Methods and Compositions for Direct Reprogramming of Somatic Cells to Stem Cells, and Uses of these Cells
a technology of somatic cells and compositions, applied in the field of compositions for direct reprogramming of somatic cells to stem cells, can solve the problems of major hurdles to overcome before induced cell technology, other challenges of cells, genetic instability and cancer risk,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
OCT4 or SOX2 Overexpression Via Lentiviral Expression De-Differentiates AF Cells
[0125]AF cells could be easily cultured and expanded in large amount from only 5 ml AF sample. The average doubling time of these cells is 12±1.6 hours. Passage 3 to 5 AF cells were plated in PLO / LN (poly-L-ornithine or poly-L-lysine) coated tissue culture plates and infected with Oct4 or Sox2 lentivirus particles at MOI of 10-100 in the presence of 8 μg / ml polybrene for 6 hours. 24-48 hours after infection, the efficiency of lentiviral infection was determined under fluorescent microscope by GFP expression. Typically the percentage of GFP positive cells can reach higher than 80%.
[0126]Transduced cells were plated to assess growth. Numerous colonies were present only 7 or 9 days post-transduction. The OCT4 transduced colonies in particular were able to expand and continue to grow over 10 passages. The inventors found that addition of small chemical compounds such as TGF-βreceptor I inhibitor II (10 uM) a...
example 2
OCT4 or SOX2 Overexpression via Lentiviral Transduction can Reprogram AF Cells to Neural Precursor Cells
[0127]After OCT4- or SOX2-induced dedifferentiation, cells were transferred to ReNcell medium plus bFGF and EGF. On Day 4 in ReNcell medium, OCT4 and SOX2-induced AF cells formed cell clusters in culture (FIG. 1C; FIG. 7, “AF”) and these clusters could be dissociated into single cells for monolayer culture. The induced cells resembled neural stem cells in morphology (FIG. 1 D; FIG. 6“SOX2”), including development of long, thin processes which are drastically different from the WT or GFP-only control cells (FIGS. 1A-B; FIG. 6“GFP” and “WT”).
[0128]Neurosphere formation is a feature of NSC growth in vitro. Not surprisingly, the induced NSC from AF cells could form typical neurospheres in low attachment dish (FIG. 2A; FIG. 7“AF”).
[0129]Next, the inventors used antibodies to Nestin and Mushashi-1, which are neural stem cell markers, to detect the expression of NSC-specific markers in t...
example 3
OCT4 Overexpression via Lentiviral Transduction can Reprogram AF Cells to Endothelial Stem Cells
[0133]After viral transduction, cells were transferred to human ES (embryonic stem cell) medium (supplemented with 20% Knockout Serum replacement, 2 mM glutamine, 0.1 mM non-essential amino acids, 10 ng / ml bFGF, 100 μM β-Mercaptoethanol) or endothelial cell medium, such as EGM2 (Lonza, Inc.). Numerous colonies appeared on day 9 that were not seen in the AF cells transduced with control lentiviruses expressing GFP. More than 90% of colonies were FLK1 positive by day 11.
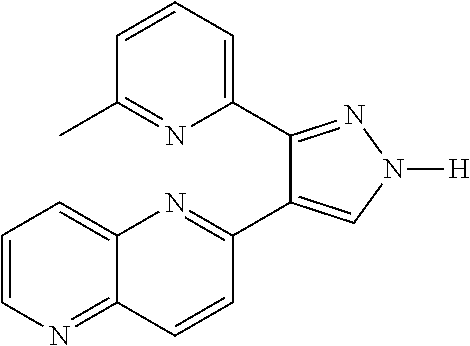
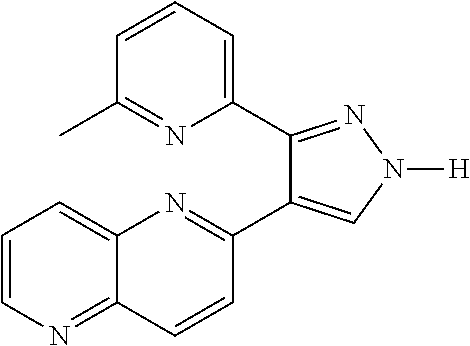
[0134]In order to increase the colony forming efficiency, the inventors tried different culture media and found that the addition of TGF-beta receptor1 inhibitor II, 616452 at 10 μM or 8-Br-cAMP at 0.1 mM in the culture media accelerated and increased the efficiency of colony formation by approximately 15%.
[0135]616452 is 2-(3-(6-Methylpyridin-2-yl)-1H-pyrazol-4-yl)-1,5-naphthyridine with structure:
[0136]8-Br-cAMP, also know...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Elongation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com