Use of umbilical cord blood regenerated particle and composition thereof in treatment of cerebral degenerative diseases
A degenerative disease, umbilical cord blood technology, applied in the field of biomedicine, can solve complex and multi-step problems, and achieve the effect of alleviating brain decline and improving cognitive and behavioral functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of umbilical cord blood regeneration particles
[0051] In this example, the preparation of umbilical cord blood regeneration particles is exemplified.
[0052]Blood source: The umbilical cord blood is collected into a blood bag containing anticoagulant. The anticoagulant is the original sodium citrate in the blood bag, and the umbilical cord blood is transported to Preparation lab.
[0053] Conditions: The process of obtaining umbilical cord blood regeneration particles requires a complete aseptic operation.
[0054] Specific steps are as follows:
[0055] (1) The umbilical cord blood was taken and centrifuged at 200g for 10 minutes. After centrifugation, it was divided into two parts, the upper layer solution A and the lower layer precipitate A.
[0056] (2) Take the lower layer of precipitate A, add PBS at a volume ratio of 1:2-5 for slight washing, and centrifuge at 200g for 10 minutes. After centrifugation, it is divided into two parts, the upper lay...
Embodiment 2
[0063] Regenerating particles migrate into the injured cerebral hemisphere in a ribbon pattern
[0064] The experimental group injected the regenerative particles (GFP marker) obtained in Example 1 into the mice with ischemic brain injury through the tail vein, and the control group injected normal saline.
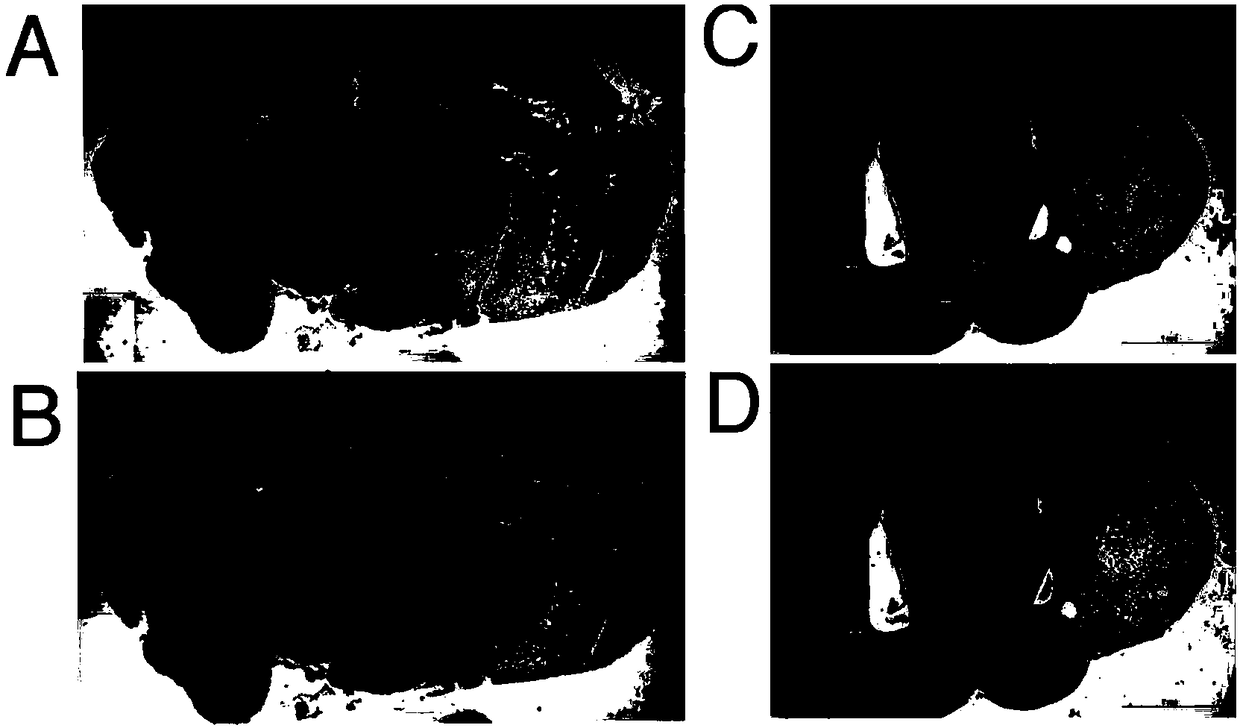
[0065] At 7 days, the sections of the control group brains were all negative for GFP ( figure 2 A), but positive for GFP in the GFP antibody reactive fraction ( figure 2 B). At 4 weeks, consecutive sections of control part brains were negative for GFP ( figure 2 C), but positive for GFP in the GFP antibody reactive fraction ( figure 2 D). At 7 days, whole brain sections showed that almost all left hemisphere tissue was ischemic and damaged, and it was enhanced and enlarged. GFP-positive material showed a banded pattern (see figure 2 B) Arranged around the injured hemisphere.
Embodiment 3
[0067] Regenerating Particles Forming Granular Cells
[0068] The experimental group injected the regenerative particles (GFP marker) obtained in Example 1 into the mice with ischemic brain injury through the tail vein, and the control group injected normal saline.
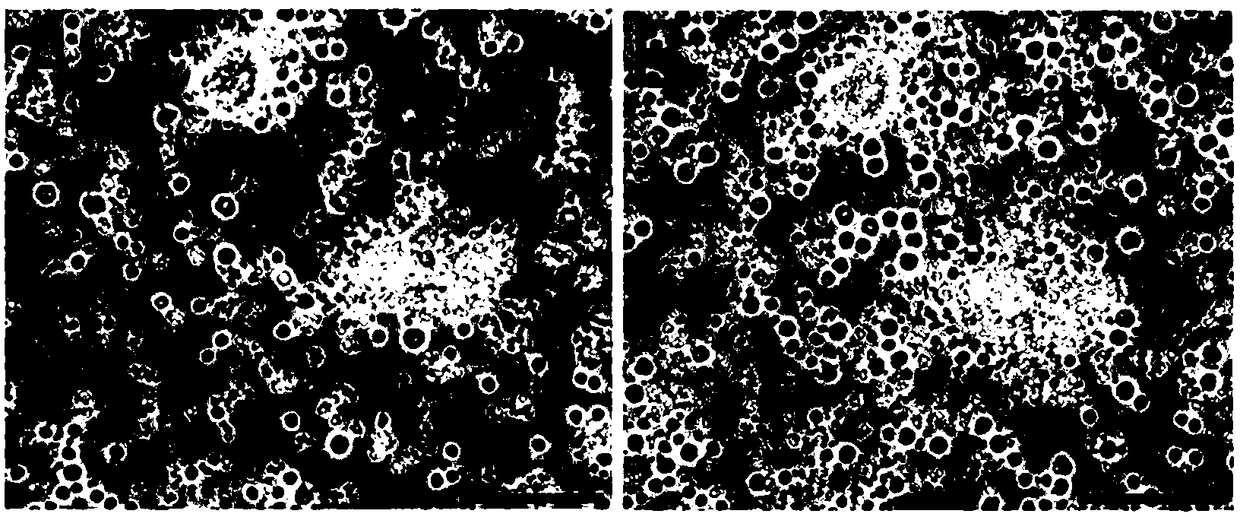
[0069] After the regenerative particles migrate into the brain tissue, they first fuse into a non-nucleated cell-like structure, and then further form nucleated cells. High-magnification microscopic observation further confirmed that regenerative particles from capillaries (see Figure 4 ) fuse together before forming granule cells and further develop to form new granule cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com