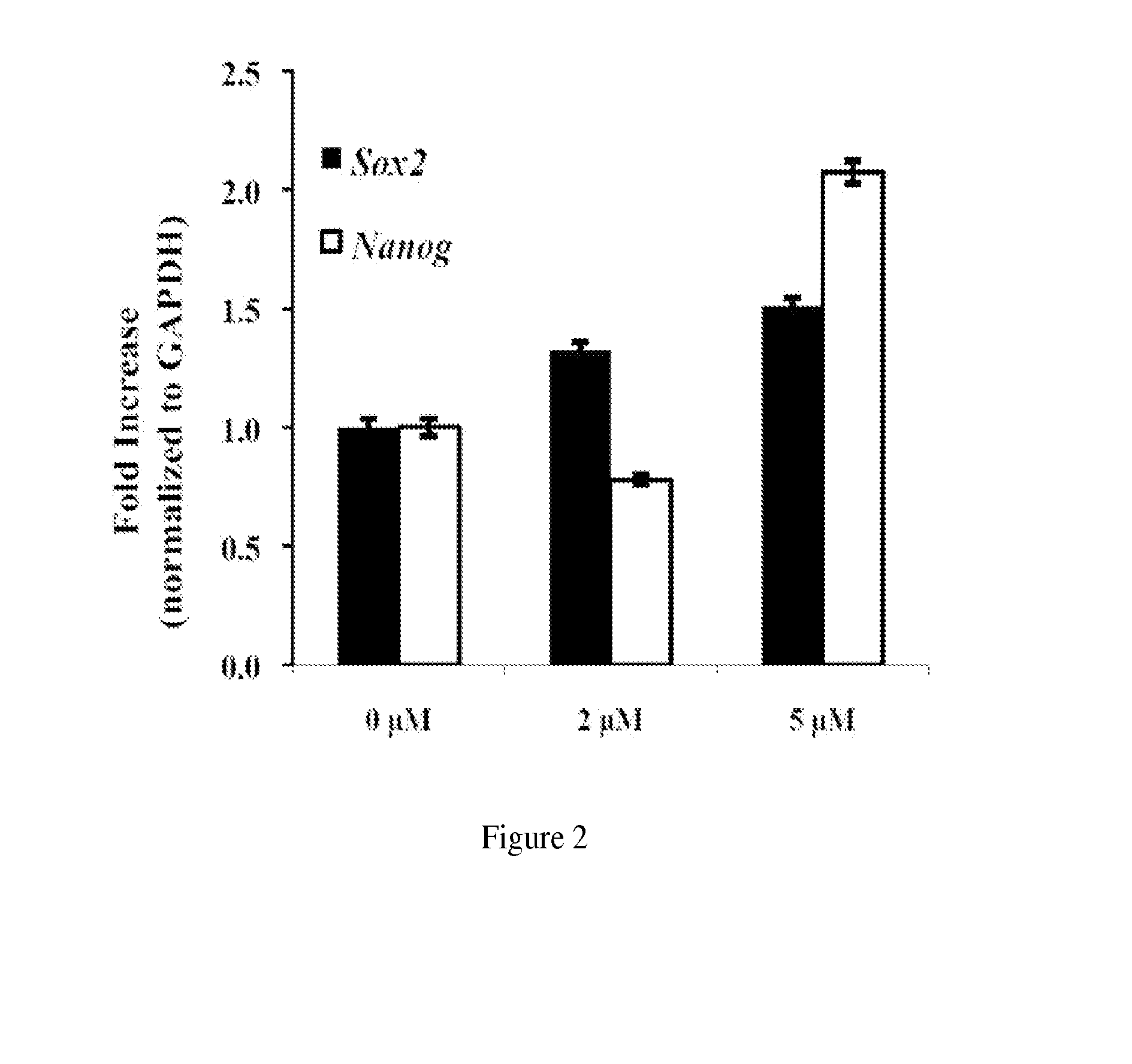
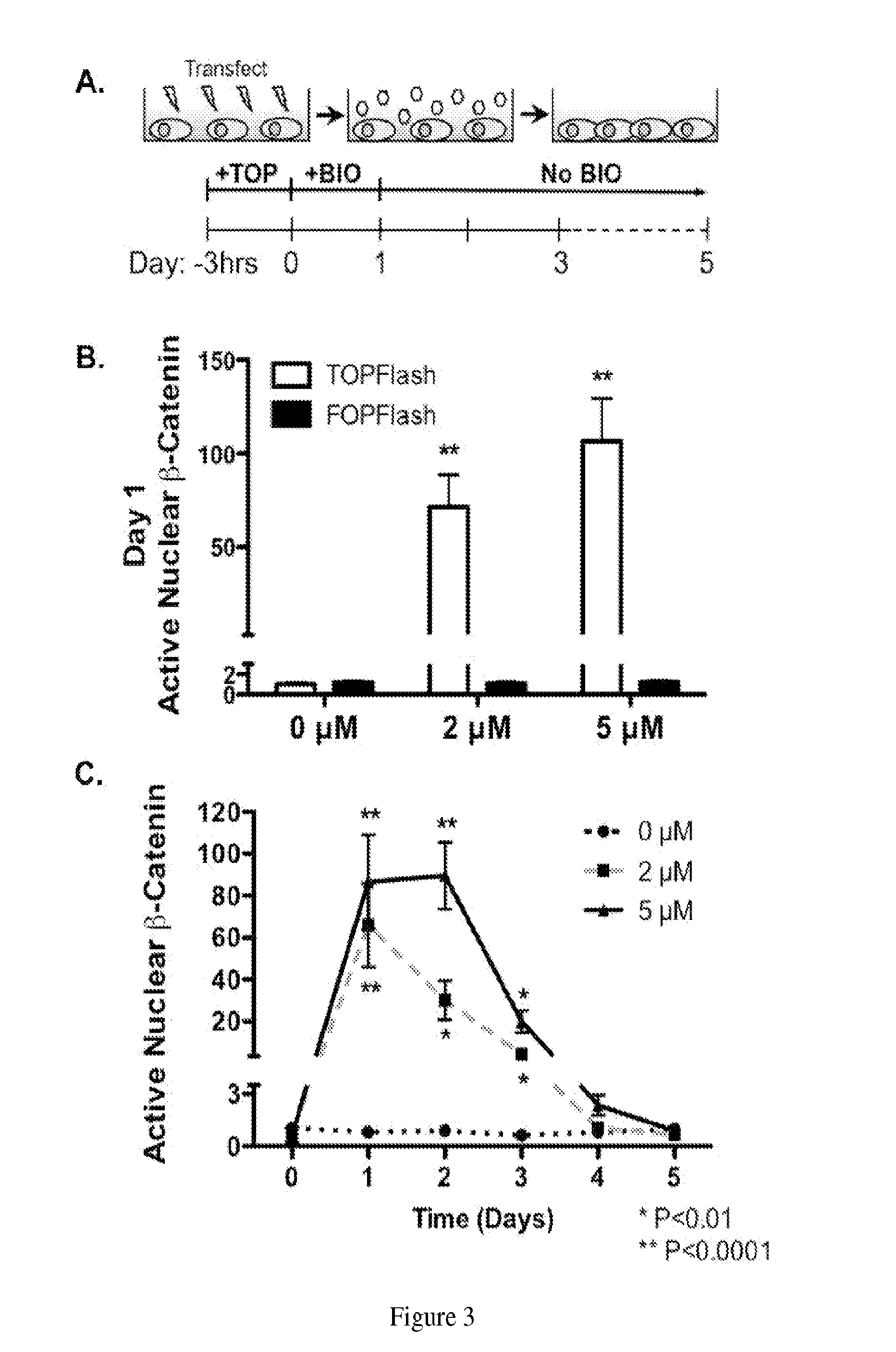
Methods and compositions for mesenchymal stem cell proliferation
InactiveUS20140234960A1Promote healingRobust expansionArtificial cell constructsSkeletal/connective tissue cellsSOX2Mesenchymal stem cell proliferation
Provided is a method for increasing proliferation of mesenchymal stem cells. The method entails contacting the cells with 6-bromoindirubin-3′-oxime (BIO). This results in robust proliferation and “sternness” in a dose-dependent fashion, as indicated by the gene expression of two stem cell genes: Sox2 and Nanog.
Owner:UNIVERSITY OF ROCHESTER
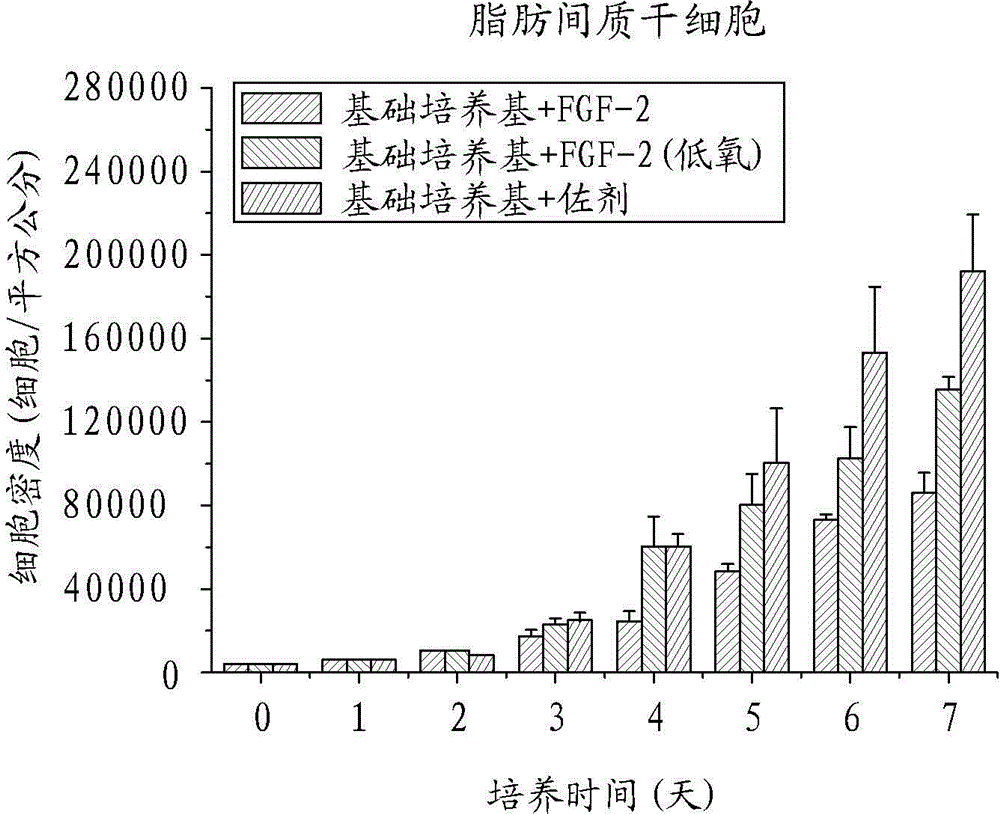
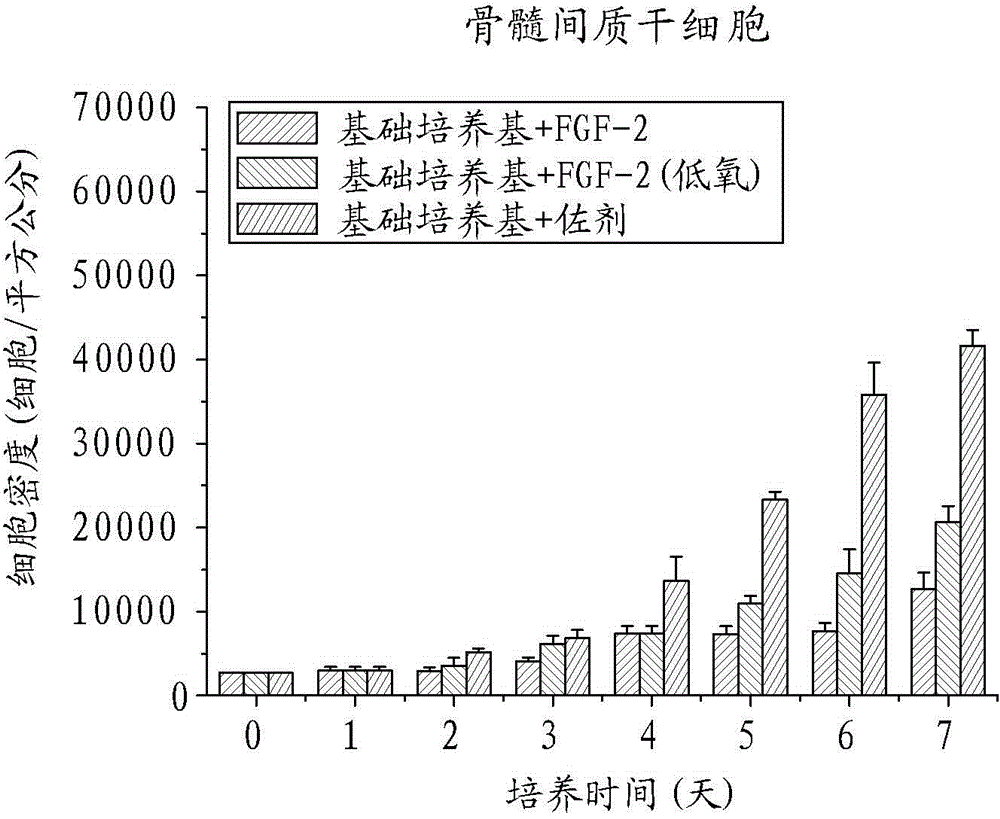
Human mesenchymal stem cell proliferation promotion adjuvant, human mesenchymal stem cell amplification, pharmaceutical composition, growth factor and use
ActiveCN104450608AThe ratio of cycle synthesis period (S phase) is increasedRaise the ratioCosmetic preparationsPeptide/protein ingredientsCell divisionAdjuvant
The invention relates to a human mesenchymal stem cell in-vitro rapid proliferation adjuvant used to solve the problem of poor cell amplification efficiency of human mesenchymal stem cell culture. The human mesenchymal stem cell culture adjuvant comprises at least an antioxidant and a second type fibroblast growth factor (FGF-2). Thereby, after the adjuvant is added into a medium containing human mesenchymal stem cells for culture in normoxic environment (with the oxygen partial pressure being about 21%), phenomena such as rapid cell division, increase of cell cycle period synthesis phase proportion, reduction of aging, improvement of the differentiation potential, and the like, the human mesenchymal stem cell in-vitro rapid proliferation adjuvant can not only rapidly expand the human mesenchymal stem cells and obtain the growth factor, can still keep the more functional characteristic of the stem cells, and can be used for the purposes of human mesenchymal stem cell culture and amplification.
Owner:BUDDHIST TZU CHI GEN HOSPITAL
Application of nuezhenoside and specnuezhenide to facilitation of multiplication of in-vitro-cultured bone marrow-derived mesenchymal stem cell and inhibition of replicative senescence
ActiveCN108823158AReduce immune rejectionPromote proliferationSkeletal/connective tissue cellsCell culture active agentsLiver fibrosisBiology
The invention discloses application of nuezhenoside and specnuezhenide to facilitation of multiplication of an in-vitro-cultured bone marrow-derived mesenchymal stem cell and inhibition of replicativesenescence. The nuezhenoside and the specnuezhenide are coexistent, the nuezhenoside and the specnuezhenide have the effects of facilitating the multiplication of the in-vitro-cultured bone marrow-derived mesenchymal stem cell and inhibiting replicative senescence, and provide sufficient stem cells for clinical treatment, wherein the nuezhenoside and the specnuezhenide are commercially availablehigh-purity nuezhenoside and specnuezhenide (content is larger than or equal to 98 percent), and can be prepared according to a conventional method. The application disclosed by the invention has theadvantages that the nuezhenoside and the specnuezhenide can be used for facilitating the multiplication of the bone marrow-derived mesenchymal stem cell, and inhibiting the replicative senescence, sufficient cells are provided for tissue engineering and stem cell treatment of bone defect, cartilage defect, liver fibrosis, myocardial infarction, pulmonary fibrosis, diabetes and the like, and meanwhile, body immunological rejection reaction is reduced.
Owner:ZHEJIANG UNIV
Serum-free polypeptide composition for promoting proliferation of mesenchymal stem cells
ActiveCN109943526AEasy to synthesizePromote proliferationCulture processSkeletal/connective tissue cellsSerum igeChemical composition
The invention provides a serum-free polypeptide composition for promoting proliferation of mesenchymal stem cells. Each liter of the serum-free polypeptide composition mainly comprises 10-100 micrograms of tripeptide-1, 1-20 micrograms of tripeptide-2, 1-20 micrograms of hexapeptide-9, 1-20 micrograms of palmitoyl hexapeptide-12 and 10-100 micrograms of laminin-derived peptide. The serum-free polypeptide composition contains clear chemical components without animal sources or serum, and can realize rapid proliferation of mesenchymal stem cells, solve the problem of insufficient cell quantity and maintain the biological characteristics and immunophenotypic stability of mesenchymal stem cells.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Non-genetically modified light control bidirectional regulation method for human umbilical cord mesenchymal stem cell proliferation
ActiveCN108624554AObviously positiveThe effect of obvious negative regulationSkeletal/connective tissue cellsElectrical/wave energy microorganism treatmentUmbilical cordMedical treatment
The invention relates to a non-genetically modified light control bidirectional regulation method for human umbilical cord mesenchymal stem cell proliferation. The method includes the steps: irradiating human umbilical cord mesenchymal stem cells at 95-105mm positions under a collimating mirror for 85-95min through the collimating mirror by the aid of blue light under the conditions of the light wavelength of 400-480nm and the light intensity of 95-105 micro-w / cm<2>, and performing negative regulation; or irradiating the human umbilical cord mesenchymal stem cells at the 95-105mm positions under the collimating mirror for 115-125min through the collimating mirror by the aid of blue light, and performing positive regulation. According to the method, the multiplication speed of the human umbilical cord mesenchymal stem cells is firstly and bi-directionally regulated only through blue light irradiation under setting of special parameters, foreign genes transferred into the stem cells areomitted, virus transfection vectors are omitted, any other substances are omitted, and the method more meets clinical application requirements of 'noninvasive light therapy' and has a wide clinical application prospect.
Owner:SHANDONG UNIV
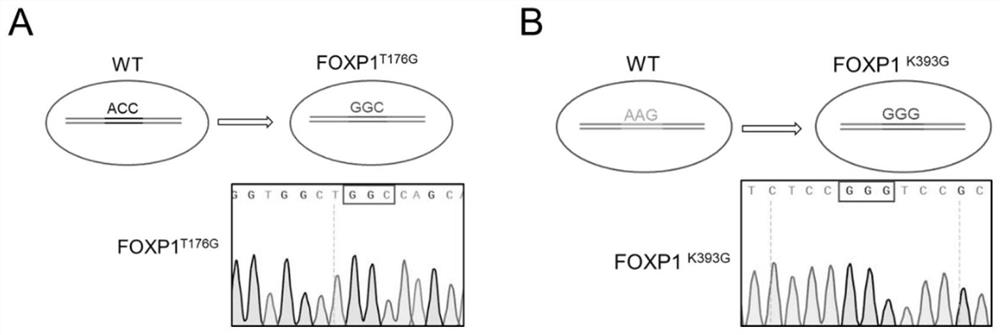
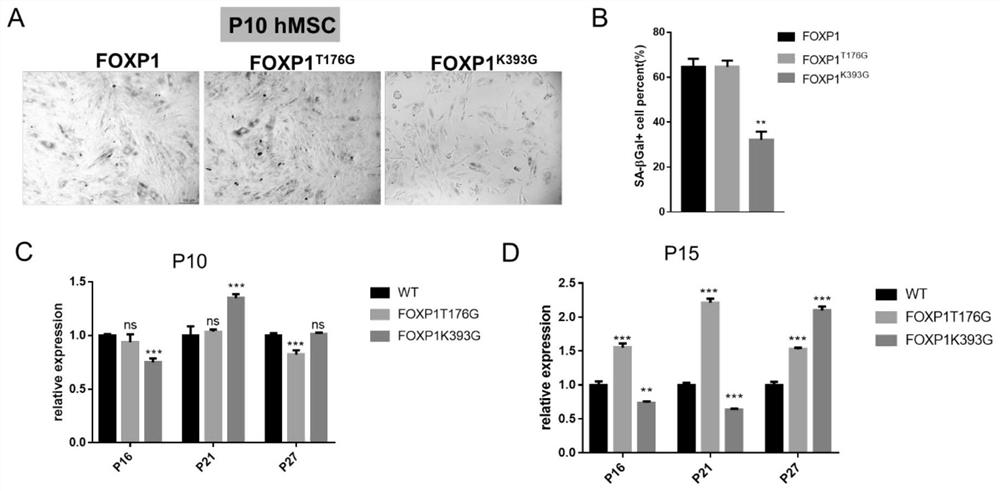
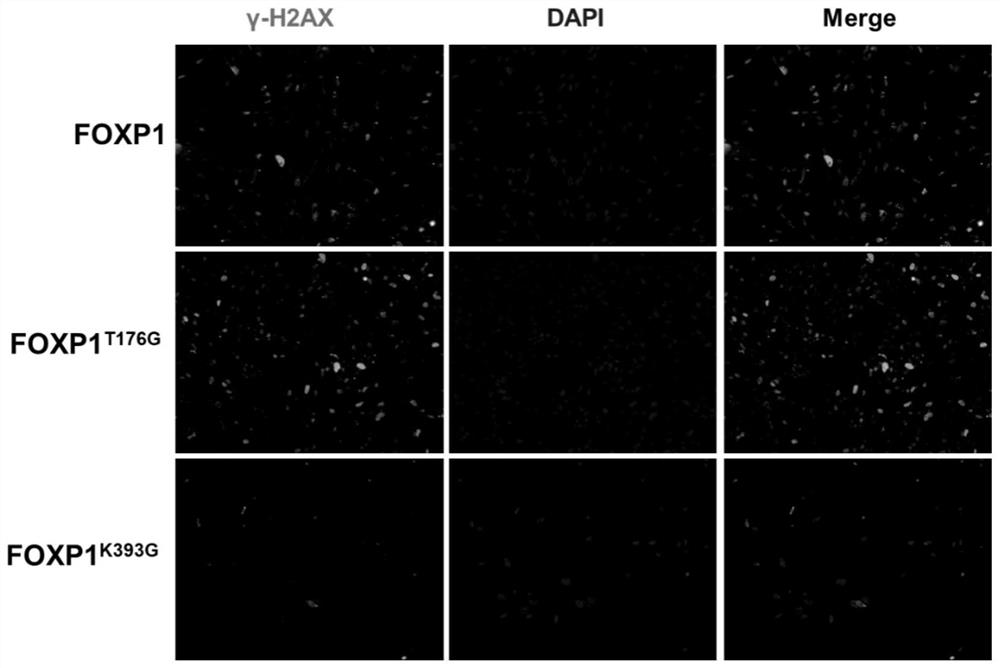
Method for delaying senescence of mesenchymal stem cells through FOXP1 gene editing and mutation
ActiveCN112941106AProliferate fastDelay and resist agingHydrolasesMicroencapsulation basedTransfer cellLipofectamine
The invention relates to the technical field of cell biology, in particular to a method for delaying senescence of mesenchymal stem cells through FOXP1 gene editing and mutation. The method includes the following steps: (1) separating and culturing human umbilical cord mesenchymal stem cells; (2) adding plasmids, donor DNA (deoxyribonucleic acid) and lipidosome into the culture solution obtained in the step (1) for transfection; (3) transferring the human umbilical cord mesenchymal stem cells transfected in the step (2) into a human umbilical cord mesenchymal stem cell culture medium containing penicillin and streptomycin, and performing culturing; (4) carrying out screening culture on the human umbilical cord mesenchymal stem cells cultured in the step (3); and (5) carrying out digestion passage on the human umbilical cord mesenchymal stem cells cultured in the step (4), and performing amplifying until stably transferred cell strains are obtained. Compared with the prior art, the method has the advantages that the proliferation speed of the mesenchymal stem cells can be obviously promoted by one time, and the cell senescence process can be delayed by 50%.
Owner:安可来(重庆)生物医药科技有限公司
Pharmaceutical composition for preventing or treating regulatory t cell-mediated diseases
ActiveUS20190022144A1Effectively inhibiting proliferationPrevent and treat and ameliorate T diseaseDigestive systemSkeletal/connective tissue cellsDiseaseRegulatory T cell
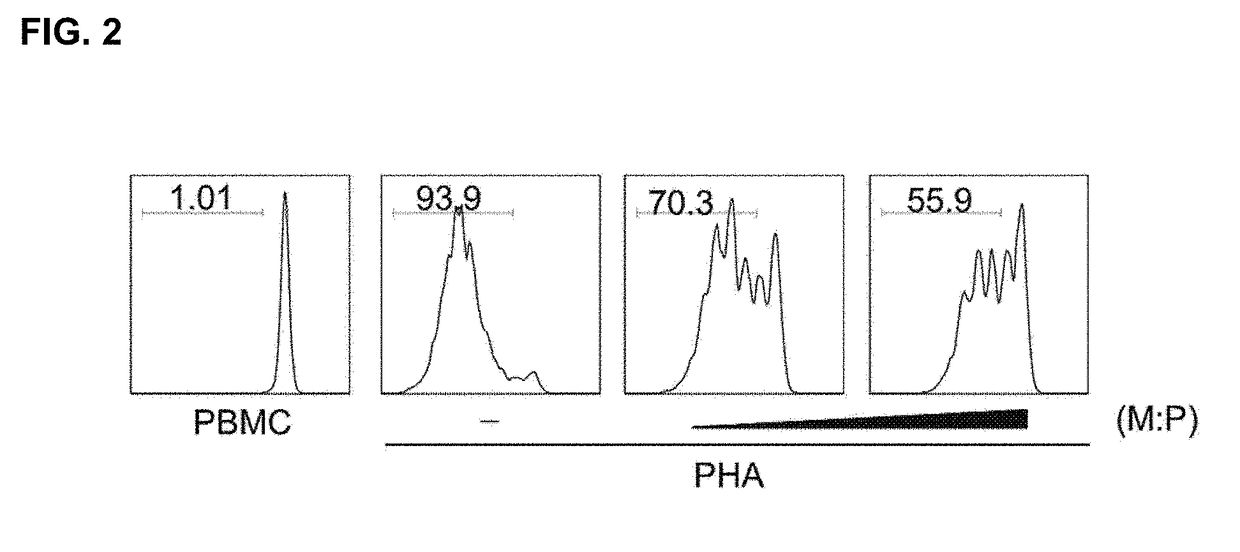
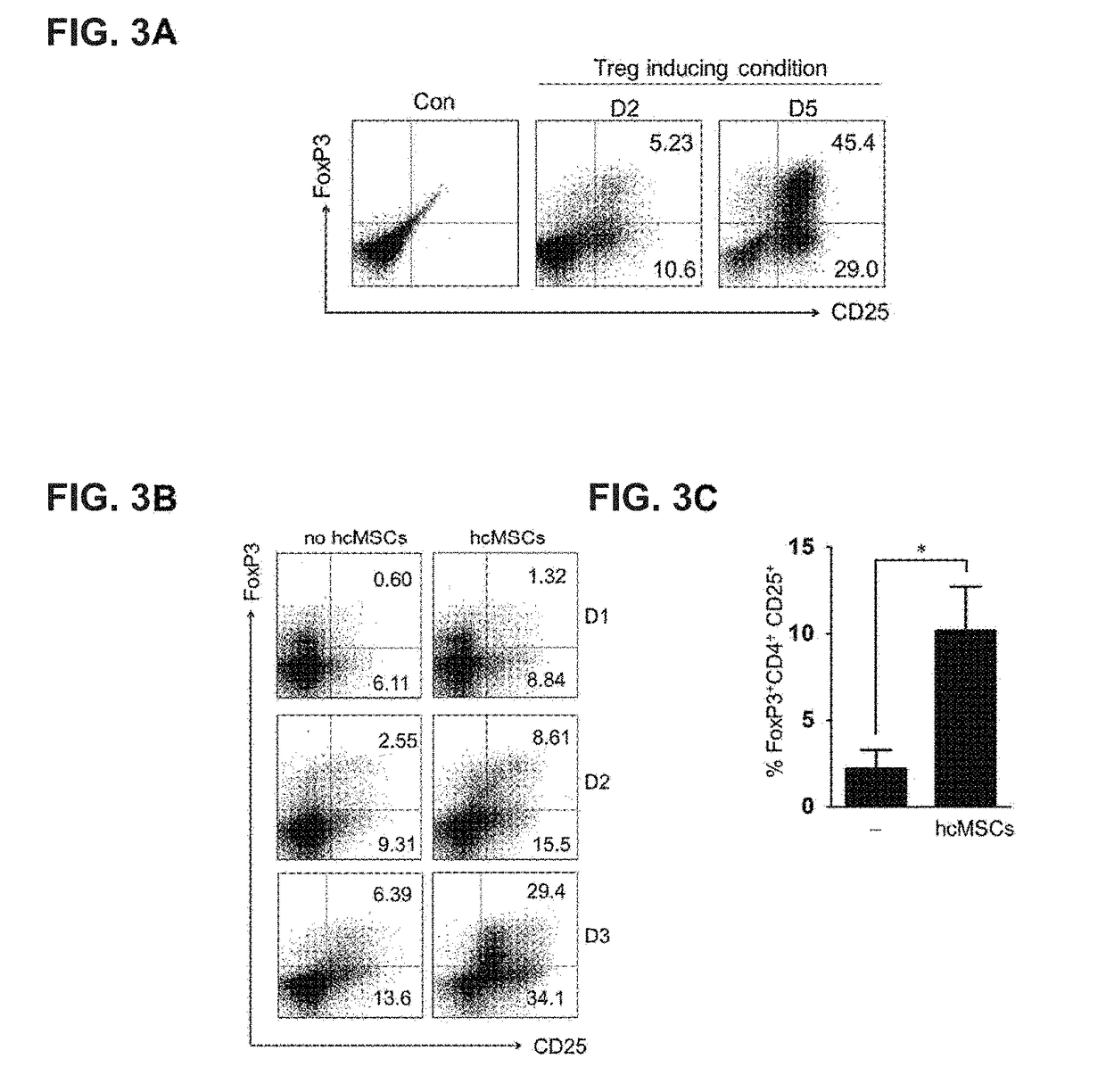
The present invention relates to a composition and a method for inducing CD4+ T cells to differentiate into regulatory T cells and proliferate through an induced T cell co-stimulator ligand (ICOSL) or an ICOSL-overexpressing mesenchymal stem cell and for preventing or treating regulatory T cell-mediated diseases. The induced T cell co-stimulator ligand (ICOSL) or ICOSL-overexpressing mesenchymal stem cell according to the present invention effectively suppresses the proliferation of PBMCs, induces the expression of an ICOS in regulatory T cells, thereby inducing the differentiation and proliferation of the regulatory T cells through a PI3K-Akt mechanism, and thus can effectively prevent, treat, or enhance regulatory T cell-mediated diseases.
Owner:SCM LIFESCI CO LTD
Application of rehmannioside C and salvianolic acid A in promoting proliferation of bone marrow mesenchymal stem cells cultured in vitro and inhibiting replicative senescence
ActiveCN108949677AReduce immune rejectionPromote proliferationSkeletal/connective tissue cellsCell culture active agentsLiver fibrosisBiology
The invention discloses an application of rehmannioside C and salvianolic acid A in promoting proliferation of bone marrow mesenchymal stem cells cultured in vitro and inhibiting replicative senescence. The rehmannioside C and the salvianolic acid A coexist. The rehmannioside C and the salvianolic acid A has the functions of promoting the proliferation of the bone marrow mesenchymal stem cells cultured in vitro and inhibiting the replicative senescence, and provide enough stem cells for clinical treatment. The rehmannioside C and the salvianolic acid A are commercially available high-purity rehmannioside C and the salvianolic acid A (with the content being greater than or equal to 98%) or can be prepared according to a conventional method. The advantages of the rehmannioside C and the salvianolic acid A are that the rehmannioside C and the salvianolic acid A can promote the proliferation of the bone marrow mesenchymal stem cells and inhibit the replicative senescence, provide sufficient cells for tissue engineering for treatment of bone defects, cartilage defects, liver fibrosis, myocardial infarction, pulmonary fibrosis, diabetes and the like and stem cell therapy, and reduce bodyimmune rejection.
Owner:ZHEJIANG UNIV
Application and method for promoting adipose-derived mesenchymal stem cell proliferation and chondrogenic differentiation through b-D-Glucopyranoside
ActiveCN113583945AIncrease proliferative activityPromote proliferationCulture processSkeletal/connective tissue cellsDexamethasoneVitamin C
The invention discloses application and a method for promoting adipose-derived mesenchymal stem cell proliferation and chondrogenic differentiation by b-D-Glucopyranoside. The method comprises the following steps: S1, acquiring primary adipose-derived mesenchymal stem cells; S2, culturing the primary adipose-derived mesenchymal stem cells in vitro; S3, promoting proliferation: digesting second-generation full adipose-derived mesenchymal stem cell with 0.25% trypsin, when the cells grow and the fusion degree reaches 80%, adding 10ng / mL eucommia ulmoides glycoside into the basic culture medium, and continuously culturing in a 5% CO2 incubator at 37 DEG C for 24 hours; S4, promoting chondrogenic differentiation: taking second-generation full adipose-derived mesenchymal stem cells, digesting the adipose-derived mesenchymal stem cell with 0.25% trypsin, adding 10 ng / mL of b-D-Glucopyranoside, 10 ng / mL of TGF-beta3, 100 nmol / L of dexamethasone, 1% of ITS-G, 50 [mu] g / mL of vitamin C and 40 [mu] g / mL of proline into a basic culture medium, changing the liquid once every 2 days, and continuously performingculturing for 14 days. According to the method, proliferation and chondrogenic differentiation of the adipose-derived stem cells are promoted, meanwhile, the cost can be reduced, and the safety is good.
Owner:GUANGDONG SECOND TRADITIONAL CHINESE MEDICINE HOSPITAL (GUANGDONG PROVINCE ENG TECH RES INST OF TCM)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com