Anti-phosphorylation Oct4 protein antibody and its application
A phosphorylation and non-phosphorylation technology, applied in anti-animal/human immunoglobulin, material inspection products, biological testing, etc., can solve the problem that embryonic totipotent cells cannot maintain development and self-repair, lack of inner cell groups, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 Preparation method of Oct4 phosphorylation site monoclonal antibody;
[0059] 1: Peptide synthesis
[0060] Synthetic phosphorylated polypeptide: Ala-Phe-Pro-Ser-Val-Pro-Val-p-Thr-Ala-Leu-Gly-Ser-Pro-Met-His50mg, non-phosphorylated polypeptide: Ala-Phe-Pro- Ser-Val-Pro-Val-Thr-Ala-Leu-Gly-Ser-Pro-Met-His 20mg.
[0061] 2: Serum Phase
[0062] After the rabbit was immunized for 5 times, the whole blood was collected, the serum was separated, and the titer of the serum was detected. Use part of the serum to detect the specificity of the reaction with the antigen by western blot method, and screen out the immunized rabbits with strong specificity.
[0063] 3: Cell Fusion
[0064] The selected spleen cells of rabbits and mouse myeloma cells SP2 / 0 were mixed together at a ratio of 5:1, added with 45% PEG solution, bathed in 37°C water for 90s, added culture medium to terminate the PEG effect, and cultured with selective medium. Only successfully fused cells...
Embodiment 2
[0071] Example 2 Preparation method of Oct4 phosphorylation site polyclonal antibody
[0072] 1: Peptide synthesis
[0073] Synthetic phosphorylated polypeptide Gly-Glu-Ala-Phe-Pro-Ser-Val-Pro-Val-p-Thr-Ala-Leu-Gly-Ser Pro-NH2 15mg, non-phosphorylated polypeptide Gly-Glu-Ala-Phe - Pro-Ser-Val-Pro-Val-Thr-Ala-Leu-Gly-Ser-Pro-NH 211 mg.
[0074] 2: Animal immunity
[0075] Select 2 healthy rabbits, collect serum 50-70ml / rat after 5ml / rat and 5 times of immunization.
[0076] 3: Identification of immune serum
[0077] Using enzyme-linked immunoassay, respectively, 10ug / ml antigen phosphorylated polypeptide Gly-Glu-Ala-Phe-Pro-Ser-Val-Pro-Val-p-Thr-Ala-Leu-Gly-Ser-Pro-NH 2 and non-phosphorylated polypeptide Gly-Glu-Ala-Phe-Pro-Ser-Val-Pro-Val-Thr-Ala-Leu-Gly-Ser-Pro-NH 2 Coat the reaction plate, add serum to the reaction plate for antigen-antibody reaction, and screen out animal sera that react positively with phosphorylated polypeptides but not negatively with phosphorylated...
Embodiment 3
[0078] Example 3 Analysis experiment of Oct4 phosphorylation site
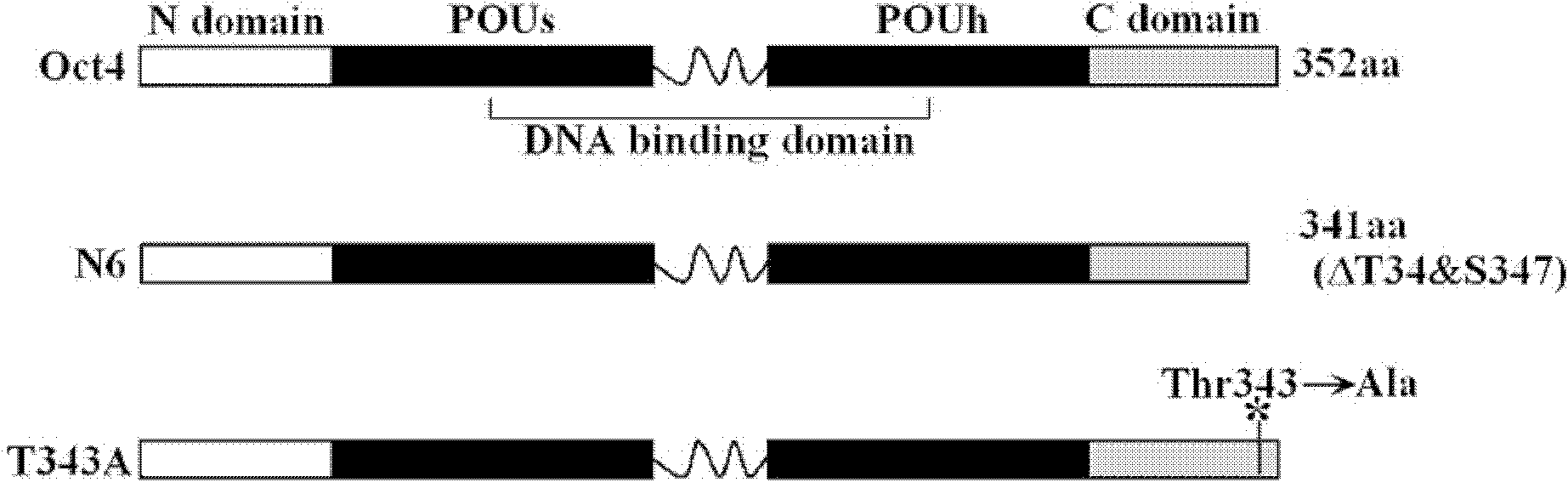
[0079] First, the amino acid residues of Oct4 protein were analyzed using NetPhos software 2.0 (Blom et al., 1999). The result of the analysis was that T343 at the C-terminus was a potential phosphorylation site.
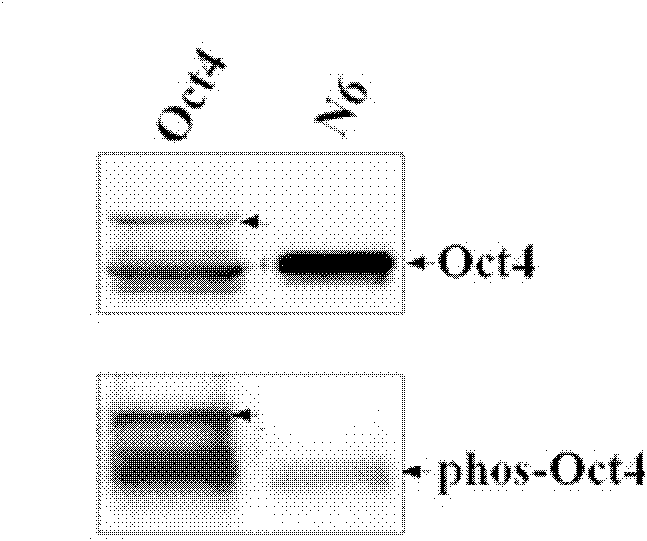
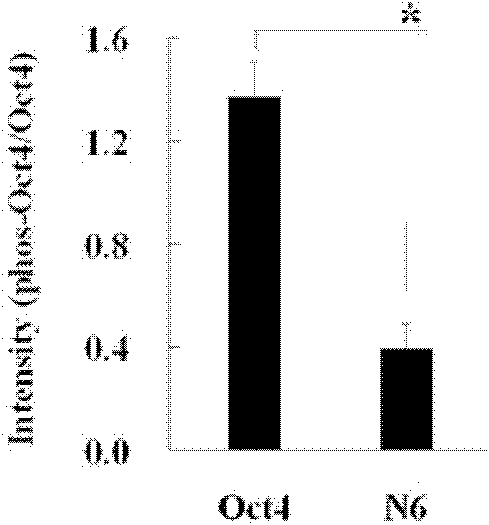
[0080] Design primers, use the polymerase chain reaction (PCR) method to truncate the T343 site, and construct the N6 protein expression plasmid. The schematic diagram of the plasmid construction is as attached figure 1 As shown, the constructed N6 protein expression plasmid was transferred into 293 cells for expression, and the cell lysate was collected, immunoprecipitated with Oct4 antibody, transferred to the hybridization membrane, and hybridized with the antibody against phosphorylated threonine. At the same time, a control group was designed. The control group was the full-length Oct4 group. figure 2 As shown, at the same time, the thickness and brightness of the bands were scanned by the Od...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com