Serum autoantibody detection kit
A technology for detection kits and autoantibodies, applied in biological tests, measuring devices, material inspection products, etc., can solve the problems of low detection sensitivity and accuracy, and achieve the effect of improving accuracy and increasing sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 , Recombinant antigenic protein expression and purification
[0051] Using the human cDNA library (purchased from Invitrogen) as a template, PCR amplification was used to prepare the required recombinant antigen protein fragments, wherein the amino acid sequence information of the recombinant antigen protein is shown in Table 1, and the primer information for PCR amplification is as follows Table 2 shows.
[0052] Table 1. Amino acid sequence information of recombinant antigen protein
[0053] recombinant antigenic protein
Amino acid sequence number
p53
SEQ ID NO:1
SEQ ID NO:2
CAGE
SEQ ID NO:3
Cyclin D
SEQ ID NO:4
NY-ESO-1
SEQ ID NO:5
GBU4‐5
SEQ ID NO:6
HuD
SEQ ID NO:7
PGP9.5
SEQ ID NO:8
MDM2
SEQ ID NO:9
GAGE7
SEQ ID NO:10
XAGE1b
SEQ ID NO: 11
SOX2
SEQ ID NO:12
MAGE A1
SEQ ID NO: 13
MAGE A...
Embodiment 2
[0060] Example 2 1. Preparation of enzyme-linked immunosorbent assay kit for detecting autoantibodies
[0061] 2.1. The composition of the ELISA kit for detecting autoantibodies
[0062] The ELISA kit (antigen immobilized) for the detection of autoantibodies includes the following components:
[0063] 1. ELISA plates coated with different antigen protein combinations;
[0064] 2. Enzyme conjugate-horseradish peroxidase-labeled goat anti-human immunoglobulin, the concentration is 0.1 μg / ml;
[0065] 3. Positive quality control (standard product): human anti-c-Myc-labeled immunoglobulin G (purchased from Tribioscience);
[0066] 4. Negative quality control products;
[0067] 5. Serum dilution buffer;
[0068] 6. TMB developer;
[0069] 7. 20×antigen washing solution;
[0070] 8. Termination solution;
[0071] 9. Parafilm.
[0072] 2.2. ELISA plate preparation and ELISA detection of serum samples
[0073] The steps of enzyme plate preparation and ELISA detection are as...
Embodiment 3
[0090] Example 3 , Antigen combination and clinical significance
[0091] 3.1. Detection of the effectiveness of candidate antigenic proteins in Chinese lung cancer patients
[0092] The recombinantly expressed and purified antigenic protein in Example 1 was tested for its effectiveness in Chinese lung cancer patients according to the method described in Example 2, including p53, Annexin1, CAGE, NY-ESO-1, HuD, Cyclin D, PGP9 .5, MDM2, GAGE7, GBU4‐5, XAGE1b, SOX2, MAGE A1, P62 and MAGE A4.
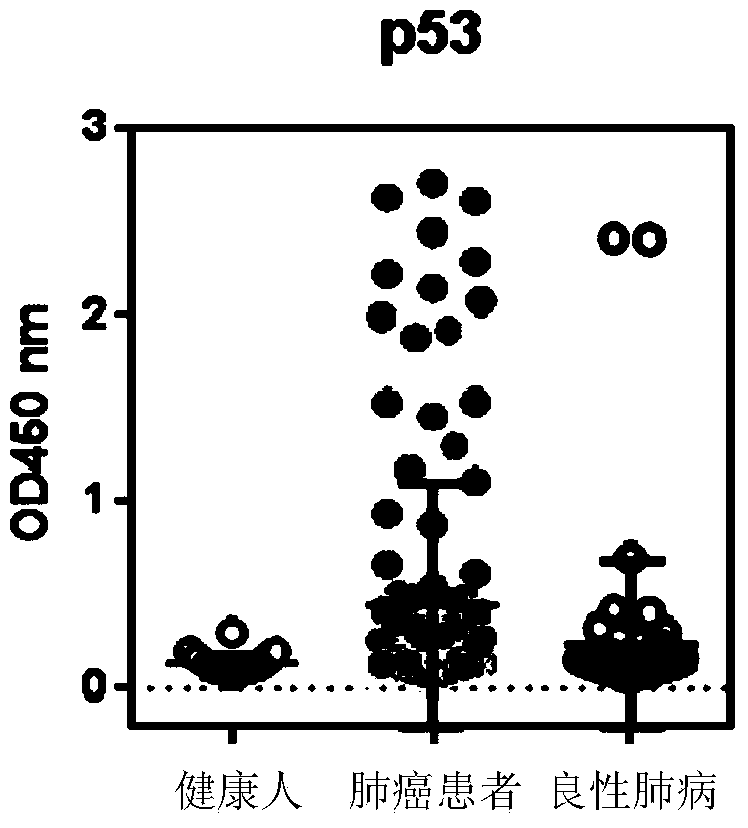
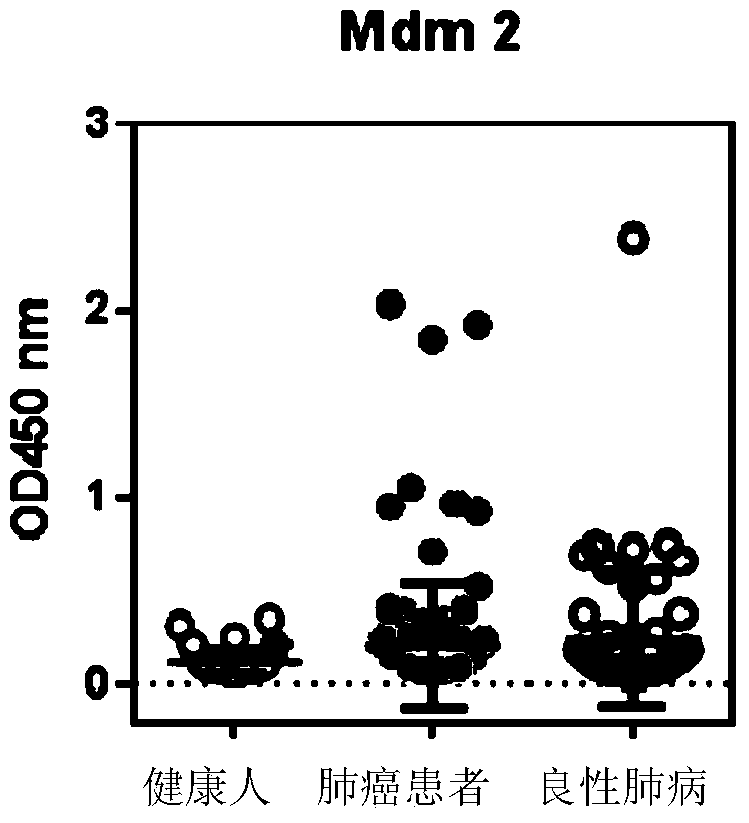
[0093] The result is shown in Figure 2, where:
[0094] 1. The OD450 of autoantibodies corresponding to p53 in patients with lung cancer was significantly higher than that in normal people and benign lung diseases, and 2 cases were positive in patients with benign lung diseases;
[0095] 2. The OD450 of autoantibodies corresponding to Mdm2 in lung cancer patients is significantly higher than that of normal people and benign lung diseases, and some samples in patients with benign lung di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com