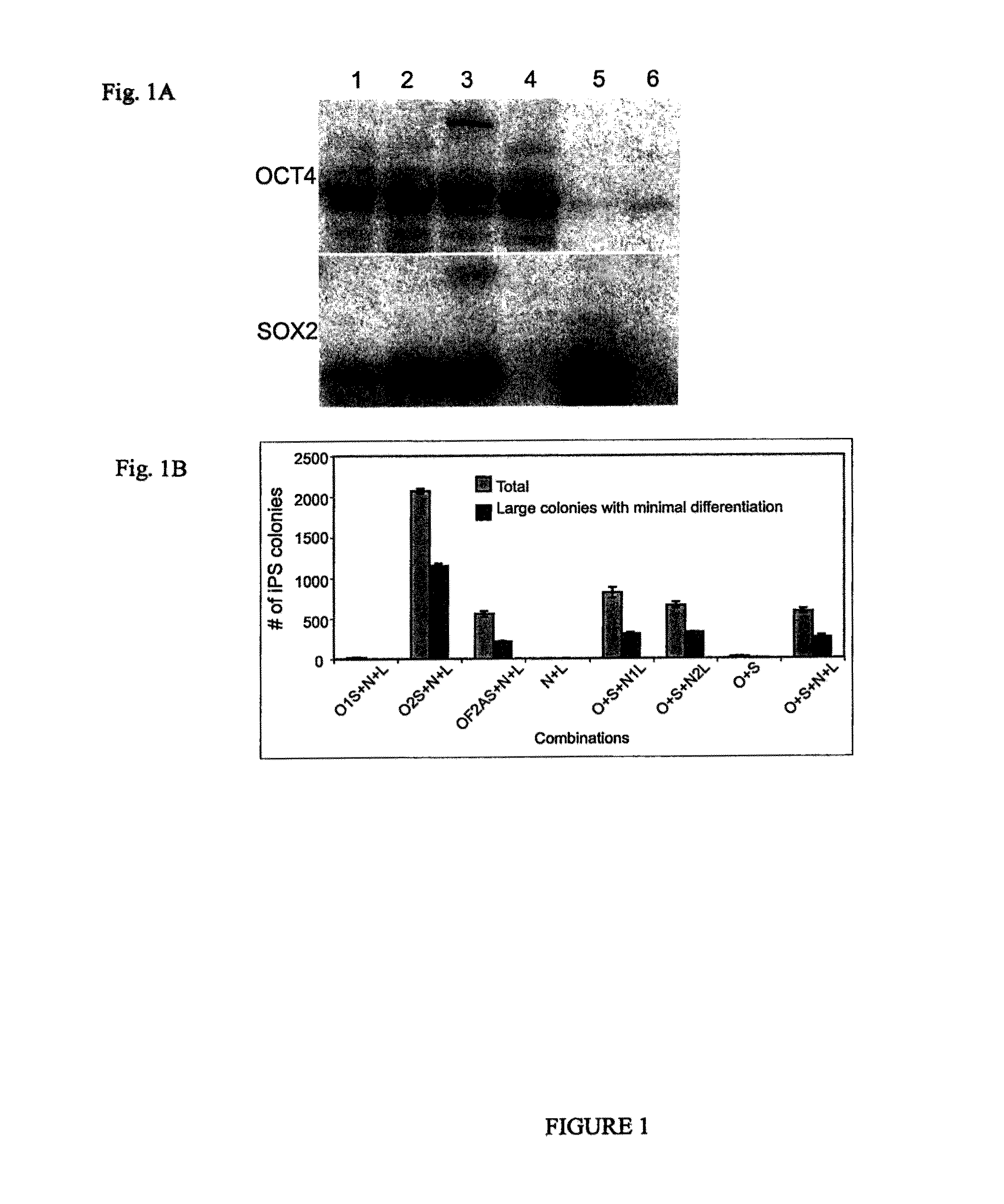
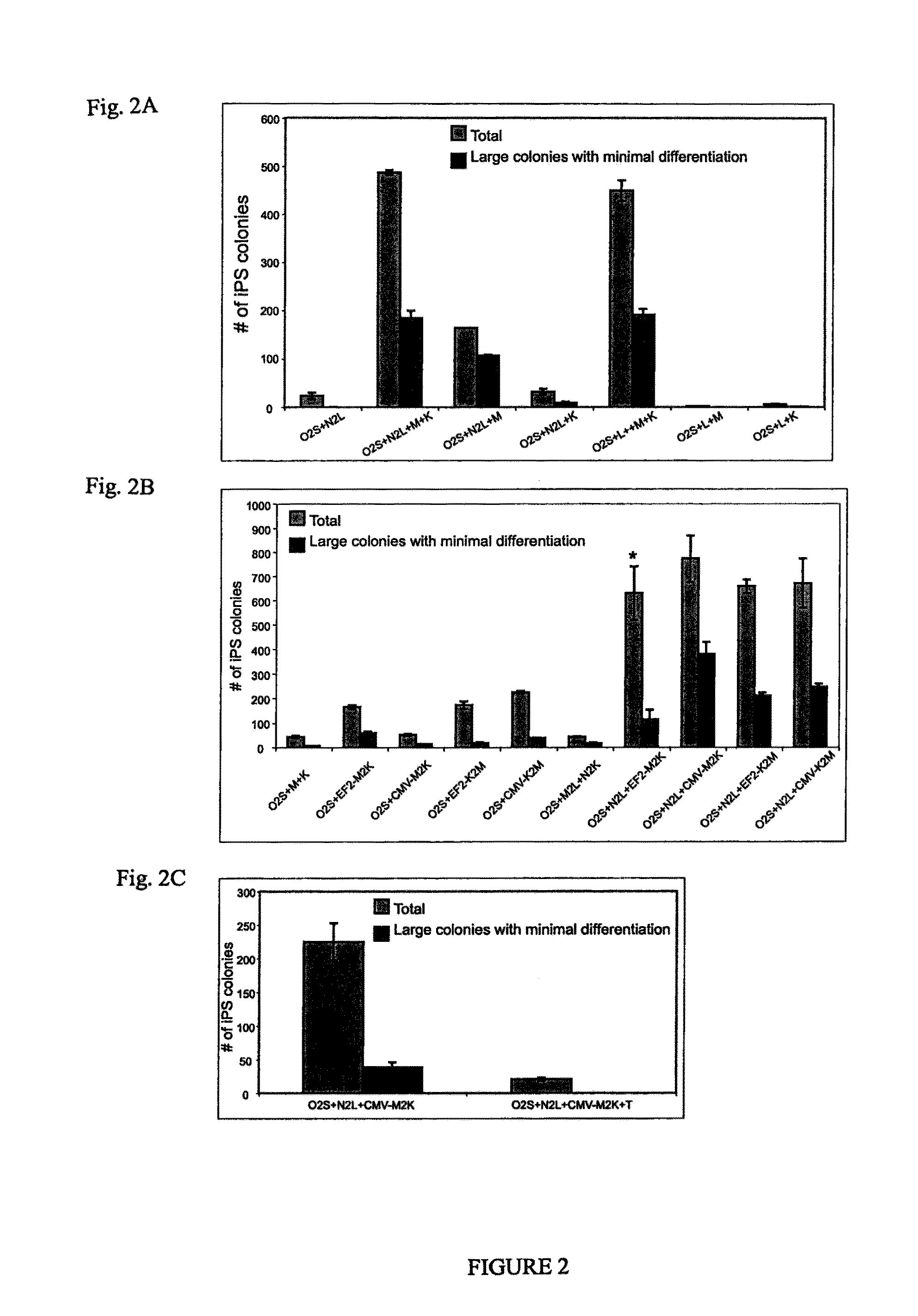
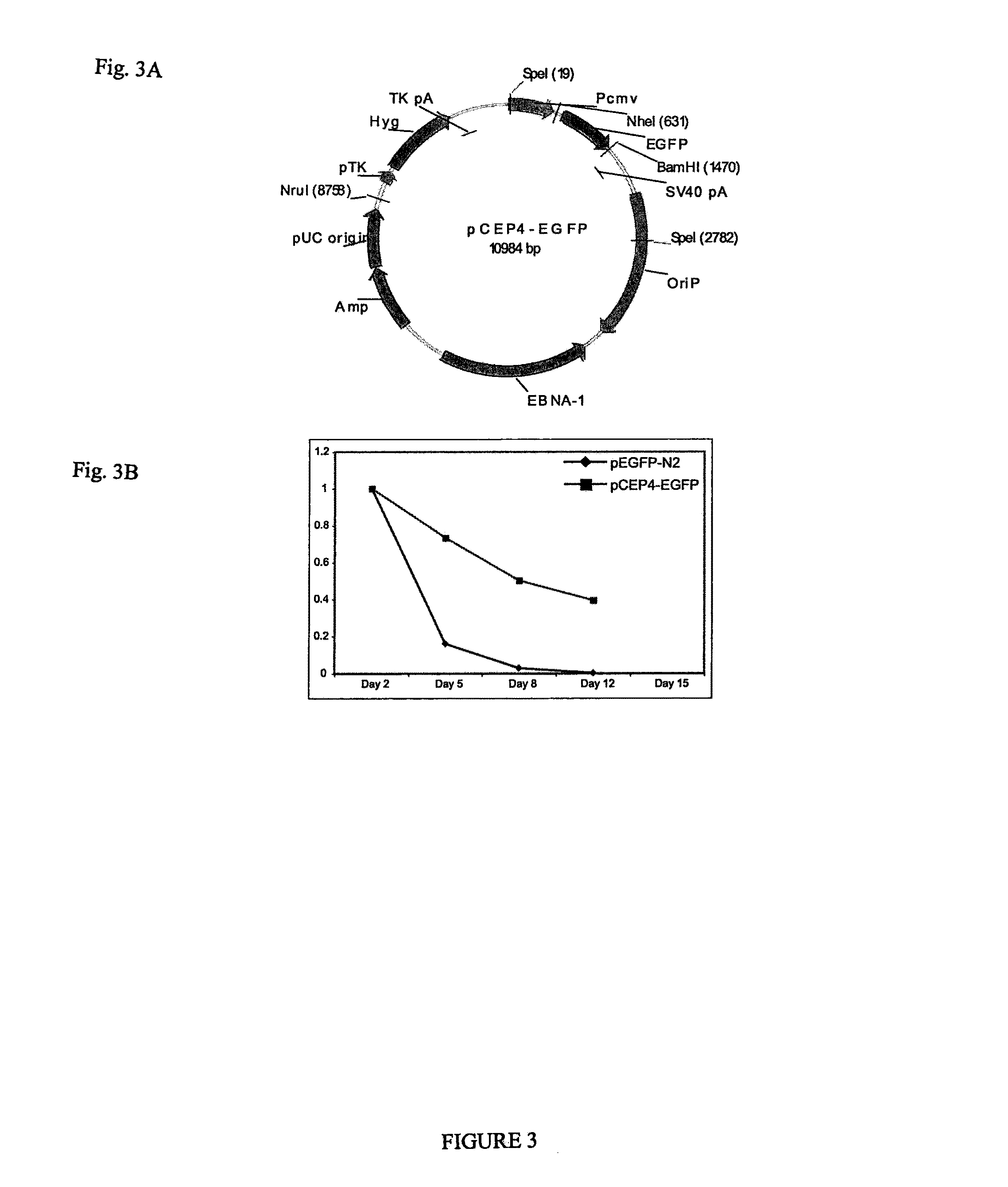
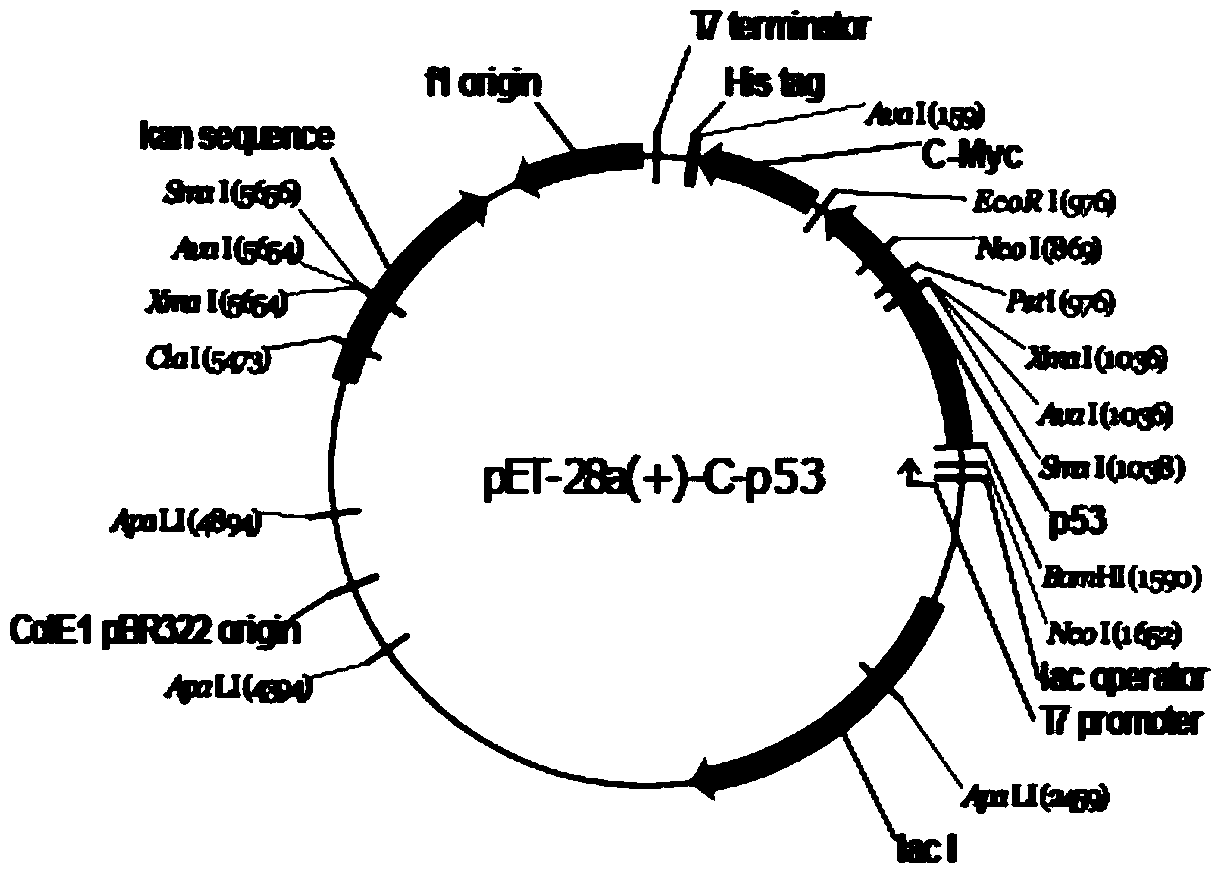
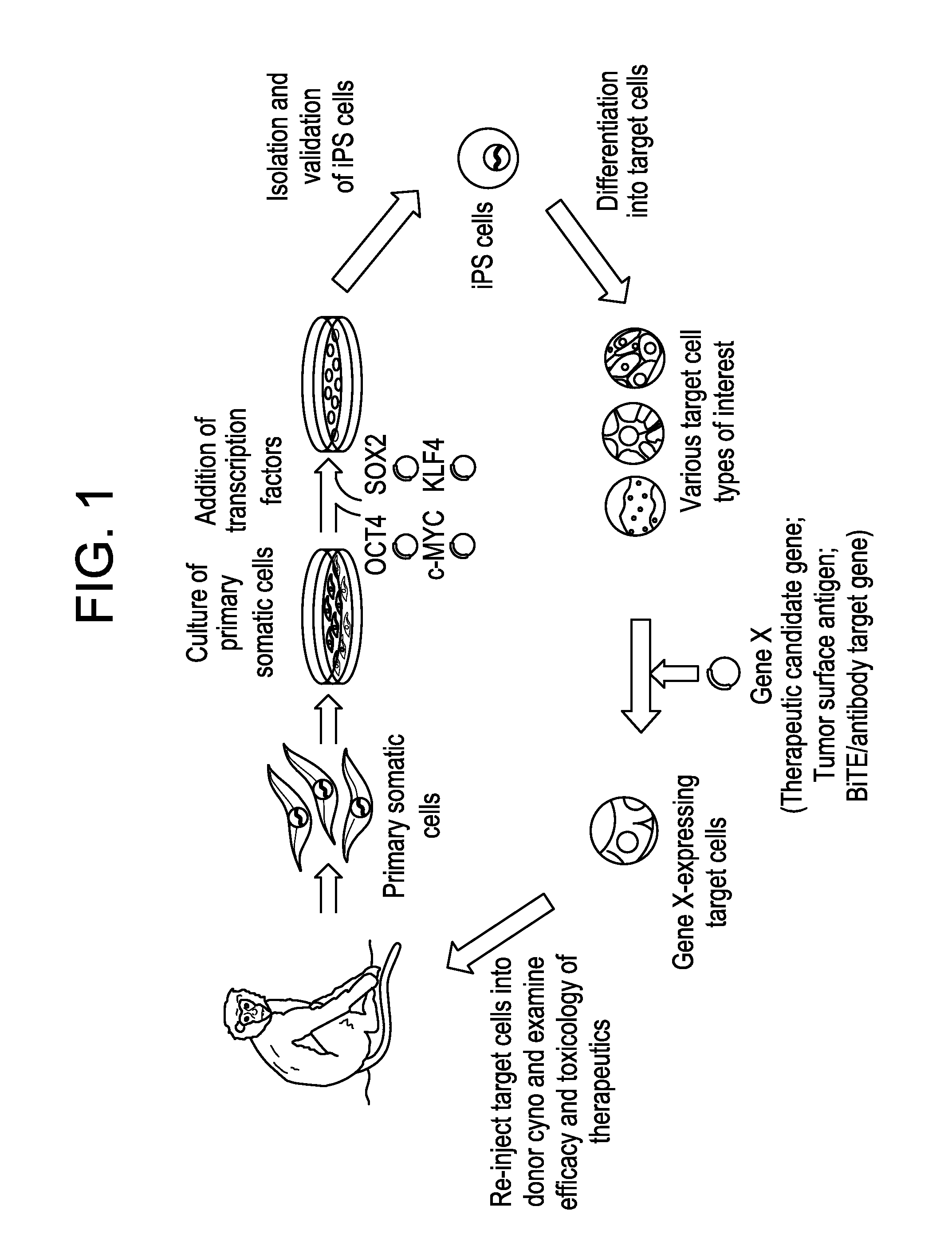
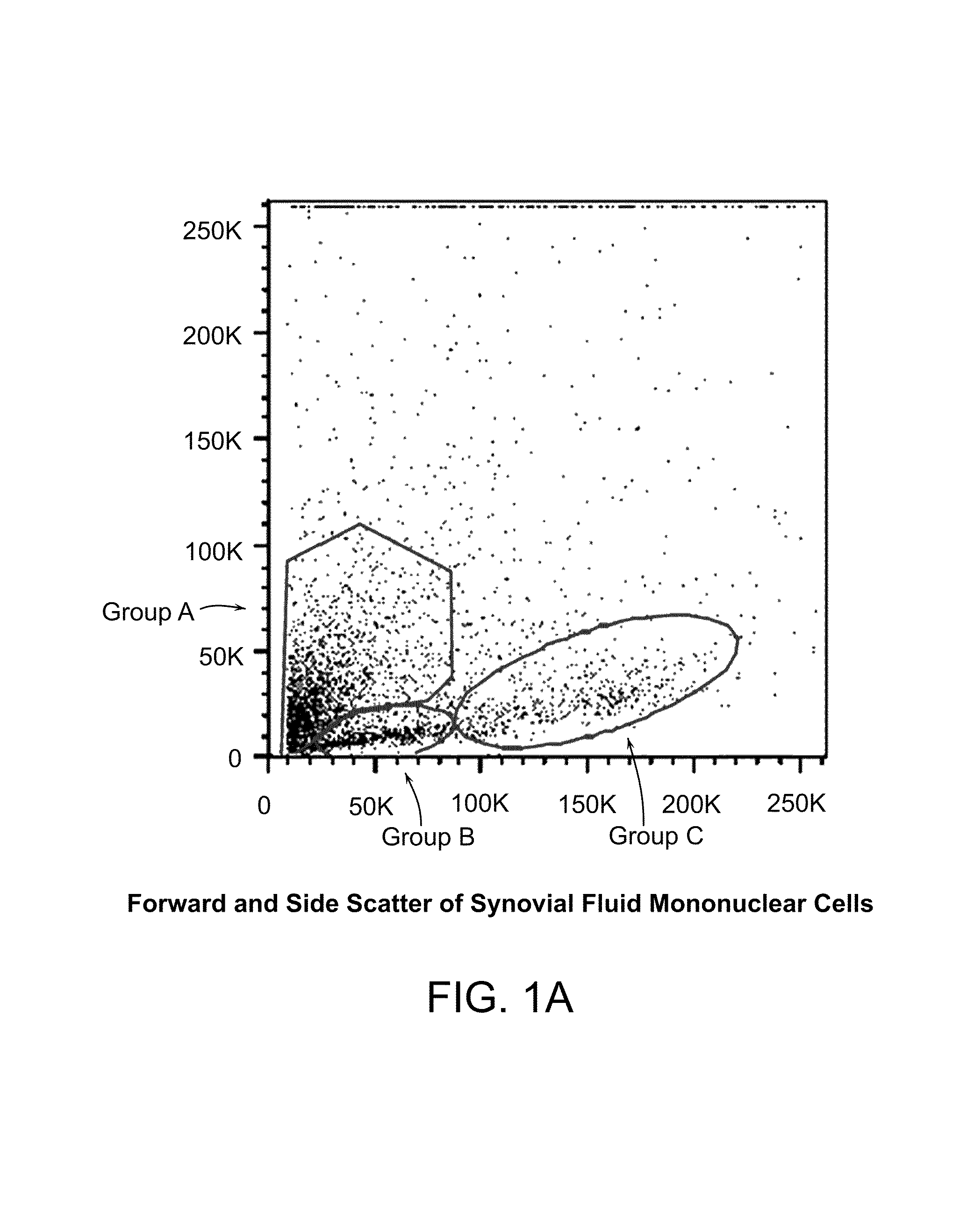
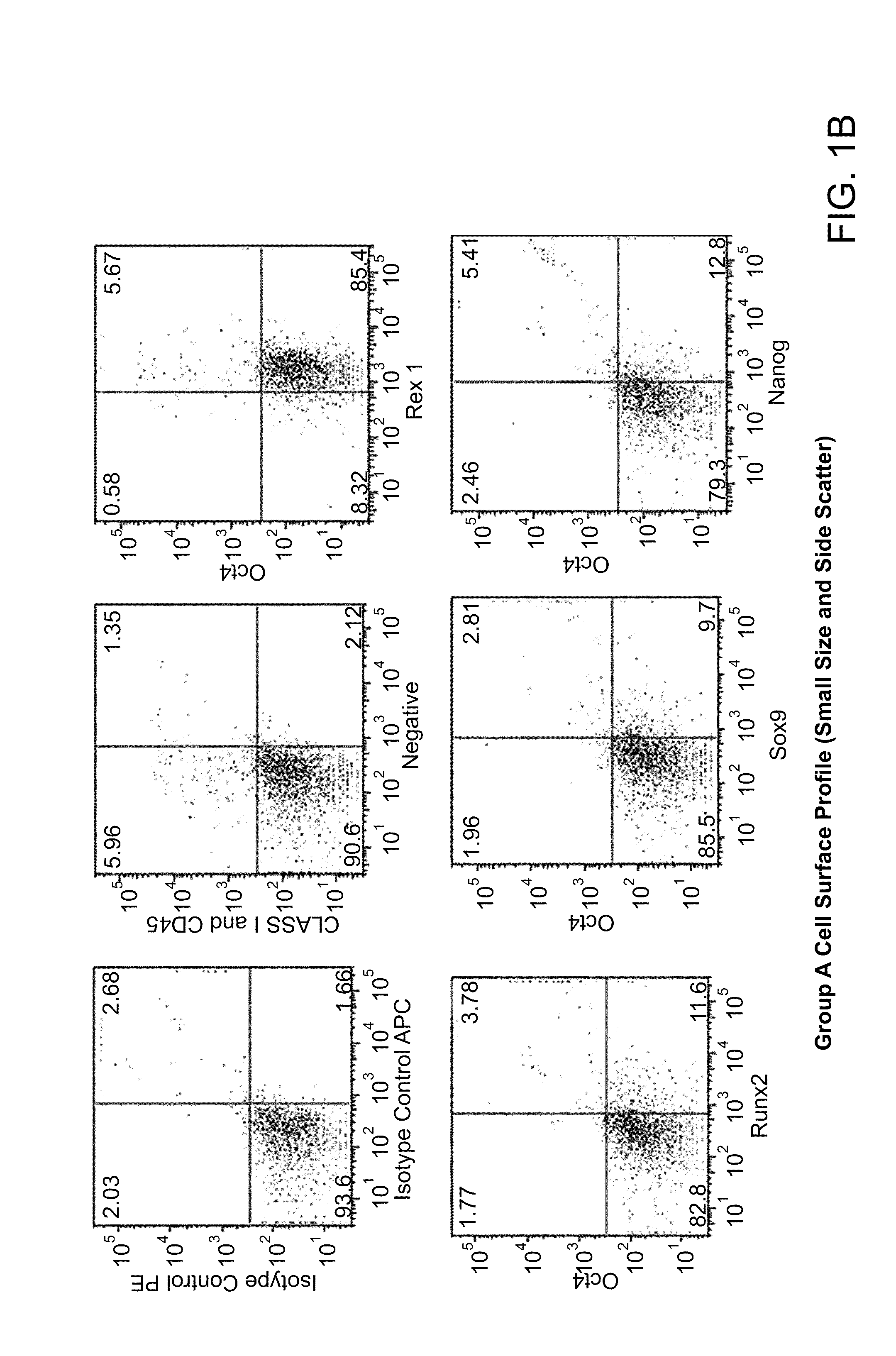
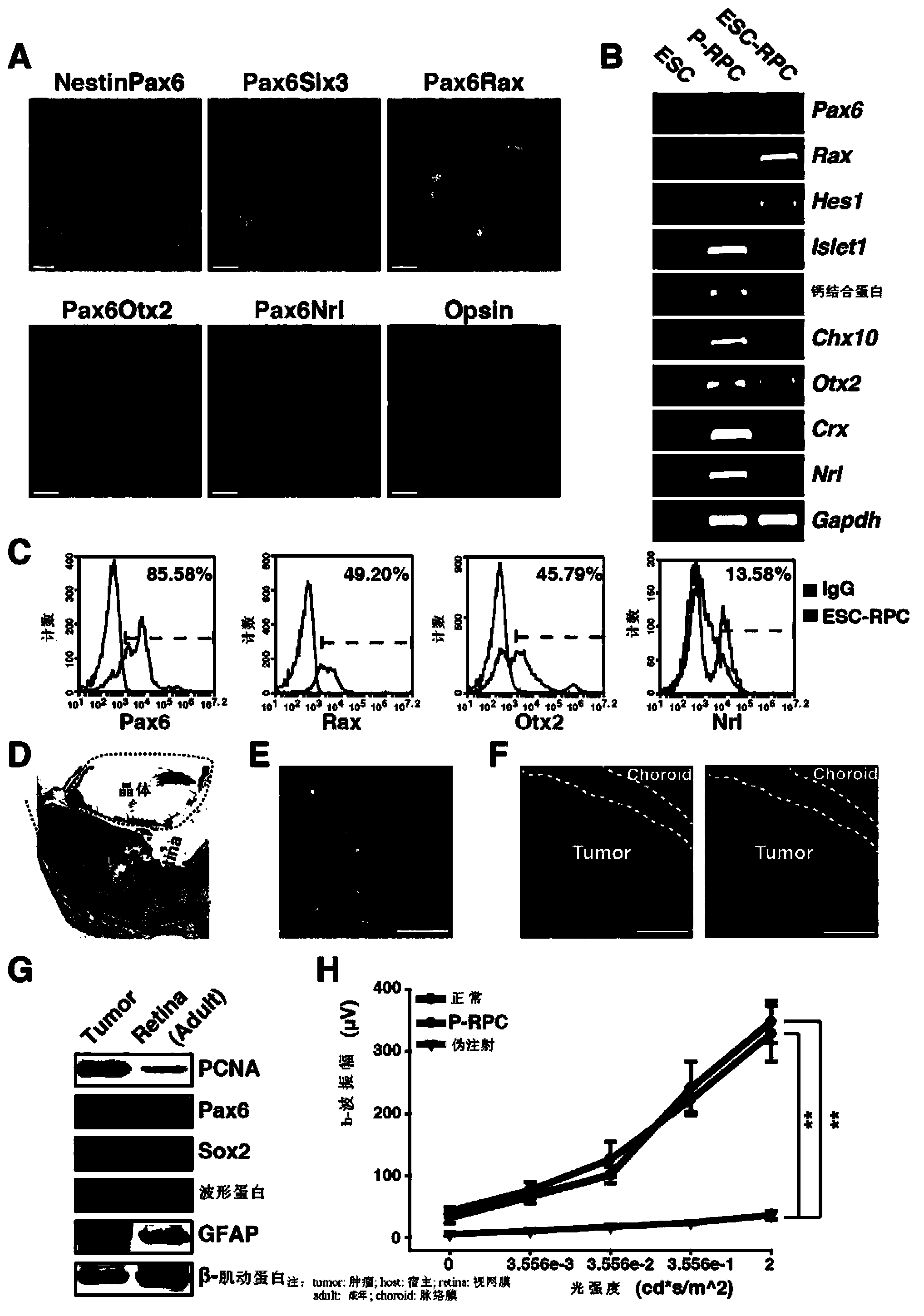
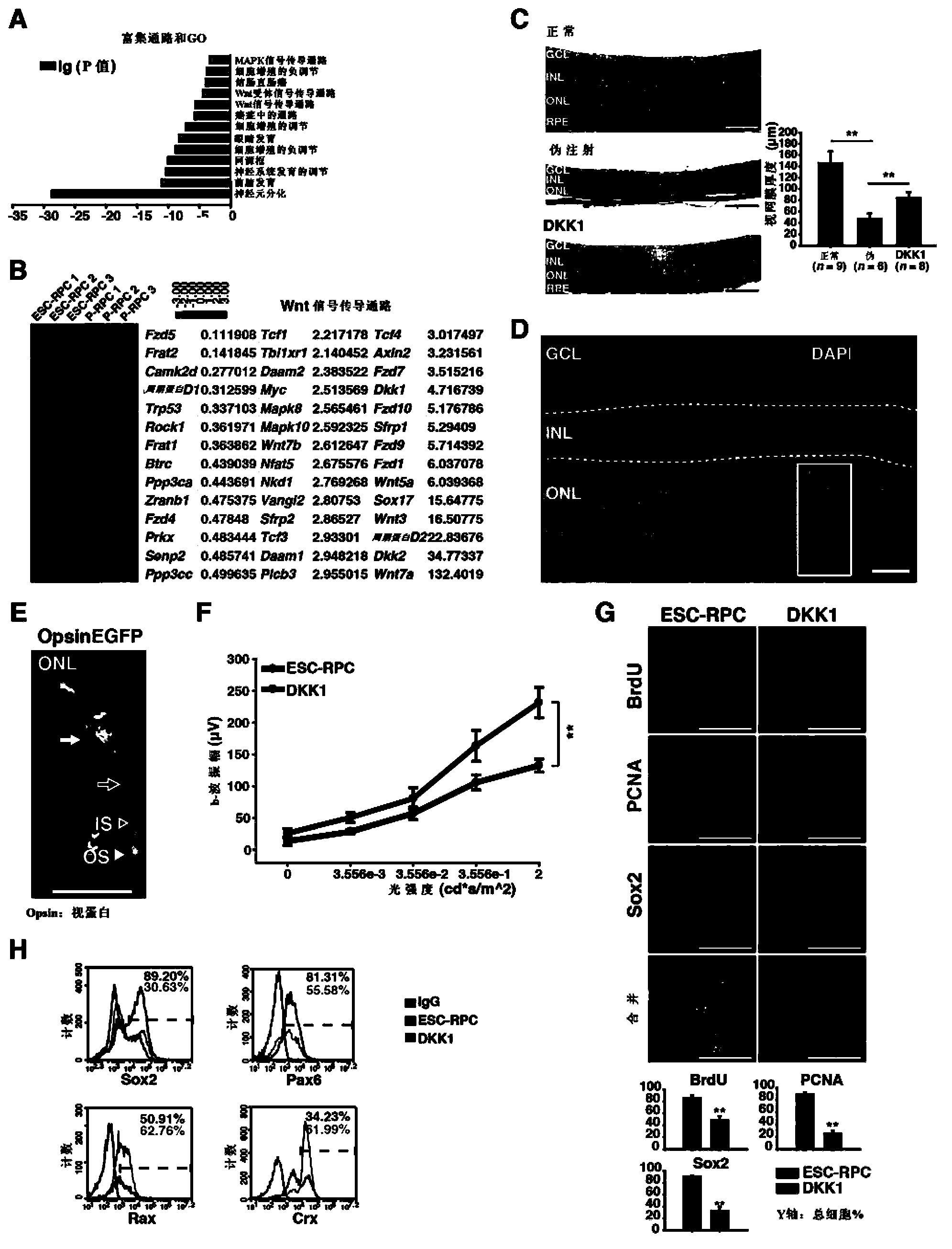
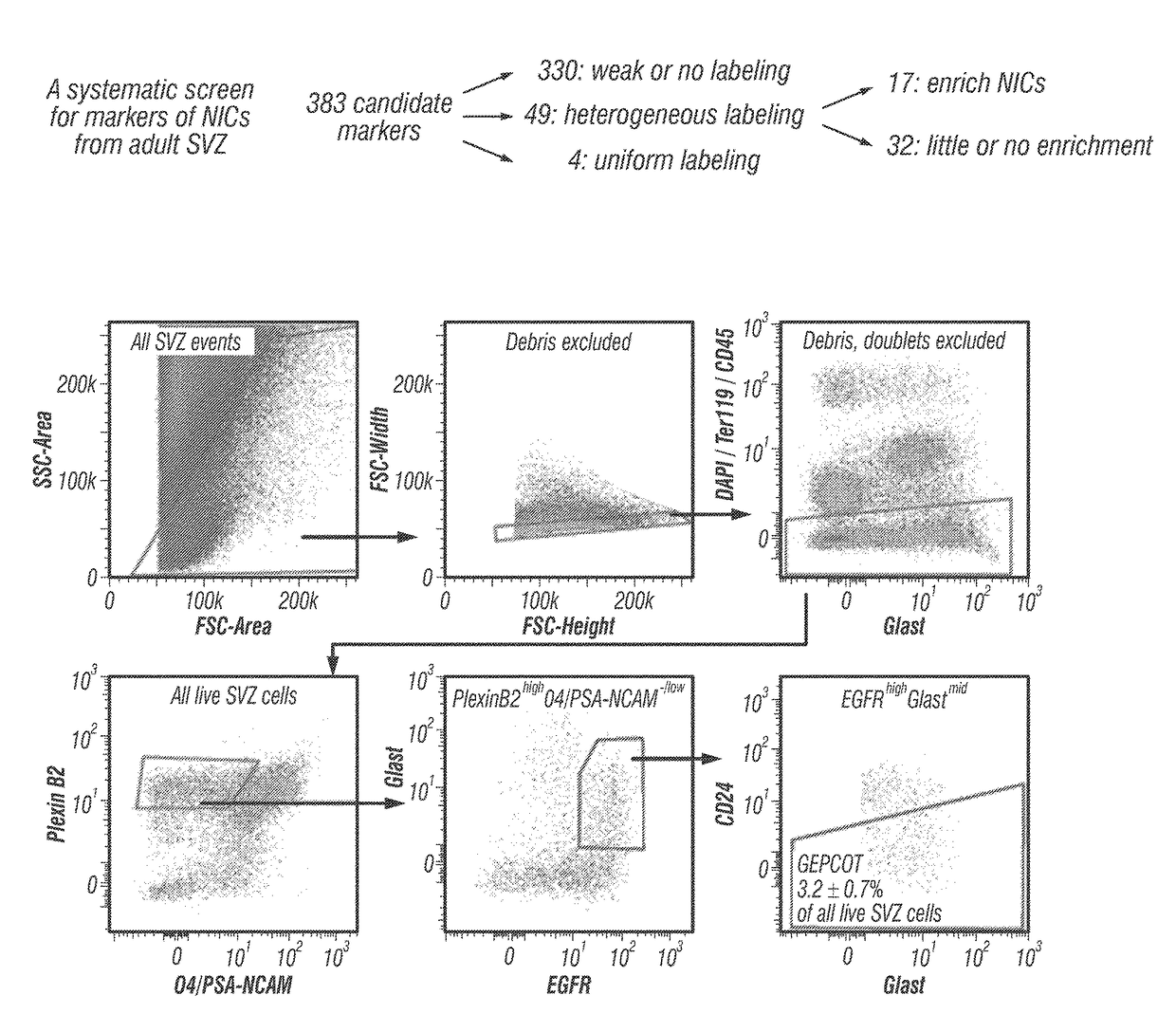
Patents
Literature
116 results about "SOX2" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
SRY (sex determining region Y)-box 2, also known as SOX2, is a transcription factor that is essential for maintaining self-renewal, or pluripotency, of undifferentiated embryonic stem cells. Sox2 has a critical role in maintenance of embryonic and neural stem cells.
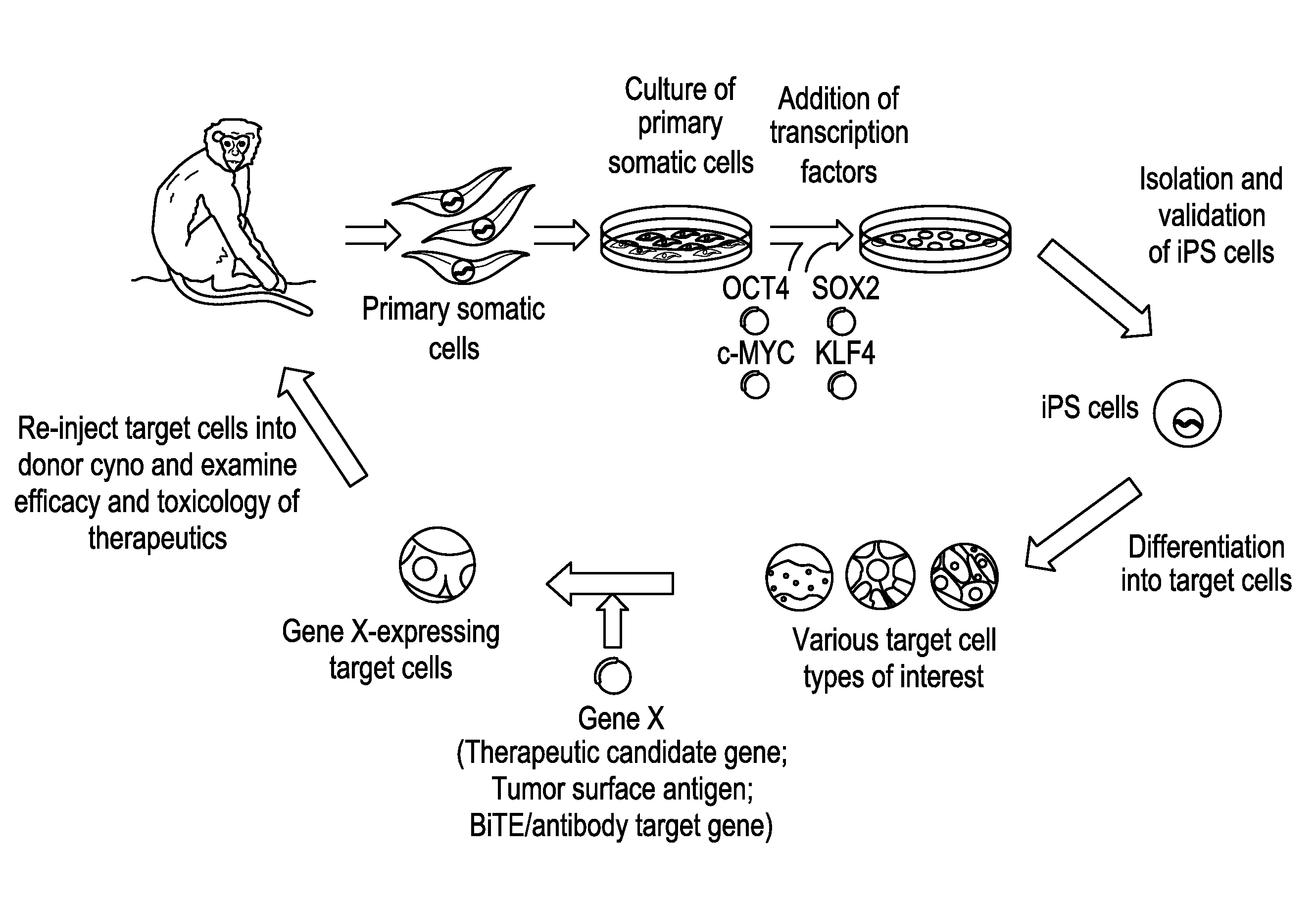
OCT4 and SOX2 with SV40 T antigen produce pluripotent stem cells from primate somatic cells
Methods for reprogramming primate somatic cells to pluripotency using an episomal vector that does not encode an infectious virus are disclosed. Pluripotent cells produced in the methods are also disclosed.
Owner:WISCONSIN ALUMNI RES FOUND
Serum autoantibody detection kit
ActiveCN103869086AEfficient identificationIncreased sensitivityDisease diagnosisBiological testingCancers diagnosisSOX2
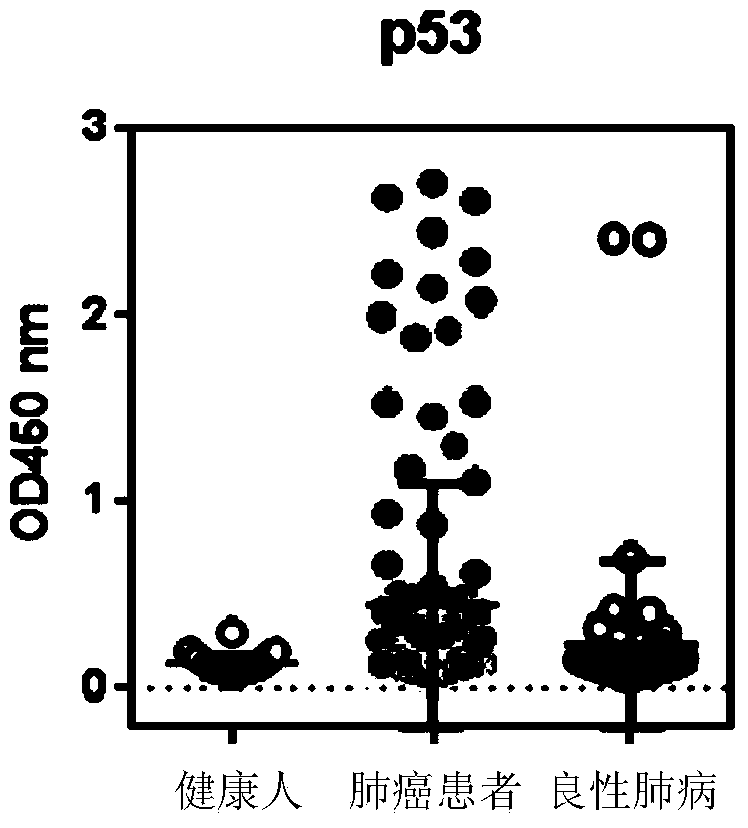
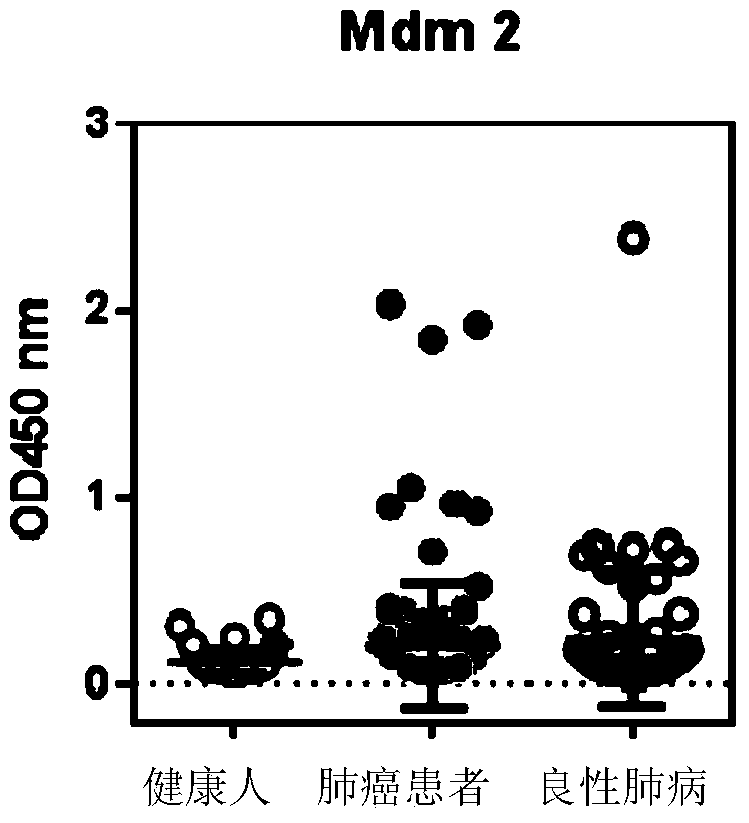
The invention discloses a detection kit used for detecting the serum autoantibody of mammals. The detection kit comprises an antigen protein, wherein the antigen protein is a combination of five or more out of p53, Annexin1, CAGE, NY-ESO-1, HuD, Cyclin D, PGP9.5, GBU4-5, MDM2, GAGE7, XAGE1b, SOX2, MAGE A1 and MAGE A4. The detection kit adopts a group of novel antigen combination corresponding to the autoantibody of a biomarker related to cancers, utilizes biotins to form characteristics of a polymer to envelope the antigen protein, and uses an anti-human label peptide as a standard of quantitative detection, thus increasing the detection sensibility and accuracy of the serum autoantibody, and providing an optimized detection method for using the autoantibody to carry out cancer diagnosis.
Owner:HANGZHOU KAIBAOLUO BIOLOGICAL SCI & TECH
Method to produce induced pluripotent stem (IPS) cells from non-embryonic human cells
The invention provides methods for generating induced pluripotent stem (iPS) cells from normal and mutant adult cells, as well as the iPS cells so generated from such methods. In some aspects, iPS cells are generated by ectopically expressing SOX2 and OCT4 nucleic acids in such adult cells. Other nucleic acids such as but not limited to MYC may also be ectopically expressed in such adult cells in the methods described herein.
Owner:CHILDRENS MEDICAL CENT CORP
Multipotent stem cells and uses thereof
ActiveUS20110070205A1Easily attainable embryonic-likePromote wound healingBiocideNervous disorderGerm layerMHC class I
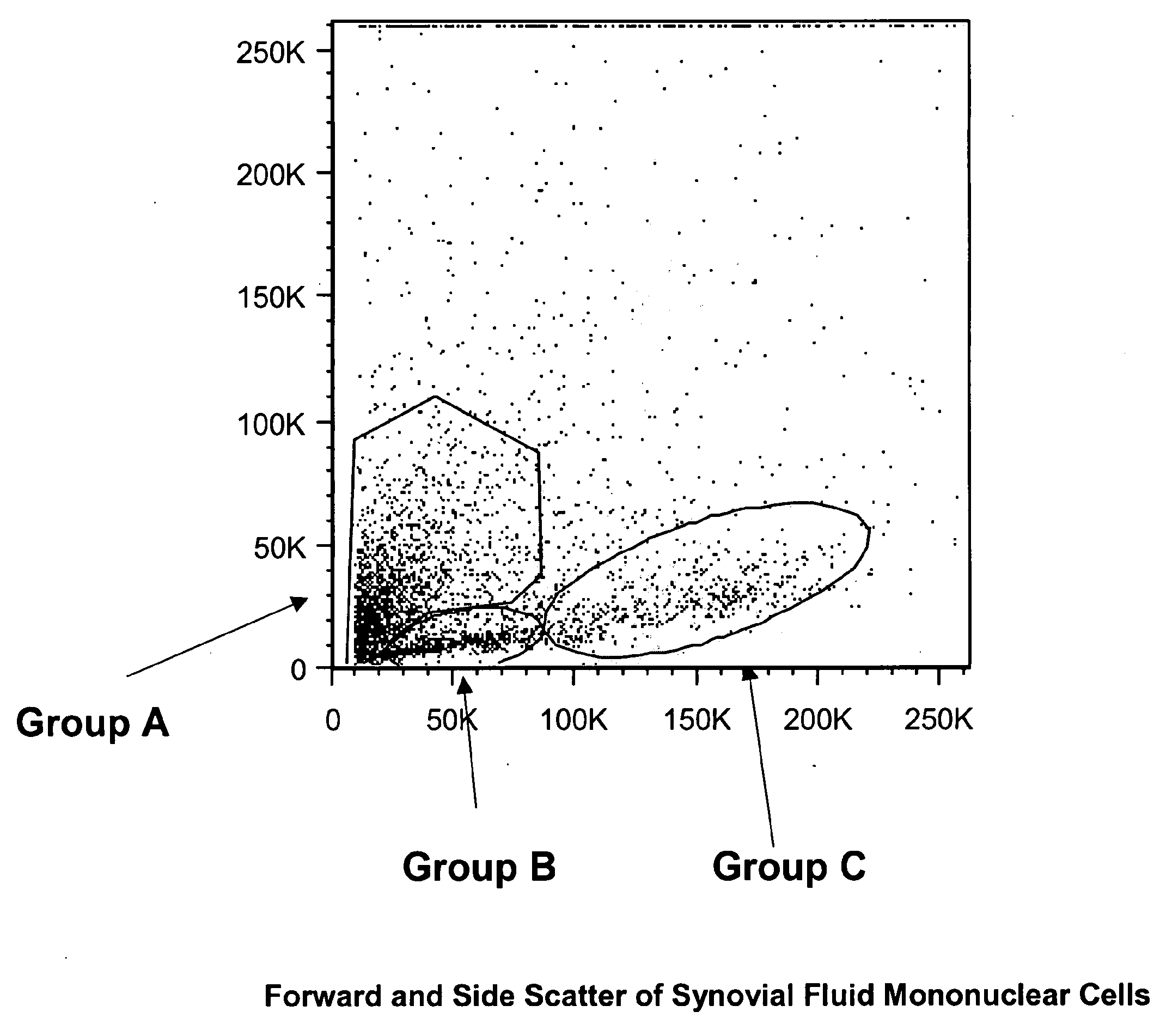
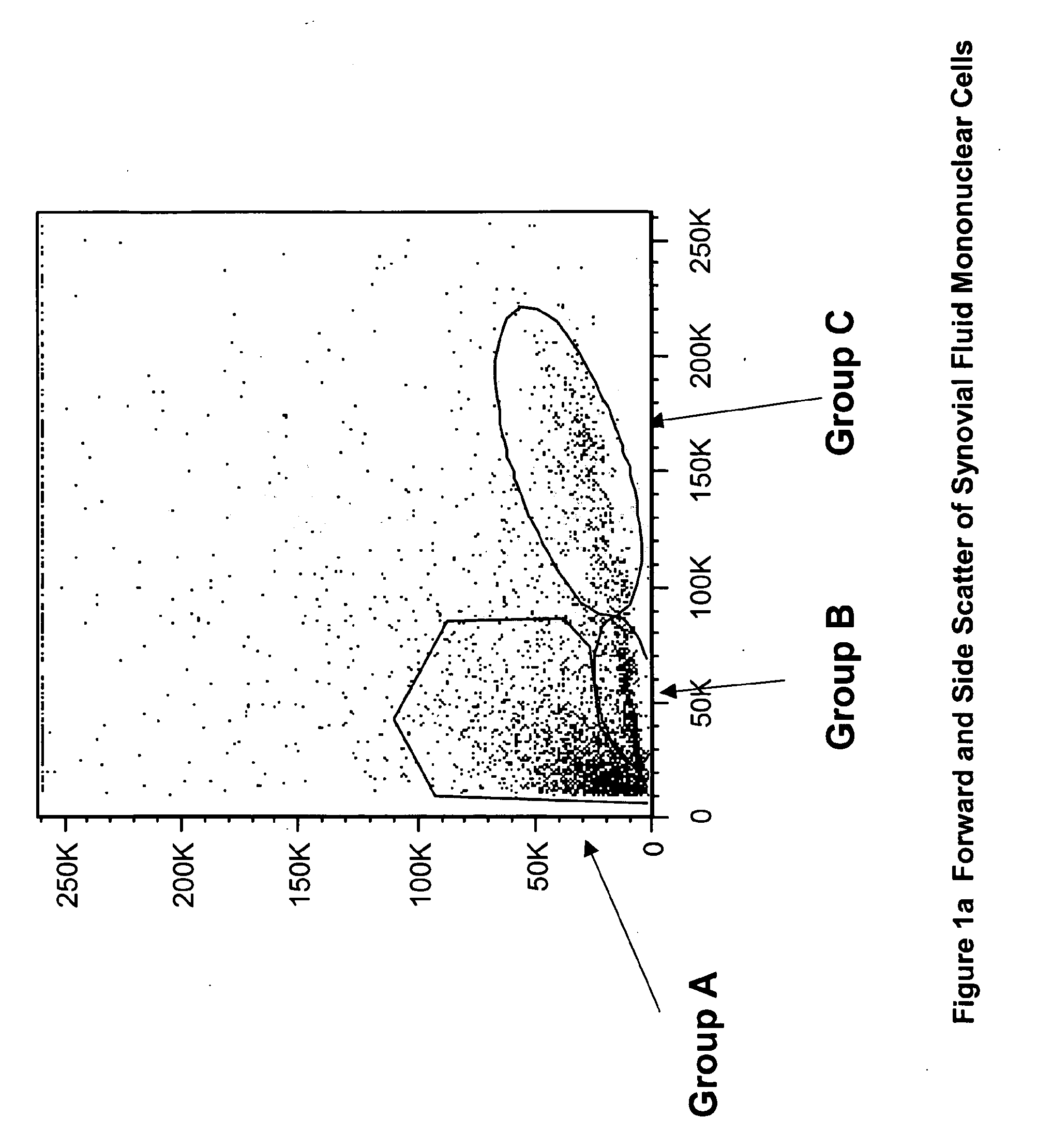
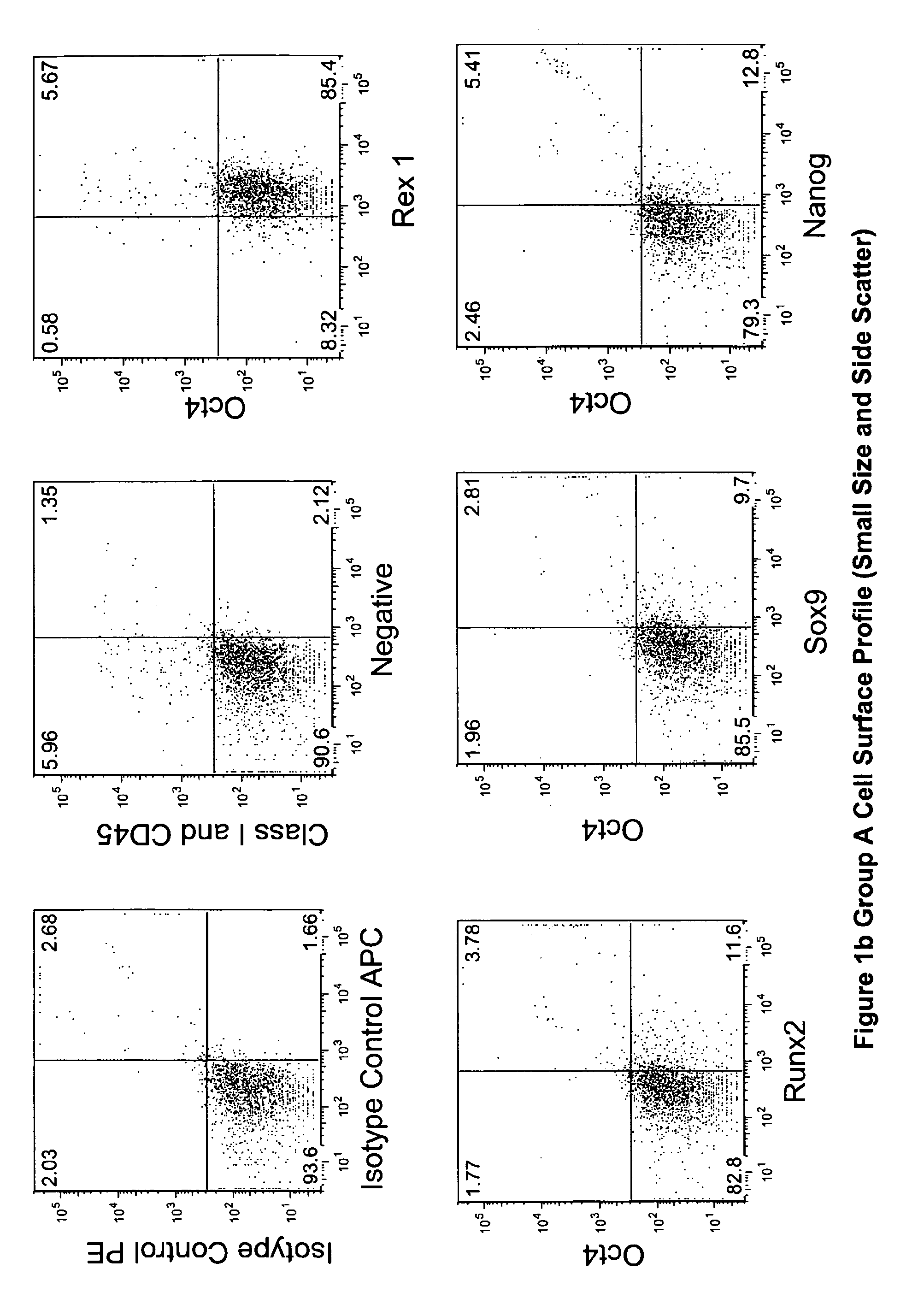
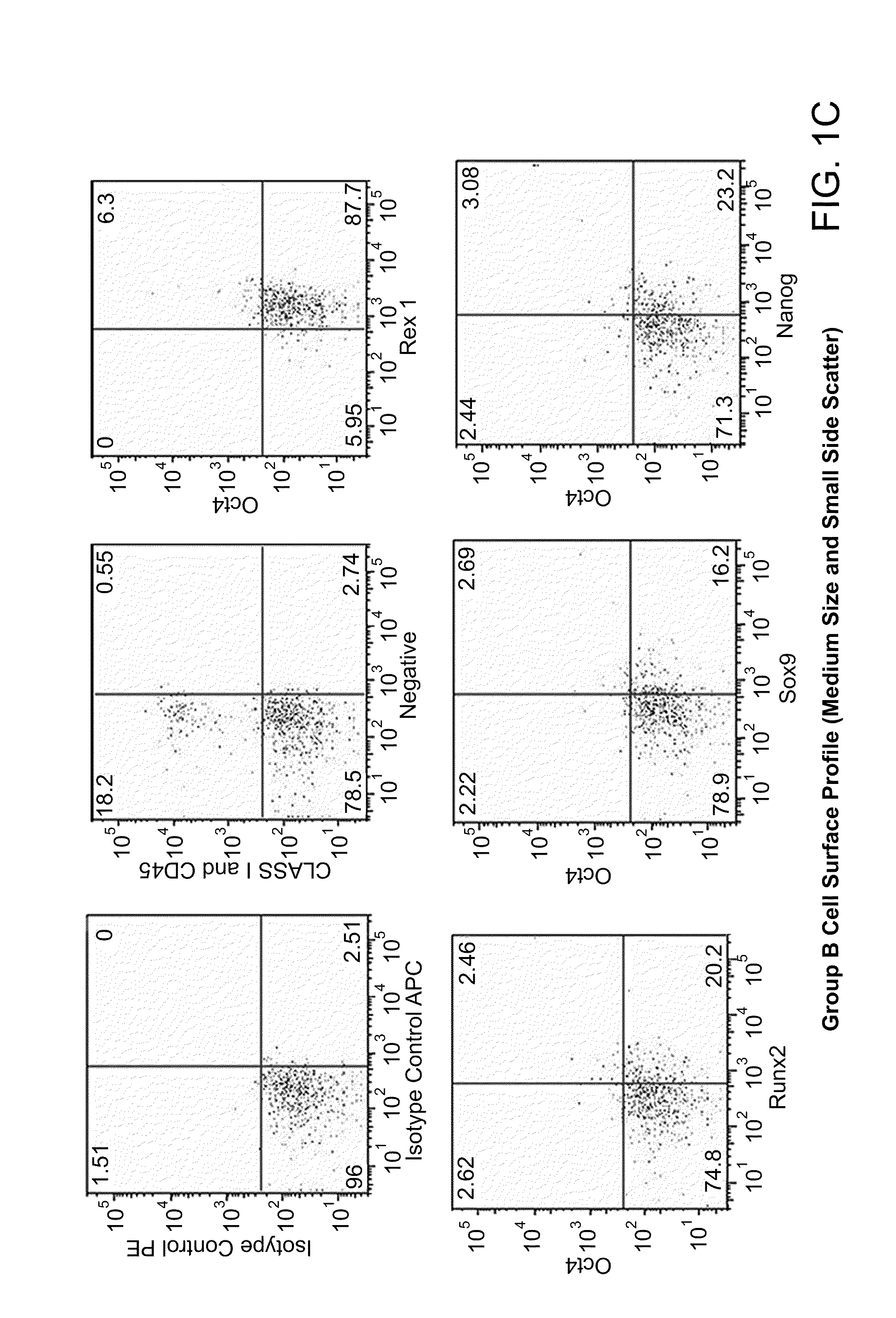
The invention provides a quiescent stem cell having the capacity to differentiate into ectoderm, mesoderm and endoderm, and which does not express cell surface markers including MHC class I, MHC class II, CD44, CD45, CD13, CD34, CD49c, CD73, CD105 CD90, CD66A, CD66E, CXCR4, CD133 or an SSEA. The invention further provides a proliferative stem cell, which expresses genes including Oct-4, Nanog, Sox2, GDF3, P16INK4, BMI, Notch, HDAC4, TERT, Rex-1, TWIST, KLF-4 and Stella but does not express cell surface markers including MHC class I, MHC class II, CD44, CD45, CD13, CD34, CD49c, CD73, CD105, CD90, CD66A, CD66E, CXCR4, CD133 or an SSEA. The cells of the invention can be isolated from adult mammals, have embryonic cell characteristics, and can form embryoid bodies. Methods for obtaining the stem cells, as well as methods of treating diseases and differentiated the stem cells, are also provided.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Multipotent stem cells and uses thereof
InactiveUS20100291042A1Easily attainable embryonic-likePromote wound healingBiocidePancreatic cellsDiseaseMHC class I
The invention provides a quiescent stem cell having the capacity to differentiate into ectoderm, mesoderm and endoderm, and which does not express cell surface markers including MHC class I, MHC class II, CD44, CD45, CD13, CD34, CD49c, CD73, CD105 and CD90. The invention further provides a proliferative stem cell, which expresses genes including Oct-4, Nanog, Sox2, GDF3, P16INK4, BMI, Notch, HDAC4, TERT, Rex-1 and TWIST but does not express cell surface markers including MHC class I, MHC class II, CD44, CD45, CD13, CD34, CD49c, CD73, CD105 and CD90. The cells of the invention can be isolated from adult mammals, have embryonic cell characteristics, and can form embryoid bodies. Methods for obtaining the stem cells, as well as methods of treating diseases and differentiated the stem cells, are also provided.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
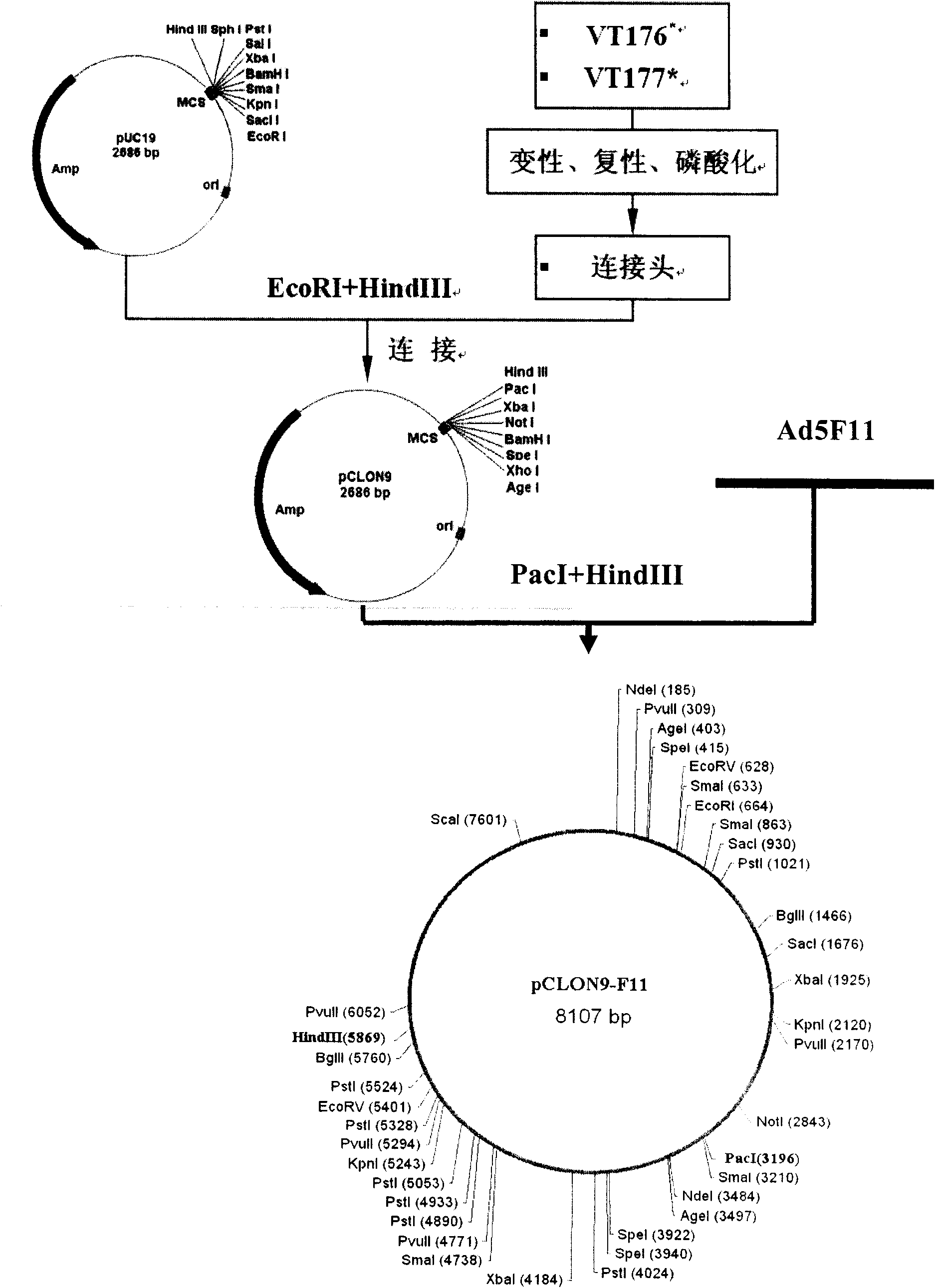
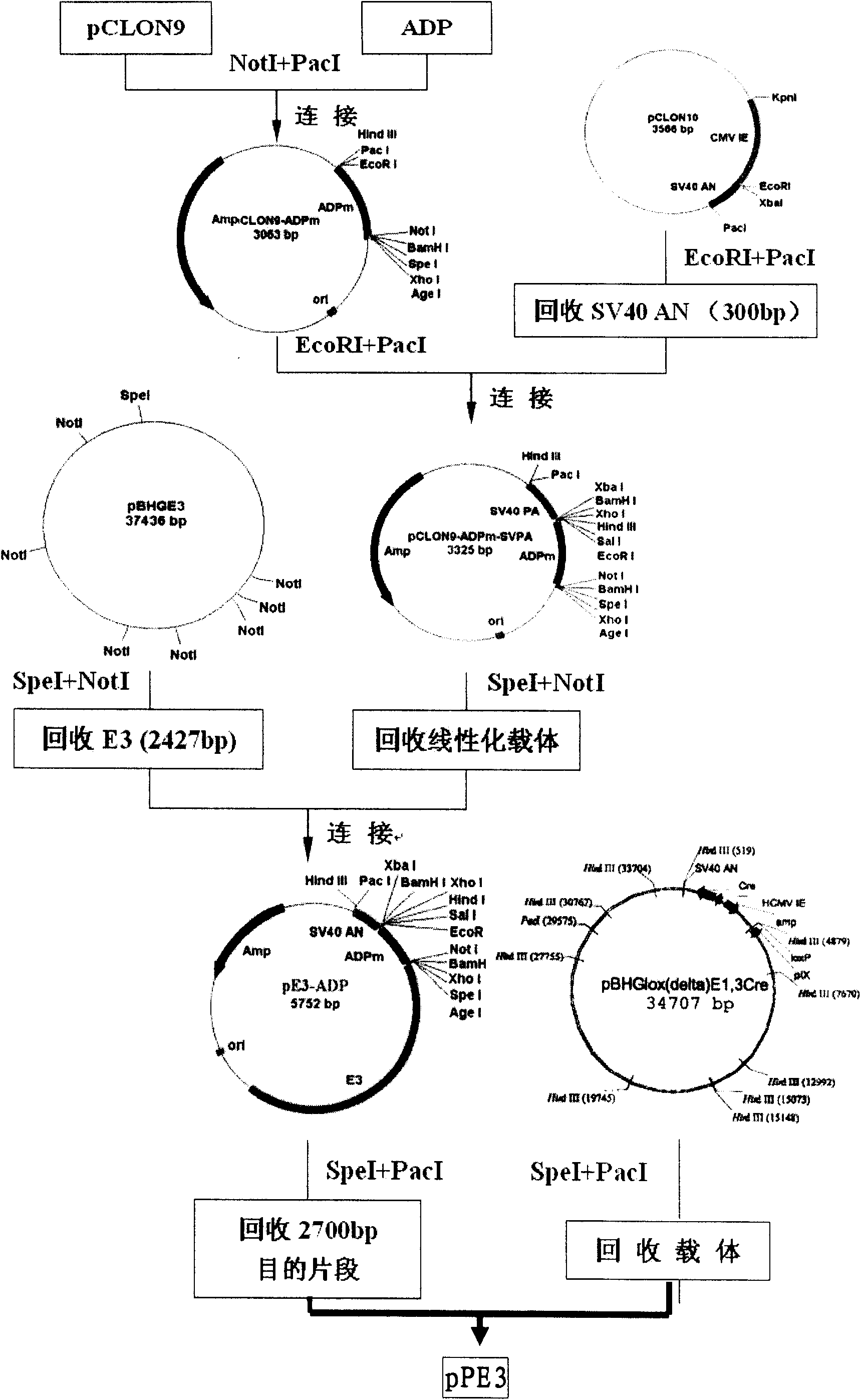
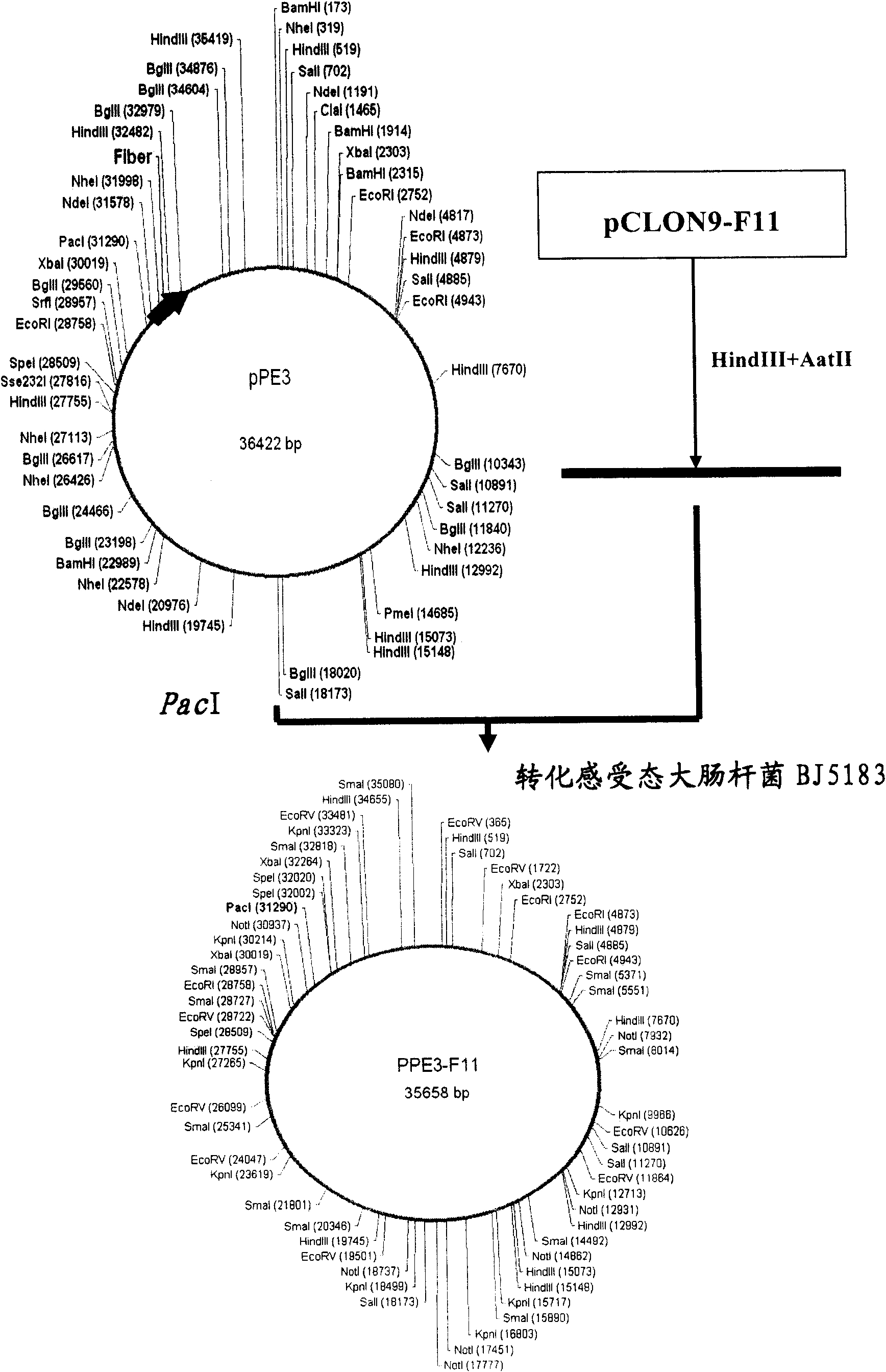
Recombinant adenovirus vector for efficiently inducing pluripotent stem cell (PS cell), method for inducing PS cell by using recombinant adenovirus vector and usage of recombinant adenovirus vector
ActiveCN101792776AWide spectrum of infectionHigh infection efficiencyCosmetic preparationsToilet preparationsDiseaseSOX2
The invention relates to a recombinant adenovirus vector for efficiently inducing a pluripotent stem cell (PS cell) and method for inducing the PS cell by using the recombinant adenovirus vector. The recombinant adenovirus vector is characterized in that the fiber genes therein are B subgroup adenovirus fiber genes, and an Sox2 gene expression cassette and an Oct4 gene expression cassette are connected within the recombinant adenovirus vector in an operating way. The terminally differentiated cell or the adult stem cell of the mammal, particularly the human is infected in vitro, the Oct4 genes and the Sox2 genes are expressed in an ectopic way, and the terminally differentiated cell or the adult stem cell of the mammal, particularly the human can be induced into the PS cell efficiently and rapidly under the synergistic effect of adjusting the epigenetic-inheritance small-molecular medicament. The PS cell of the human can be used for the cell replacement therapy to treat diseases, and the PS cell of the mammal can be used for preparing the transgenic animal model and the animal disease model.
Owner:HEPATOBILIARY SURGERY HOSPITAL SECOND MILITARY MEDICAL UNIV +1
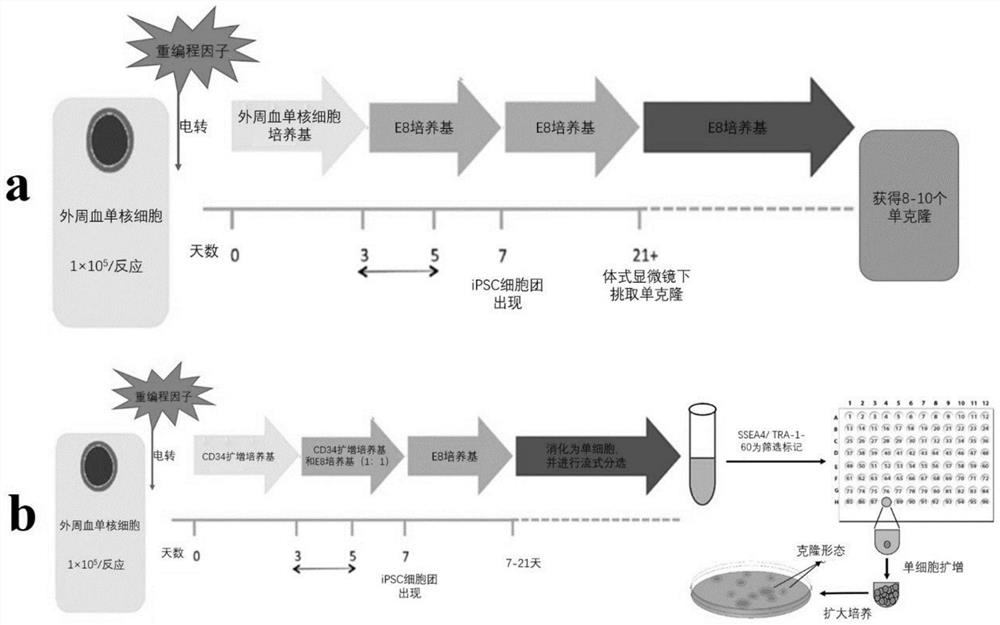
Efficient genetic-modification-free iPSC induced and industrialized monoclonal picking platform and application
ActiveCN113462638AEasy to operateImprove induction efficiencyGenetically modified cellsUnknown materialsReprogrammingSOX2
The invention discloses an efficient genetic-modification-free iPSC induced and industrialized monoclonal picking platform and application. The platform can efficiently perform reprogramming and only needs to use a minimum number of reprogramming factors (OCT4, SOX2, E6 and E7). In a monoclonal separation stage, SSEA4 / TRA-1-60 is taken as a selection marker, a large number of single cell clones are obtained through a flow cytometry sorting technology. The platform has the advantages of being high in reprogramming efficiency, high in safety, easy to operate, capable of achieving large-scale production and the like.
Owner:CHENGNUO REGENERATIVE MEDICINE TECH (ZHUHAI HENGQIN NEW AREA) CO LTD
Neurons directly induced from human skin cells and preparation method for neurons
InactiveCN102796696AHigh biosecurityImprove motor functionNervous system cellsArtificial cell constructsASCL1SOX2
The invention belongs to the field of biomedicine and relates to neurons directly induced from human skin cells and a preparation method for the neurons. By the method, induced human neurons which can perform stable passage are directly prepared by importing complementary deoxyribonucleic acid (cDNA) containing reprogramming transcription factors, namely Sox2, Ascl1 and Myt1l into isolated adult skin cells, screening cells which express a human neuron marker and cloning the cells which express the human neuron marker. The invention is characterized in that (1) the neurons express human neuron-specific marker protein in vitro; and (2) the neurons can survive in vivo and exert functions. The human neurons with the functions are obtained without the process of multipotential stem cells, so that the technology is relatively simple and effective; a heterologous feeder layer is avoided, so that the neutrons have a good clinical application prospect; and the report related to the tumorigenesis is not known about the adopted transcription factors, so the neurons have relatively high biosafety.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Protein chip, protein chip diagnostic kit, preparation method and using method
ActiveCN105527435AFacilitate early diagnosisConvenient census screeningMaterial analysisProtein markersHigh risk populations
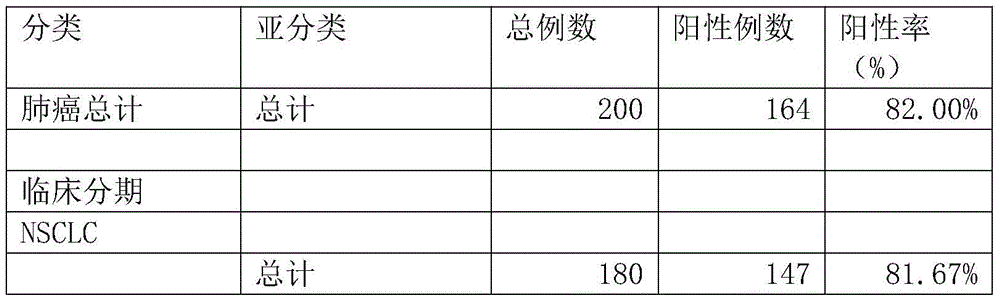
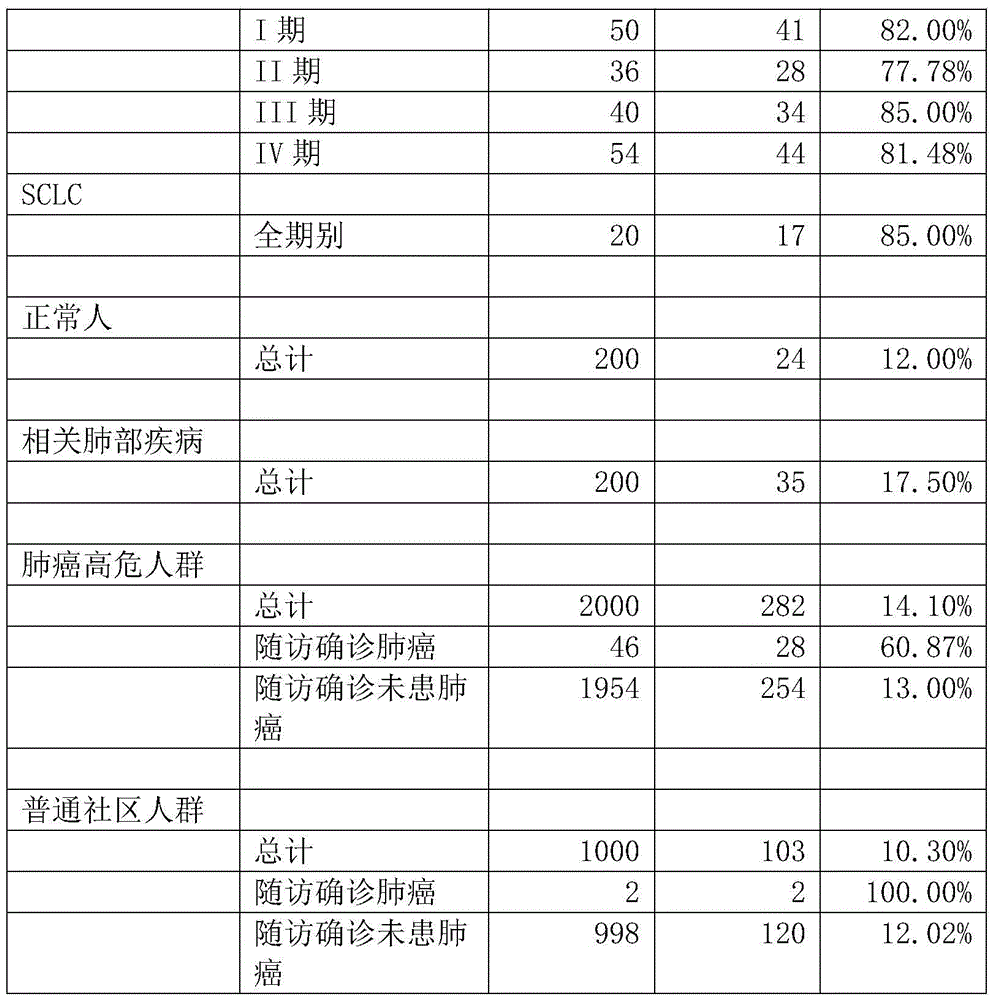
The invention relates to a protein chip, a protein chip diagnostic kit, a preparation method and a using method. The protein chip contains the following protein markers of autologous antigen protein: a P53 antigen fragment, an SOX2 antigen fragment, a COPB1 antigen fragment, an EFHD2 antigen fragment, an EIF4G3 antigen fragment and a PCNA antigen fragment, wherein the characteristic amino acid sequence of the P53 antigen fragment is as shown in ID.1sequence; the characteristic amino acid sequence of the SOX2 antigen fragment is as shown in ID.2sequence; the characteristic amino acid sequence of the COPB1 antigen fragment is as shown in ID.3sequence; the characteristic amino acid sequence of the EFHD2 fragment is as shown in picture 4seeqeunce; the characteristic amino acid sequence of the EIF4G3 antigen fragment is as shown in ID.5seqeunce; the characteristic amino acid sequence of the PCNA antigen fragment is as shown in ID.6sequence. Early diagnosis or general investigation screening for suspected patients of lung cancer or high-risk population of lung cancer can be carried out extremely conveniently, quickly, noninvasively and efficiently.
Owner:GUANGZHOU BIO BLUE TECH CO LTD
Methods and Compositions for Direct Reprogramming of Somatic Cells to Stem Cells, and Uses of these Cells
Presented herein are methods of generating an induced stem cell (iSC) from a somatic cell, by contacting the somatic cell with an induction factor that reprograms the somatic cell to generate an iSC. The induction factor can be a genetic construct or a fusion protein. Where the induction factor is a genetic construct, the construct bears one or more nucleotide sequences encoding one or more reprogramming elements selected from OCT4, SOX2, NANOG, and a Notch pathway molecule, or an active fragment or derivative thereof. The genetic construct can have a lentiviral or episomal vector backbone. The induction factor can also be a fusion protein, with the reprogramming element being a protein selected from OCT4, SOX2, NANOG, or a Notch pathway molecule, or an active fragment or derivative thereof. The fusion protein can be TAT protein or an active fragment or derivative thereof.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Establishment of a human embryonic stem cell line using mammalian cells
InactiveUS7811817B2Improve matchIncrease valueBiocideMicrobiological testing/measurementGerm layerFGF4
Purified preparations of human embryonic stem cells with certain population-specific characteristics are disclosed. This preparation is characterized by the positive expression of the following pluripotent cell surface markers: SSEA-1 (−); SSEA-4 (+); TRA-1-60 (+); TRA-1-81 (+); alkaline phosphatase (+), as well as a set of ES cell markers including Oct-4, Nanog, Rex1, Sox2, Thy1, FGF4, ABCG2, Dppa5, UTF1, Cripto1, hTERT, Connexin-43 and Connexin-45. The cells of the preparation are negative for lineage specific markers like Keratin 8, Sox-1, NFH (ectoderm), MyoD, brachyury, cardiac-actin (mesoderm), HNF-3 beta, albumin, and PDX1 (endoderm). The cells of the preparation are human embryonic stem cells, have normal karyotypes, exhibit high telomerase activity and continue to proliferate in an undifferentiated state after continuous culture for over 40 passages. The embryonic stem cell line Relicell™ hES1 also retains the ability, throughout the culture, to differentiate into cell and tissue types derived from all three embryonic germ layers (endoderm, mesoderm and ectoderm). Methods for isolating a human embryonic stem cell line are also disclosed.
Owner:RELIANCE LIFE SCI PVT
Autologous Mammalian Models Derived from Induced Pluripotent Stem Cells and Related Methods
InactiveUS20150201588A1Wide applicabilityEfficient and reliableGastrointestinal cellsMicrobiological testing/measurementSOX2Reprogramming
Disclosed is an autologous non-human mammalian model system derived from induced pluripotent stem (iPS) cells. Also disclosed are methods of differentiating non-human primate iPS cells, which can result in populations of cells enriched for SOX2+ or PDX1+ foregut-like cells, for CDX2+ hindgut-like cells, for CD34+ hematopoietic progenitor-like cells, or epithelial-like cells. Also disclosed is a non-human primate containing an autologous cell type of interest, which is differentiated in vitro from an induced pluripotent stem cell reprogrammed from a primary somatic cell. Methods of monitoring exogenously introduced cells within a non-human mammal are also disclosed.
Owner:AMGEN INC
Multipotent stem cells and uses thereof
ActiveUS8574567B2Easily attainable embryonic-likePromote wound healingBiocideNervous disorderMHC class ISurface marker
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Method for screening donor cell line based on blastocyst gene expression level
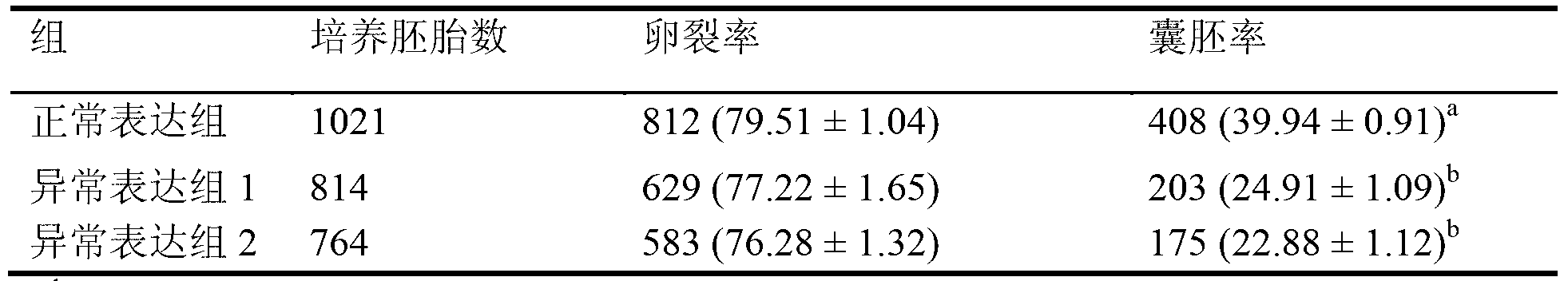
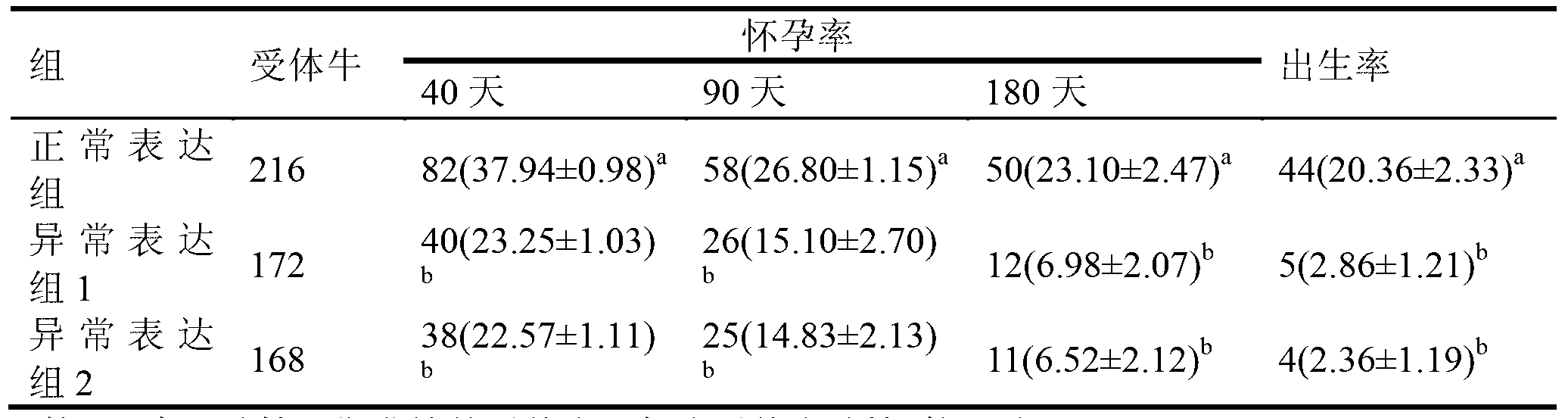
ActiveCN103305551AImprove cloning efficiencyMicrobiological testing/measurementFermentationSOX2Gene expression level
The invention discloses a method for screening a donor cell line based on blastocyst gene expression level. The method comprises the steps of: detecting expression levels of TCF7, DGCR8, SENP7, DNMT3B, Vimentin, IFI6, EFNA2, F5, IRAK1, PRICKLE1, TNFRSF1A, ARG2, NEK6, OCT4, NANOG, SOX2, CDX2, XIST and IGF2R at a blastula stage after carrying out nuclear transferring on the donor cell lines from different individuals; and selecting the donor cell line which is not significant in difference of all expression levels of these genes on cloned blastocysts and the expression level of normally growing in vitro fertilization blastocyst as a nuclear donor cell. The cloning efficiency of bovine somatic cells can be significantly improved.
Owner:NORTHWEST A & F UNIV +1
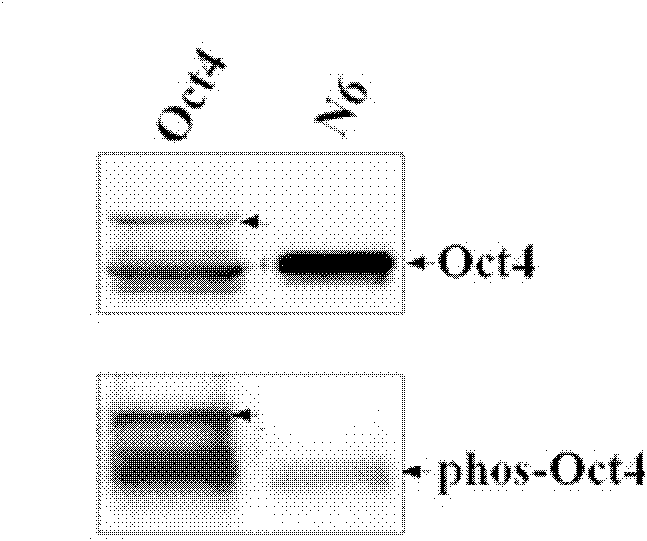
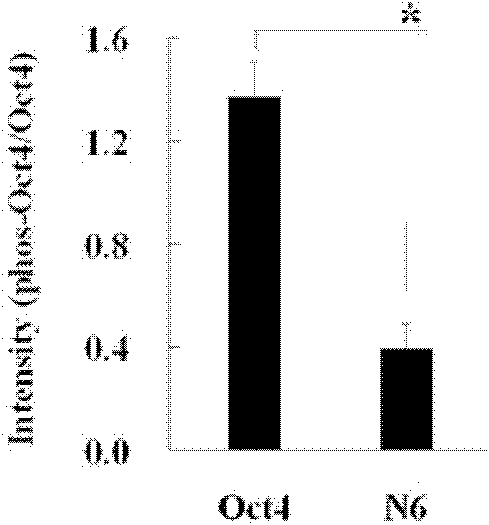
Anti-phosphorylation Oct4 protein antibody and its application
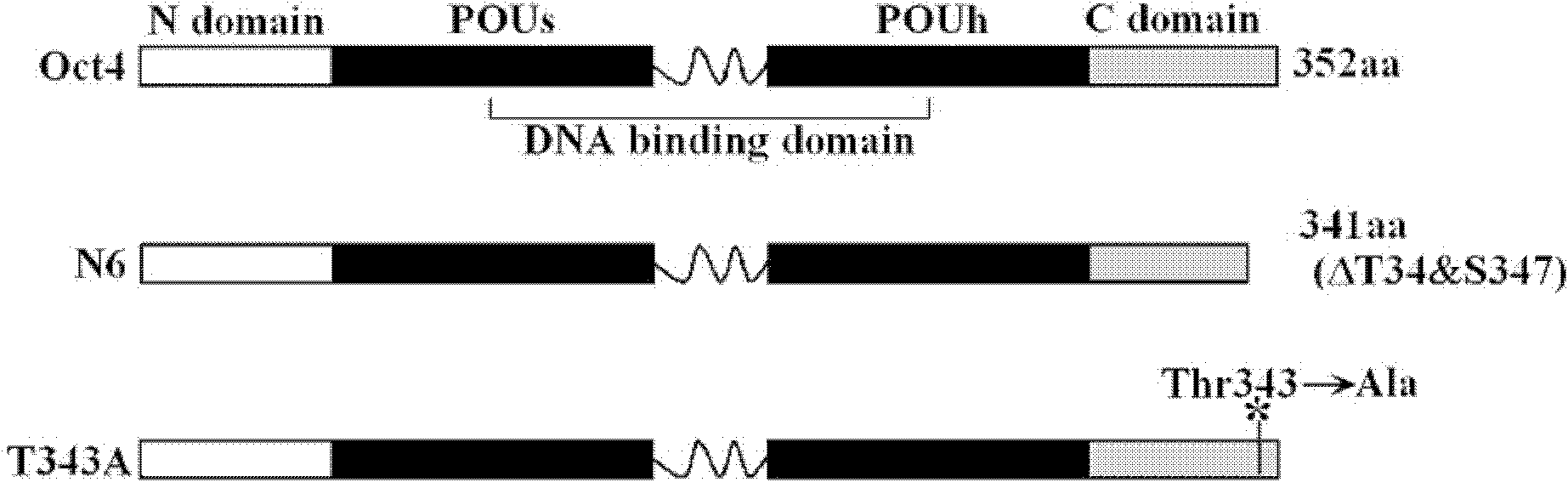
InactiveCN102731653ADecreased ability to positively regulateImmunoglobulins against animals/humansBiological testingSOX2Threonine
The invention relates to Oct4 protein phosphorylation sites and an anti-phosphorylation Oct4 protein antibody, concretely relates to Oct4 protein 343rd site phosphorylation and an antibody aimed at Oct4 protein 343rd site phosphorylation. The antibody is a monoclonal antibody or a polyclonal antibody. The invention also relates to an application of the antibody used for detecting phosphorylation of Oct4 protein 343rd site threonine. When the Oct4 protein 343rd site threonine is subjected to phosphorylation, the positive regulation and control capability of Oct4 protein on downstream genes Nanong and Sox2 is decreased, thereby the capability for keeping self-replicating type stem cells and pluripotent differentiation is lost. The application of the invention comprises the application of the antibody in detection of stem cells proliferation, cell differentiation, stem cell pluripotency and stem cells self-replicating capability.
Owner:李凌松 +1
Defined media for expansion and maintenance of pluripotent stem cells
The present invention provides methods to promote the proliferation of undifferentiated pluripotent stem cells in defined media. Specifically, the invention provides a defined cell culture formulation for the culture, maintenance, and expansion of pluripotent stem cells, wherein culturing stem cells in the defined cell culture formulation maintains the pluripotency and karyotypic stability of the cells for at least 10 passages. Further disclosed is a cell population grown under defined media conditions that express OCT4, SOX2, NANOG, and FOXA2.
Owner:JANSSEN BIOTECH INC
Methods of generating pluripotent stem cells
InactiveUS20130059385A1Prevent and delay activationOther chemical processesGenetically modified cellsHeterologousSOX2
Disclosed are methods, compositions, and kits of producing an induced pluripotent stem cell from a mammalian non-pluripotent cell that does not endogenously or heterologously express Sox2 and is not in contact with a Sox2 polypeptide.
Owner:THE SCRIPPS RES INST
Embryonic stem cell culture medium and application thereof
InactiveCN102732476AImprove versatilityExcellent self-renewal abilityEmbryonic cellsGerm cellsNanog ProteinSOX2
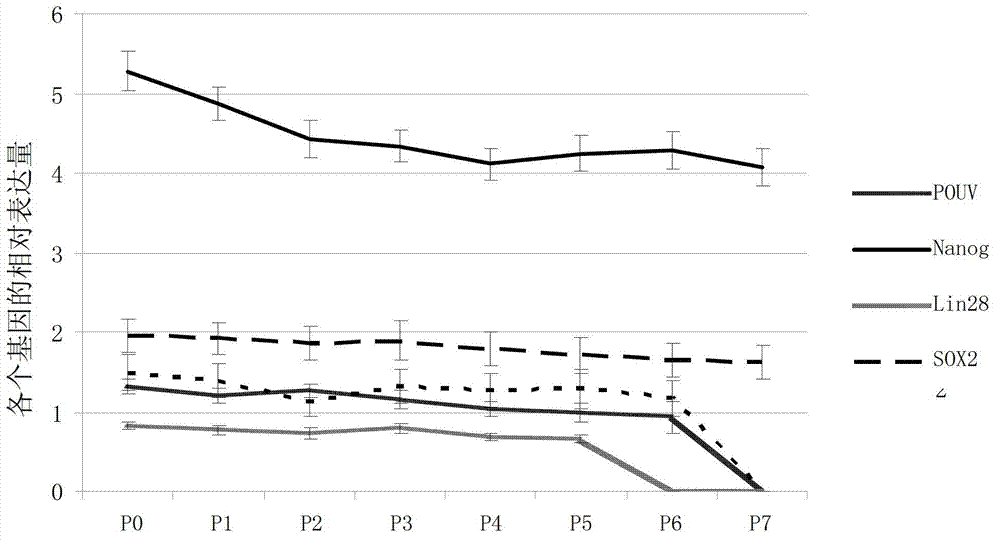
The invention discloses an embryonic stem cell culture medium and application thereof. The embryonic stem cell culture medium comprises the following ingredients: a protein A containing an SOX2 protein, a protein B containing a POUV protein, a protein C containing a Nanog protein, a protein D containing a cLIN28 protein, a cell factor LIF, a cell factor SCF and a cell factor bFGF, wherein the SOX2 protein is specifically shown in Sequence 5; the POUV protein is specifically shown in Sequence 7; the Nanog protein is specifically shown in Sequence 9; and the cLIN28 protein is specifically shown in Sequence 11. When the embryonic stem cell culture medium provided by the invention is adopted to culture embryonic stem cells, the embryonic stem cells can be expanded in quantity for a long term and maintain good totipotency.
Owner:CHINA AGRI UNIV
Detection primer group for identifying pluripotent stem cell endogenous and exogenetic pluripotent gene expression, kit and detection method thereof
InactiveCN107988329AStable detectionEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationSOX2Quantitative determination
The invention relates to a primer composite for identifying human induced pluripotent stem cell pluripotent genes, a kit and a detection method. The primer group comprises a primer group A, a primer group B and a confidential reference primer, wherein the primer group A comprises one or several kinds of primer pairs for amplifying human inducted pluripotent stem cell endogenous genes Oct4, Sox2 and Nanog; the primer group B comprises one or several kinds of primer pairs for amplifying Oct4, Sox2 and Nanog general genes; the confidential reference primer is a primer for amplifying a GAPDH gene.Concretely, RNA (ribonucleic acid) is extracted from the pluripotent stem cells; a real-time PCR(polymerase chain reaction) technology is used by the detection method and the kit for performing quantitative determination on the pluripotent gene so as to identify the endogenous and exogenetic pluripotent gene expression differences. By using the identification method provided by the invention, theoperation is simple; the cost is low; the accuracy and the sensitivity are high.
Owner:GUANGDONG XTEM BIOTECH CO LTD
Stable reprogrammed cells
InactiveUS20130259842A1Rapidly and efficiently producedImprove efficiencyBiocideGenetic material ingredientsEpigenomeSOX2
The present invention relates to a stable pluripotent reprogrammed cells, and compositions and methods of isolation and uses thereof, wherein the stable pluripotent reprogrammed cells is derived from a somatic cell and has undergone incomplete remodeling of the epigenome. In some embodiments, the stable reprogrammed cell is a human stable reprogrammed cell. In some embodiments, the stable reprogrammed cell has a statistically significant lower level of expression of one or any combination of Nanog, Dnmt3b, Lefty2 as compared to an induced pluripotent stem cell. In some embodiments, the stable reprogrammed cell has a statistically significant higher level of expression of one or any combination of Tdgf1, Tert or endogenous Sox2 as compared to a somatic cell from which it was derived. In some embodiments, the stable reprogrammed cell has a statistically significant faster rate of doubling as compared to an induced pluripotent stem cell (iPSC) or an embryonic stem (ES) cell. Other aspects of the invention relate to compositions comprising the reprogrammed cell, method of isolation and method of use.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Method for preparation of induced dopaminergic progenitors using direct reprogramming
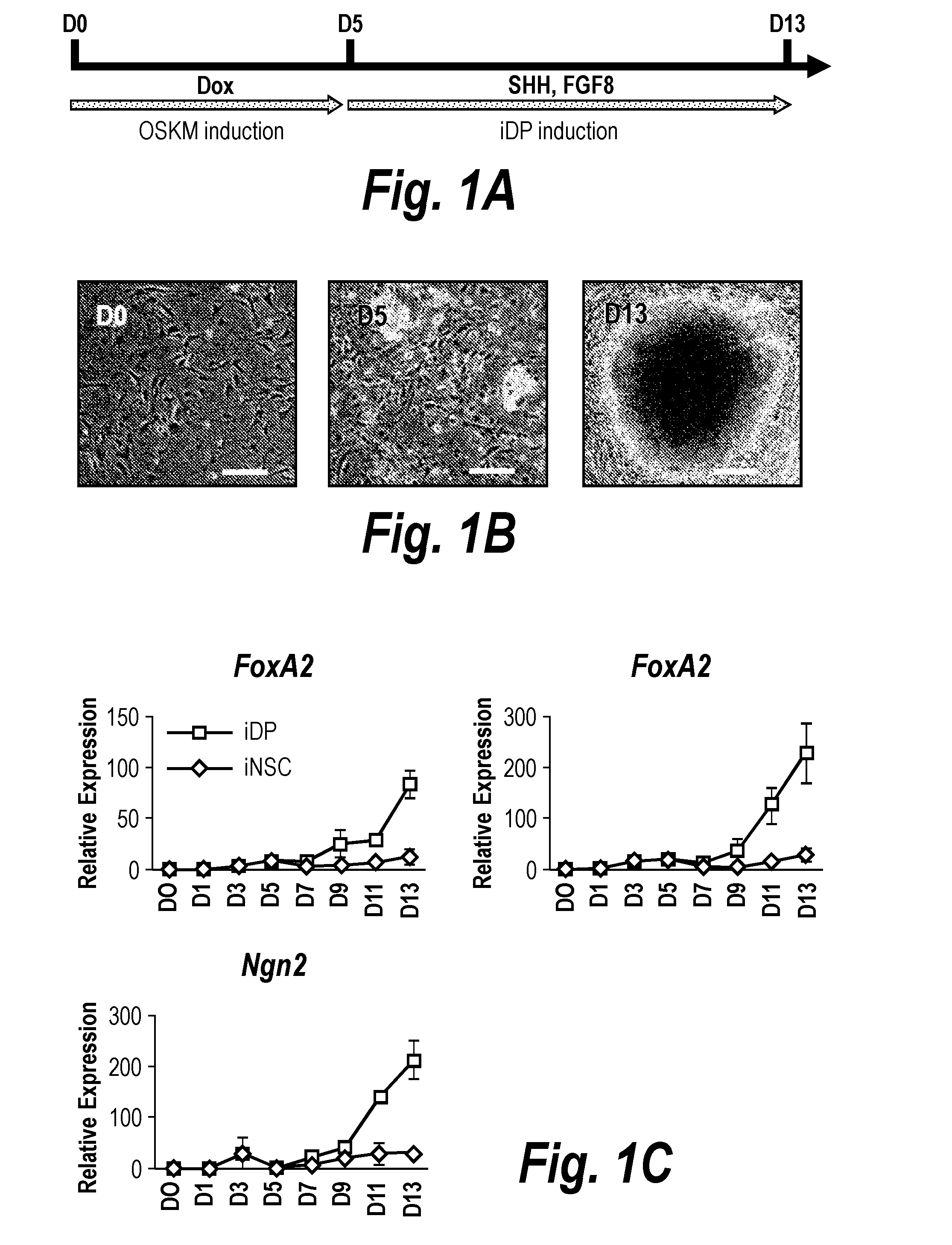
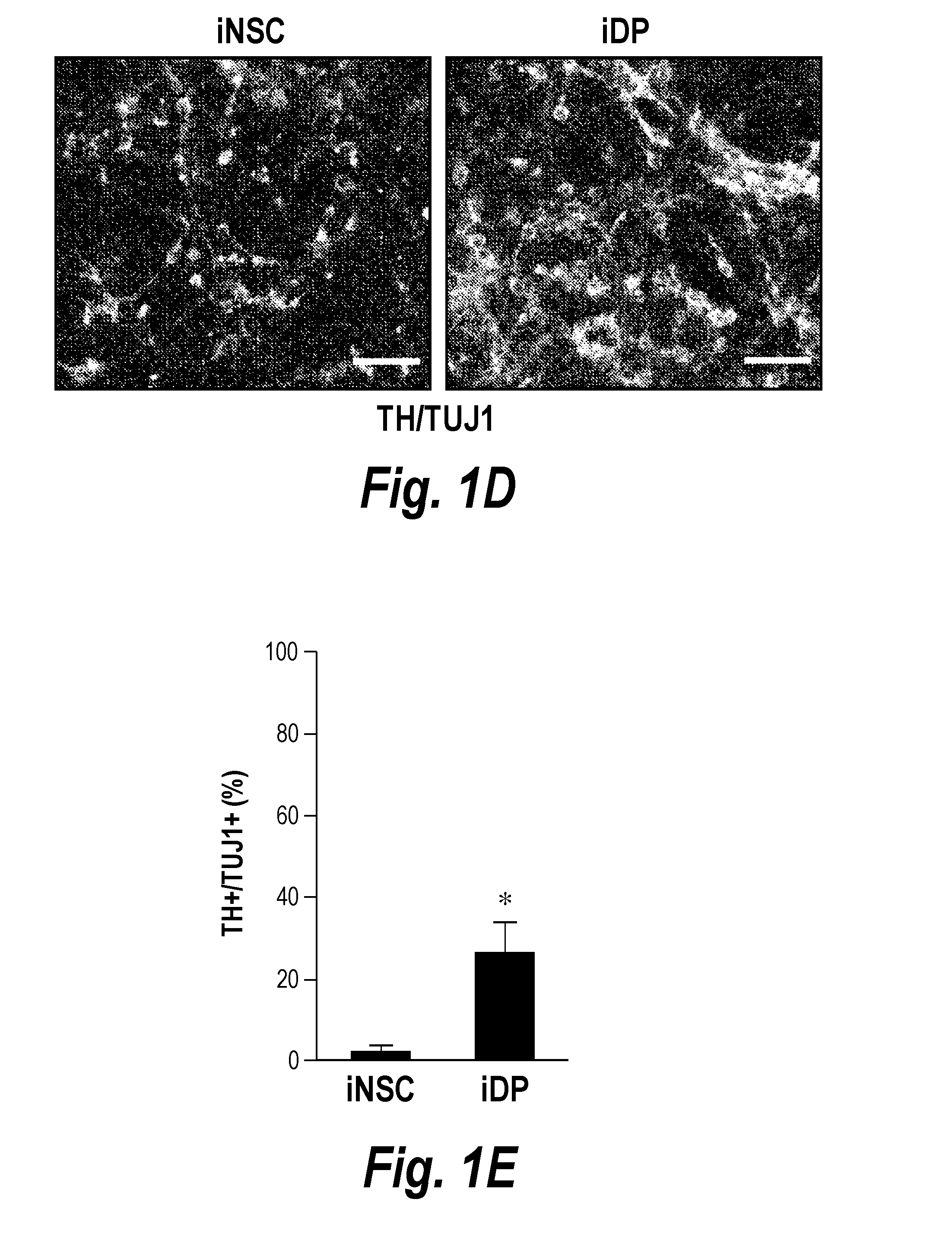
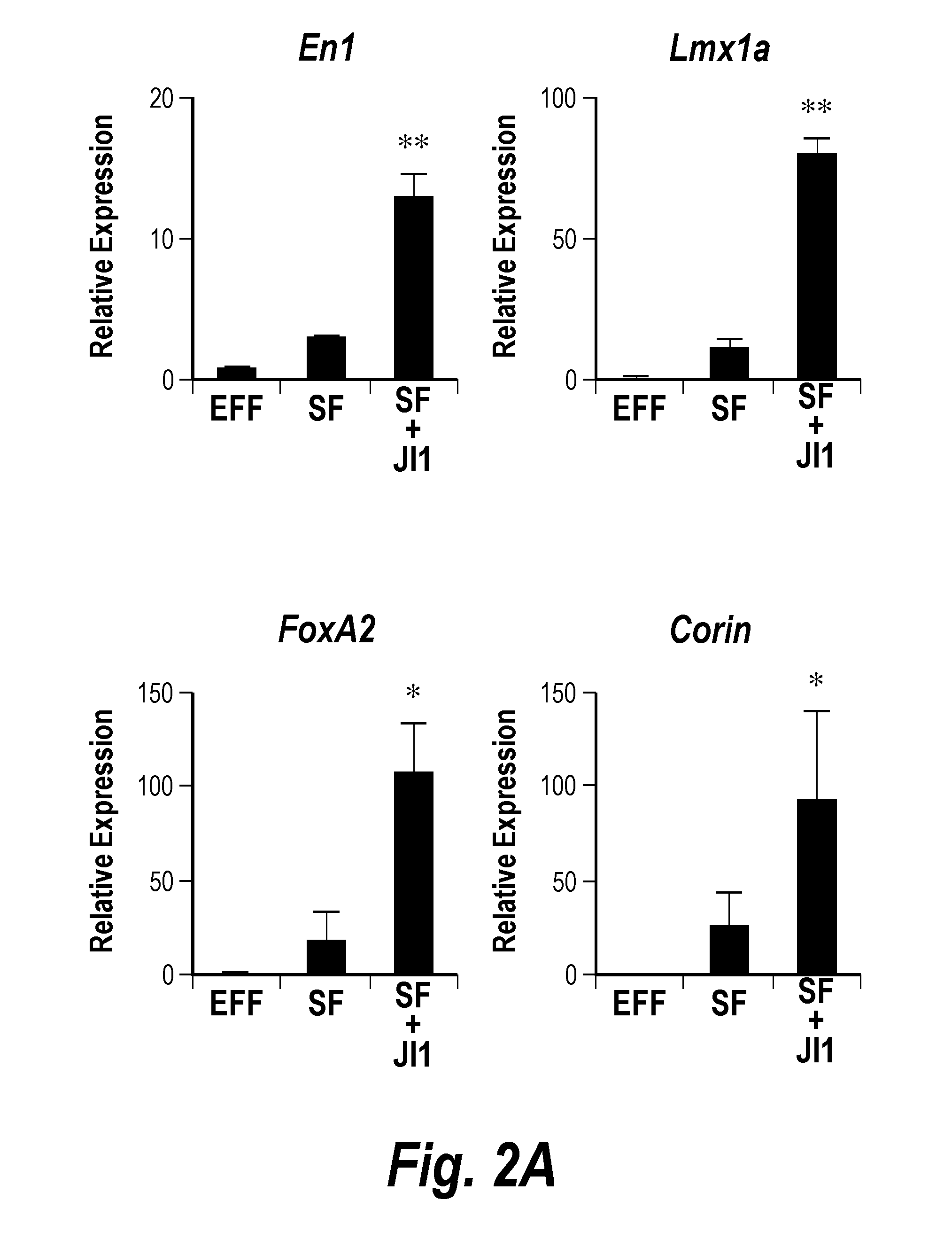
ActiveUS20160256495A1High differentiation efficiencyImprove efficiencyCompounds screening/testingGenetically modified cellsProgenitorSide effect
The present invention relates to a method for preparing induced dopaminergic neuronal progenitors (iDPs) comprising inducing an expression of Oct4, Sox2, Klf4, and c-Myc genes in adult cells and direct reprogramming of the adult cells to the iDPs by treating the cells with sonic hedgehog (SHH) and fibroblast growth factor 8 (FGF8); and a cell therapy product and a composition for treating or preventing Parkinson's Disease (PD) which comprises the iDPs as active ingredients. In addition to this, the present invention relates to a method for preparing midbrain dopaminergic neurons comprising isolating NSC-like colony by culturing the iDPs, dissociating the isolated NSC-like colony, and culturing the cells on the neural cell differentiation medium. Further, the present invention relates to a method for treating PD or a method for screening medicine for preventing or treating PD using the cells of the present invention. The progenitors prepared by the method for preparing the iDPs of the present invention can be widely used in the known art since the neuronal progenitors have significantly higher efficiency in differentiating to the dopaminergic neurons compared to conventional methods and lack side effects such as immunogenicity and ethical issues because patients' own somatic cells can be used.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Four-branch polypeptide and application thereof
InactiveCN105504070AImproving immunogenicityExtended half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsMedicineHalf-life
The invention provides a synthetic functional four-branch polypeptide (AA1-AA2)2-(Lys-NH2)2-Lys-SER-NH2, wherein AA1 and AA2 are 15-AA peptides of SOX2 and OCT4 respectively. The branched synthetic polypeptide product provided by the invention has the effects of comprehensively improving the immunogenicity of an organism and prolonging the half life of the organism, and has broad application prospect in preparation of an anti-tumor medicament or vaccine.
Owner:深圳市中美康士生物科技有限公司
Yap1 inhibitors that target the interaction of yap1 with oct4
Binding of the transcriptional co-activator, YAP1, to the transcription factor Oct4, induces Sox2, which is a transcription actor necessary for the self-renewal of stem-like cells from non-small celllung cancer. The WW domain of YAP1 binds to the PPxY motif of Oct4 to induce Sox2. Delivering a peptide corresponding to the WW domain could prevent the induction of Sox2 and stemness. Similarly, peptides and mimetics of the PPxY motif would be able to inhibit stemness. Disclosed are compounds that affect the Yap1:Oct4 interaction.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC
Microrna as a cancer progression predictor and its use for treating cancer
ActiveUS20120255043A1Modulated tumor-initiating propertiesHigh sensitivityOrganic active ingredientsSugar derivativesAbnormal tissue growthAdenylyl cyclase
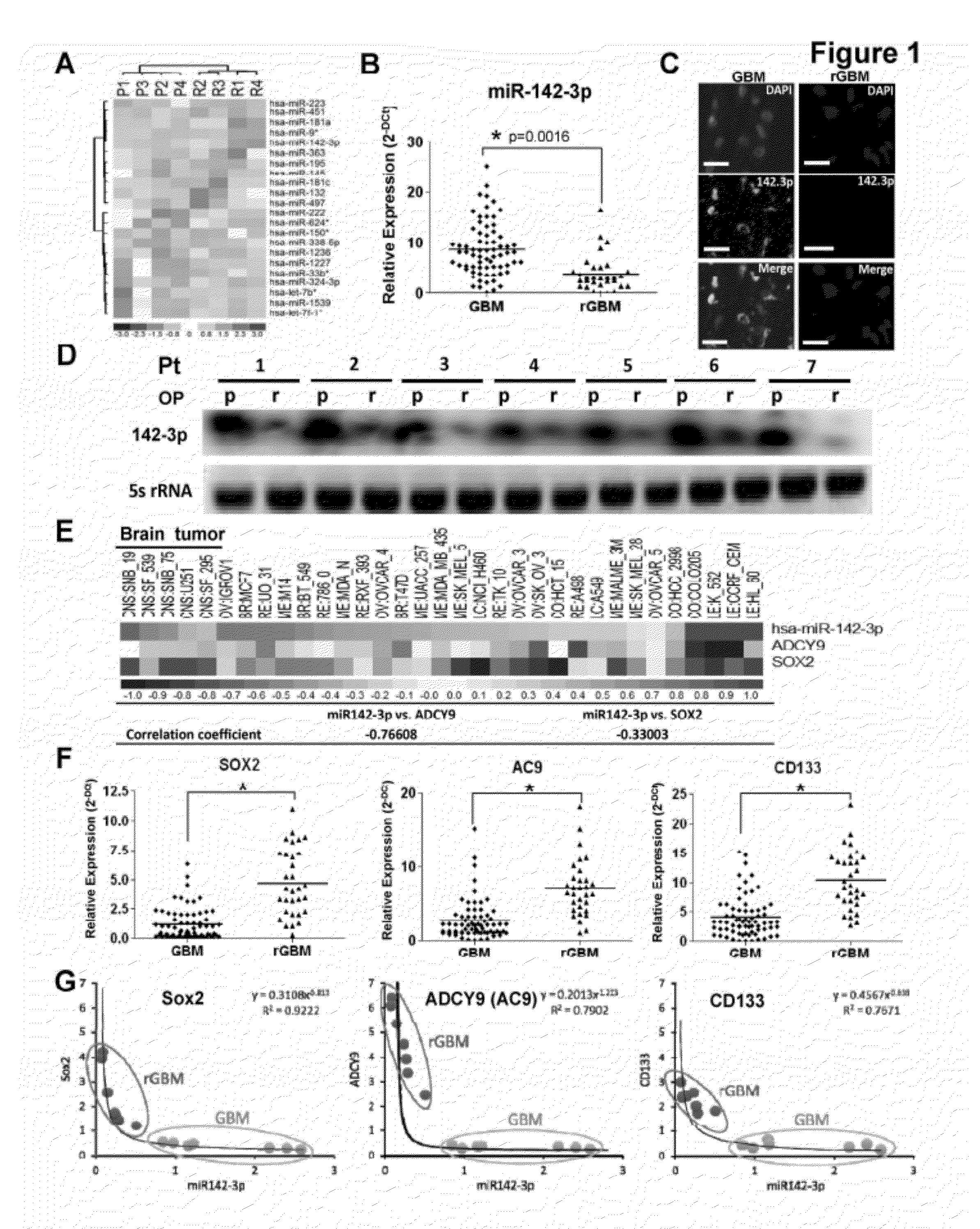
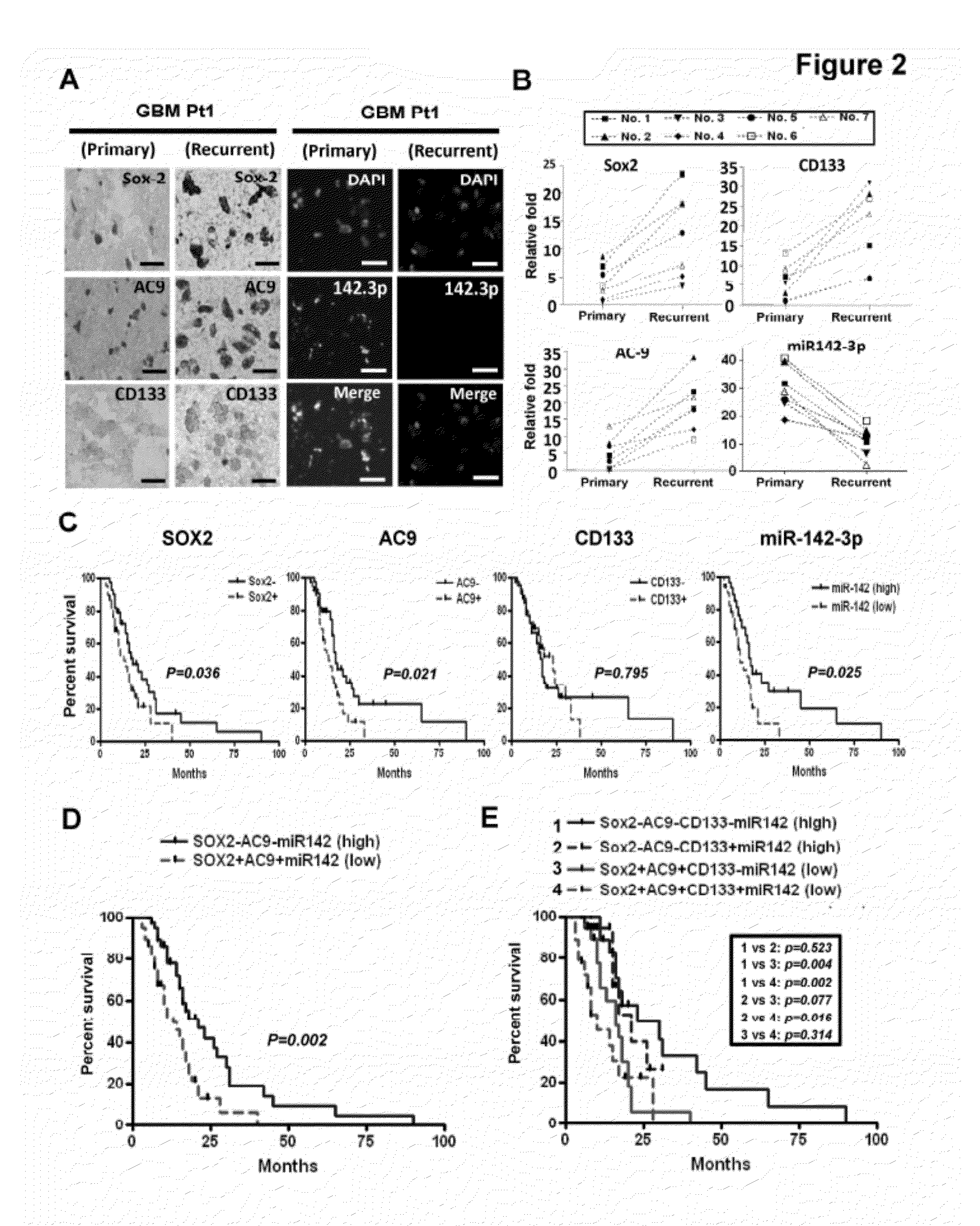
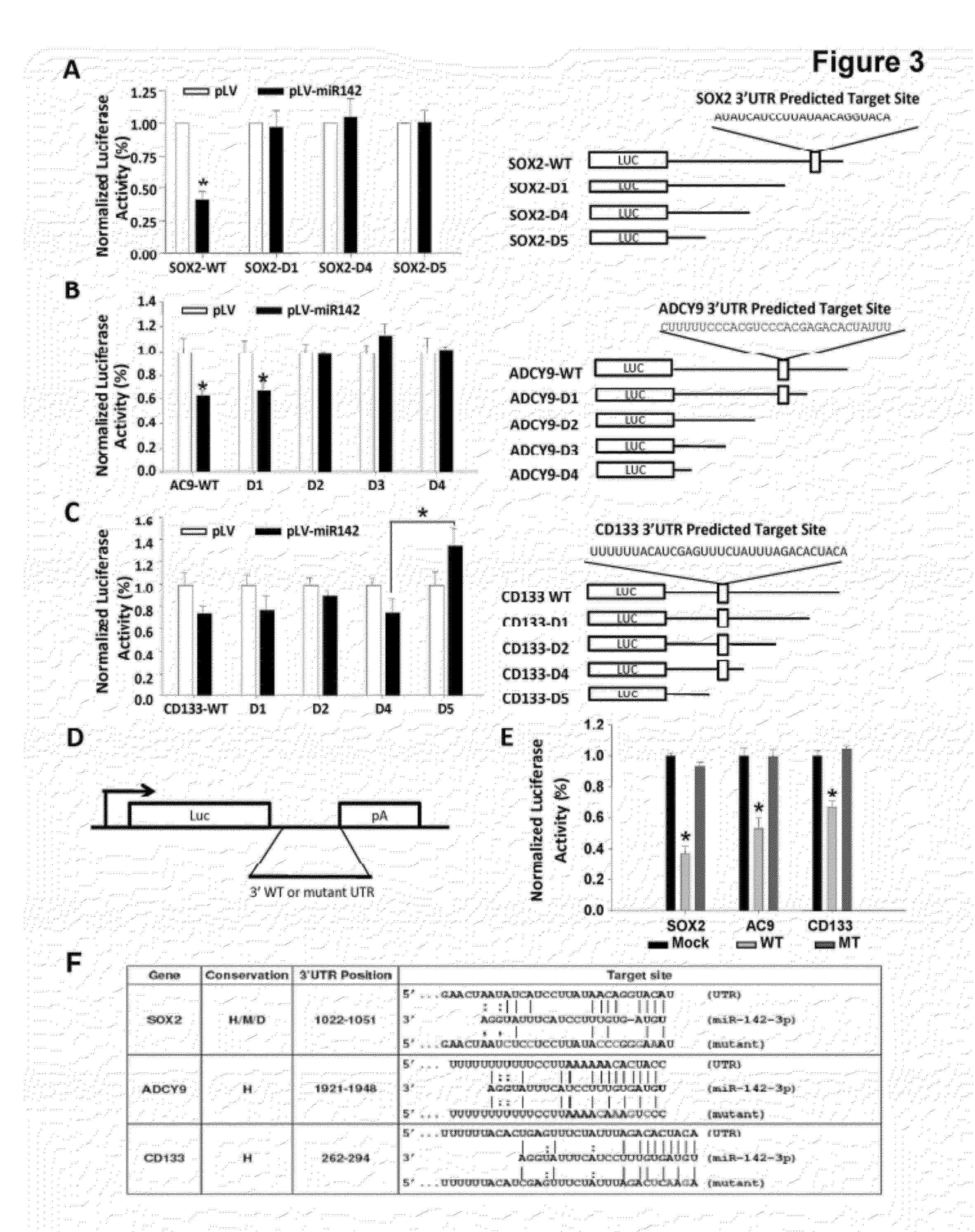
The present invention is based on the findings that a novel function for miR142-3p in the regulation of Sox2, adenylyl cyclase 9 (AC9), and CD133 expressions, and consequently the overall stemness of recurrent GBM cells as well as CSCs, and that miR142-3p modulated tumor-initiating properties in recurrent GBM. The present invention consequently supports the development of novel miRNA-based strategies for brain tumor treatment.
Owner:VETERANS GEN HOSPITAL TAIPEI
Method of regulating and controlling retinal progenitor cell generated by inducing
ActiveCN103571793AAvoid formingGood treatment effectSenses disorderUnknown materialsSOX2Retinal precursor cells
The invention provides a retinal progenitor cell generated by separating and inducing as well as a composition including the cell. The cell is transplanted to a host, so that integration rate and oncogenicity of the retina are greatly improved. The invention further provides a method of preparing the retinal progenitor cell by restraining a classical Wnt signal channel as well as a method of preparing the retinal progenitor cell suitable for transplanting. The invention further provides a composition consisting: (a), a Tcf1 protein, (b), a beta-catenin protein, and (c), a Sox2 protein as well as an application of the composition in screening a drug for lowering cell oncogenicity generated by inducing. The invention further provides a method of screening the drug for lowering cell oncogenicity generated by inducing. The invention opens up a novel way for cell treatment.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI +2
Identification and isolation of neural stem cells and neurosphere initiating cells
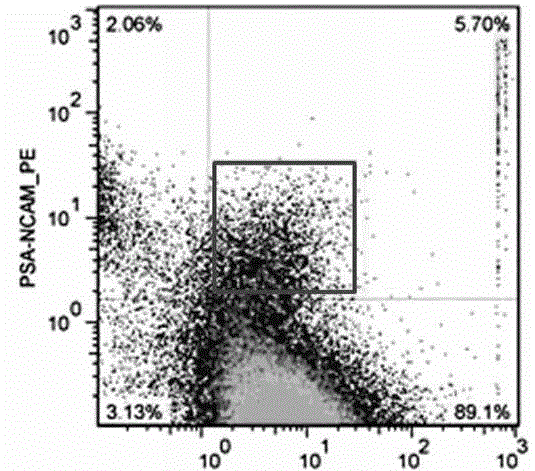
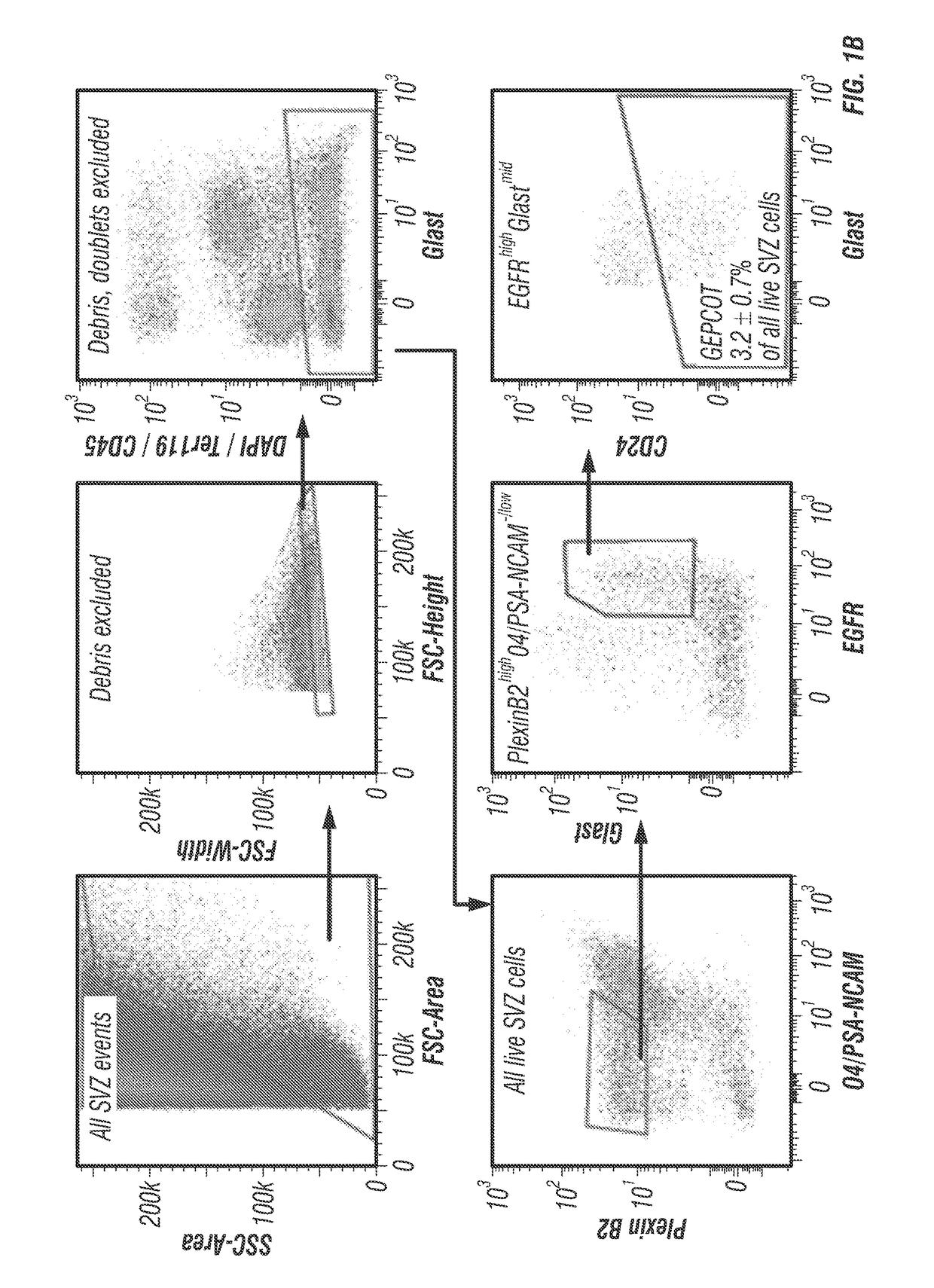
The disclosure reports on the identification and isolation of adult mouse lateral ventricle subventricular zone (SVZ) neurosphere initiating cells (NICs) by flow cytometry on the basis of GlastmidEGFRhighPlexinB2highCD24− / lowO4 / PSA-NCAM− / lowTer-119 / CD45− markers (GEPCOT cells). These cells are highly mitotic and short-lived in vivo based on fate-mapping with Ascl1CreERT2 and Dlx1CreERT2. In contrast, pre-GEPCOT cells were quiescent, expressed higher Glast, and lower EGFR and PlexinB2. Pre-GEP-COT cells could not form neurospheres but expressed the stem cell markers Glast-CreERT, GFAP-CreERT2, Sox2CreERT2, and Gli1C-reERT2 and were long-lived in vivo. While GEPCOT NICs were ablated by temozolomide, pre-GEPCOT cells survived and repopulated the SVZ. Conditional deletion of the Bmi-1 polycomb protein depleted pre-GEPCOT and GEPCOT cells, though pre-GEPCOT cells were more dependent upon Bmi-1 for p16Ink4a repression. These data distinguish quiescent NSCs from NICs and make it possible to study their properties in vivo.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
A method of targeting patient-specific oncogenes in extrachromosomal DNA to treat glioblastoma
PendingUS20190360029A1Inhibits tumor glioma growthCompounds screening/testingMicrobiological testing/measurementBAP1Present method
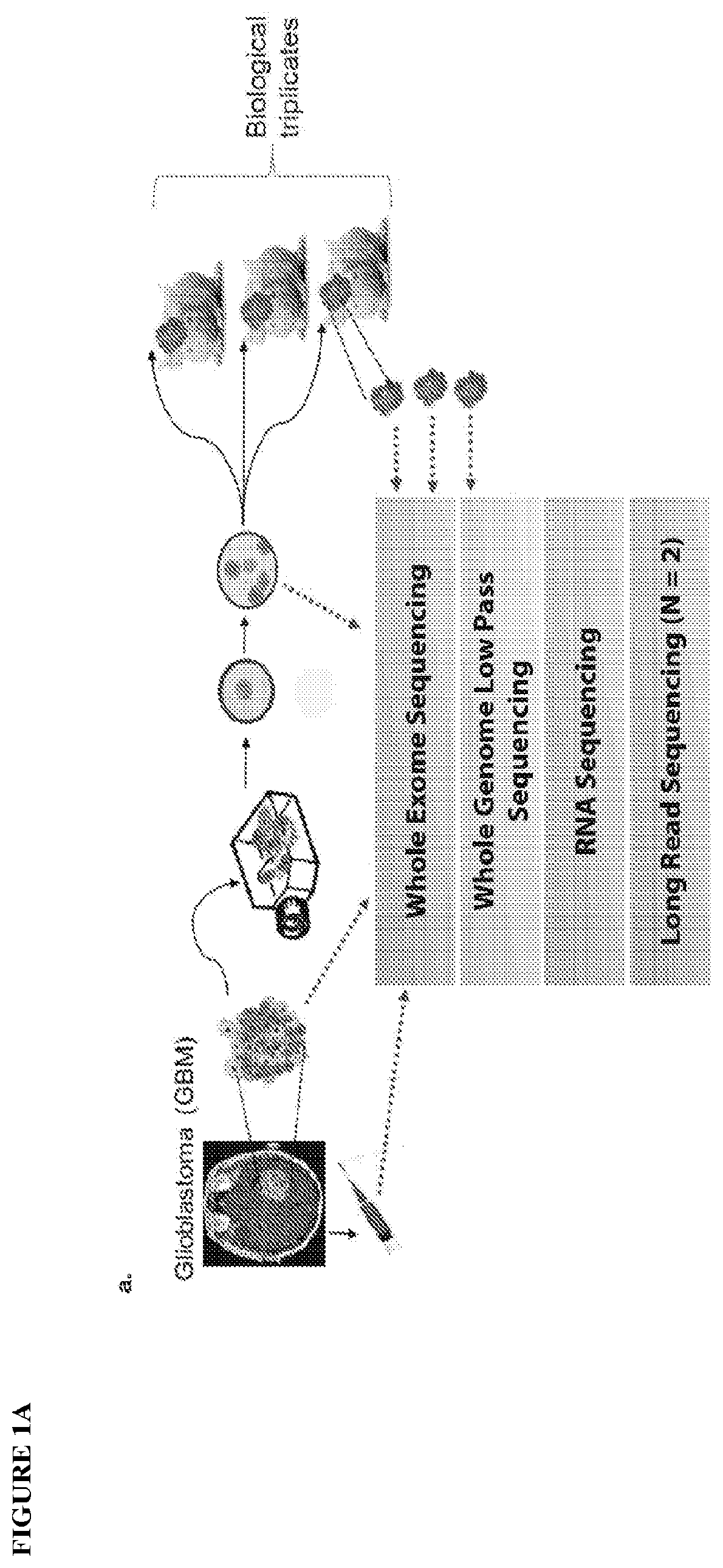
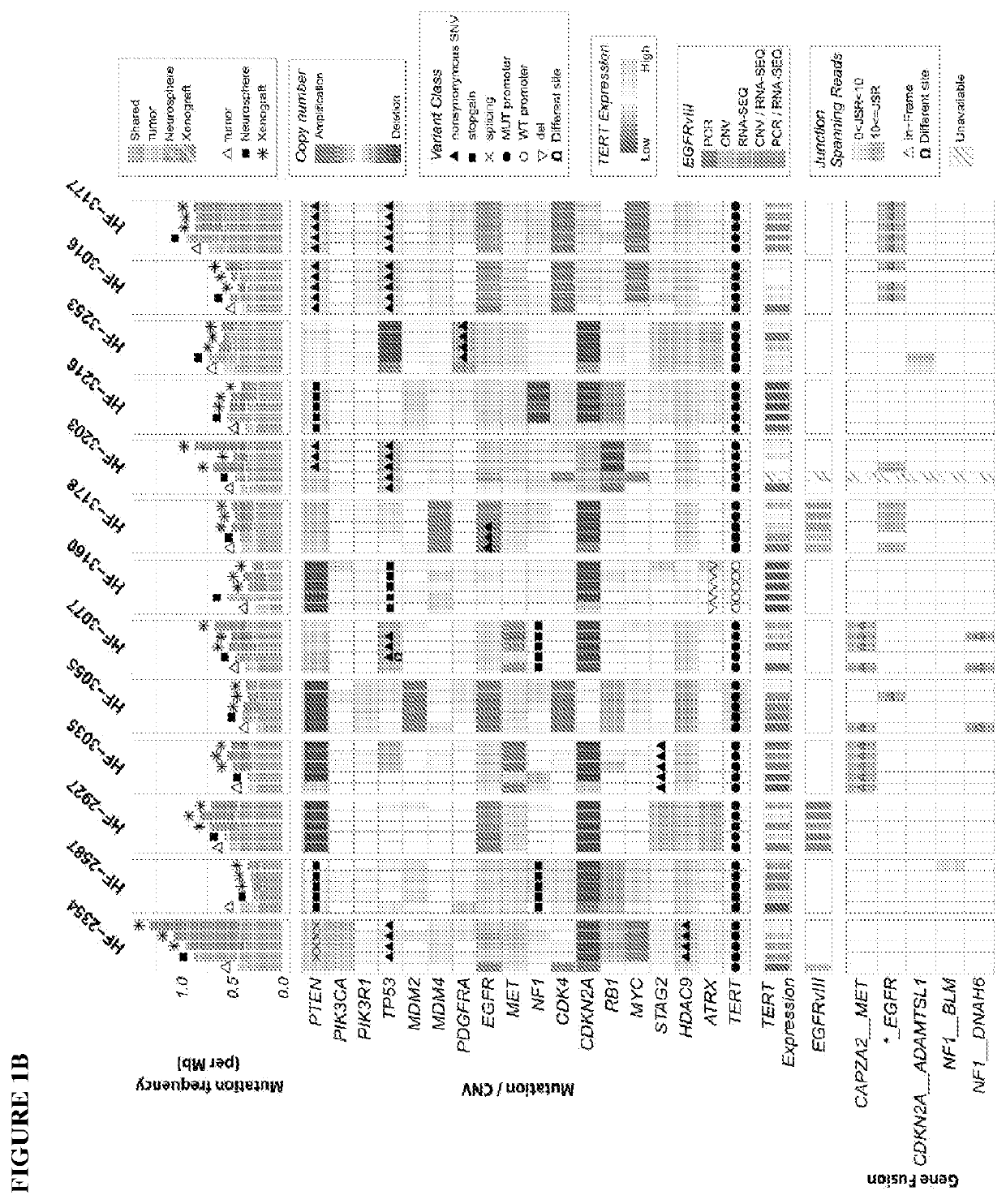
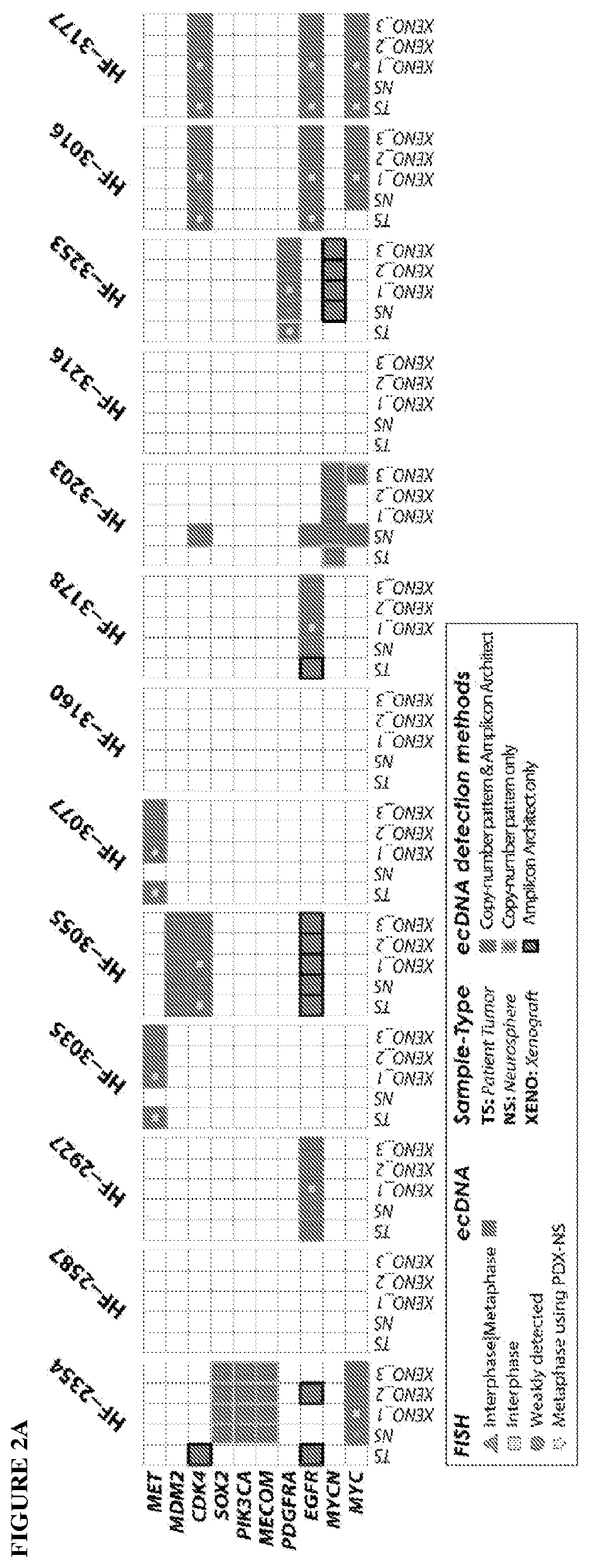
Provided are methods of targeting patient-specific oncogenes in extrachromosomal DNA (ecDNA) to treat glioma in a human. The present methods include identifying a drug that targets against an oncogene present in ecDNA of a human suffering from glioma, such as glioblastoma. The identified oncogenes present in ecDNA include MET, MET / CAPZA2, MDM2, CDK4, SOX2, PIK3CA, MECOM, PDGFRA, EGFR, MYCN, MYC, TERT, SMARCA4, RP56, FBXW7, CDK6, CCND2, ERBB2, BRCA1, and BAP1. The present methods include identifying a drug targeted against the ecDNA oncogene, which drug inhibits the function of the identified oncogene, so as to inhibit tumor growth or progression of the glioma in the human. Also provided are PDX mouse models to further identify and / or confirm patient-specific drugs that target the identified oncogene(s) present in ecDNA. Also provided are methods of diagnosing gliomas or recurrent gliomas and methods of screening or monitoring for recurrence of gliomas. Further provided are methods of validating a predicted presence of ecDNA in a brain tumor using fluorescence in situ hybridization (FISH). Also provided are methods of screening drug candidates for a patient by implanting different identified drugs that target an identified oncogene into PDX mouse models.
Owner:HENRY FORD HEALTH SYST
Use of umbilical cord blood regenerated particle and composition thereof in treatment of cerebral degenerative diseases
InactiveCN108531454AFacilitate cognitionFunction increaseNervous disorderNervous system cellsSOX2Brain degeneration
The invention discloses use of an umbilical cord blood regenerated particle and a composition thereof in treatment of cerebral degenerative diseases. The umbilical cord blood regenerated particle hasthe following characteristics that: the diameter size of the umbilical cord blood regenerated particle is 1-5micrometers; the umbilical cord blood regenerated particle contains trace DNA component; and the umbilical cord blood regenerated particle expresses Oct3 / 4, Nanog and Sox2 proteins. Research finds that the therapeutic agent prepared from the umbilical cord blood regenerated particle and thecomposition thereof can effectively treat cerebral degenerative diseases, and is especially suitable for regeneration of brain nerve cells and delaying of brain degeneration.
Owner:孔五一
System for predicting prognosis of patient with lung squamous cell carcinoma
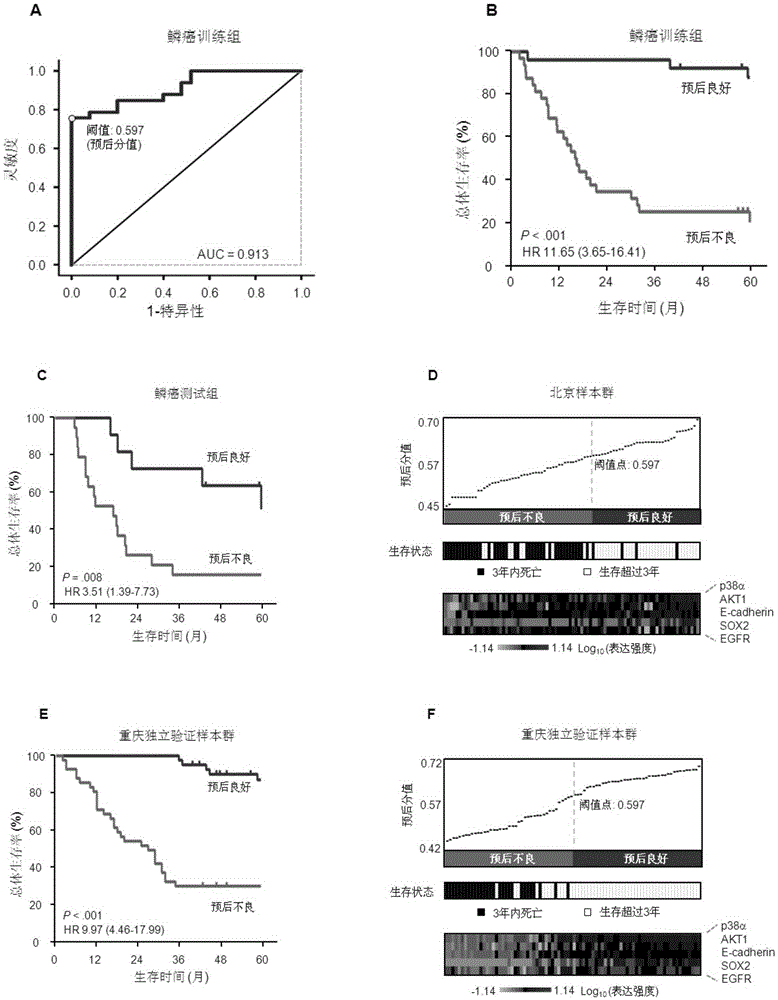
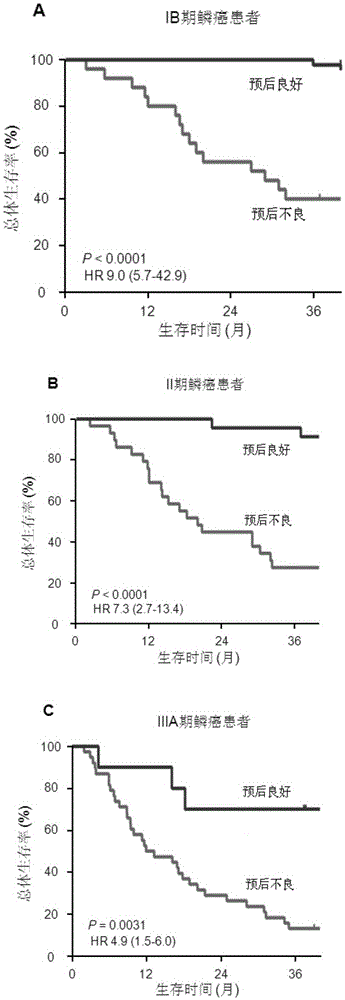
ActiveCN106442990AAccurately predict clinical prognosisImproving the level of prognosis predictionBiological material analysisSquamous CarcinomasSOX2
The invention discloses a system for predicting the prognosis of a patient with lung squamous cell carcinoma. The system includes a subsystem for detecting the expression quantity of such five proteins as EGFR, p38alpha, AKT1, SOX2 and E-cadherin and a protein expression quantity data processing system. The subsystem for detecting the expression quantity of the five proteins is allowed to measure the expression quantity of the proteins through an immunohistochemistry staining method; the protein expression quantity data processing system is allowed to convert the expression quantity of the five proteins from the squamous cell carcinoma tissues separated from the patient with the lung squamous cell carcinoma to a prognostic score; based on the prognostic score, the prognosis of a patient with the lung squamous cell carcinoma is predicted.
Owner:BIOMEDICAL ANALYSIS CENT OF ACADEMY OF MILITARY MEDICAL SCI
Multipotent stem cells and uses thereof
InactiveUS20120003737A1Easily attainable embryonic-likePromote wound healingPancreatic cellsMammal material medical ingredientsGerm layerDisease
The invention provides a quiescent stem cell having the capacity to differentiate into ectoderm, mesoderm and endoderm, and which does not express cell surface markers including MHC class I, MHC class II, CD44, CD45, CD13, CD34, CD49c, CD73, CD105 and CD90. The invention further provides a proliferative stem cell, which expresses genes including Oct-4, Nanog, Sox2, GDF3, P16INK4, BMI, Notch, HDAC4, TERT, Rex-1 and TWIST but does not express cell surface markers including MHC class I, MHC class II, CD44, CD45, CD13, CD34, CD49c, CD73, CD105 and CD90. The cells of the invention can be isolated from adult mammals, have embryonic cell characteristics, and can form embryoid bodies. Methods for obtaining the stem cells, as well as methods of treating diseases and differentiated the stem cells, are also provided.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com