Method for preparing antibody-maytansine alkaloid medicine conjugate
A drug conjugate, maytansine technology, applied in the biological field, can solve the problems of reduced plasma clearance and toxicity, increased cost, etc., to achieve the effect of improving efficiency and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1. Preparation of trastuzumab-SMCC-DM1 conjugate, verifying the effect of adding a surfactant on the coupling efficiency.
[0062] The preparation method of trastuzumab-SMCC-DM1 conjugate, the steps are as follows:
[0063] (1) Antibody replacement buffer
[0064] Using desalting chromatography (Sephadex TM G25) to replace the trastuzumab monoantigen solution into the reaction buffer A1 (0.1M sodium phosphate, pH7.5), and concentrate the antibody concentration to 20mg / ml to prepare the trastuzumab antibody A1;
[0065] Using desalting chromatography (Sephadex TM G25) to replace the trastuzumab monoantigen solution into the reaction buffer A2 (0.1M sodium phosphate, pH7.5, 0.05% v / v Tween 20), and concentrate the antibody concentration to 20mg / ml to prepare trastuzumab Antibody A2;
[0066] (2) Preparation of SMCC-DM1 mother liquor
[0067] Weigh SMCC-DM1 and fully dissolve it with dimethylacetamide (DMA) to prepare 10mg / ml SMCC-DM1 mother liquor;
[0068...
Embodiment 2
[0081] Example 2, adding anion exchange chromatography in the coupling process can reduce the content of conjugated product aggregates and endotoxin content.
[0082] The preparation of trastuzumab-SMCC-DM1 conjugates, the steps are as follows:
[0083] (1) Antibody replacement buffer: first use desalting chromatography (Sephadex TM G25) to replace the trastuzumab antibody stock solution into the reaction buffer (0.1M sodium phosphate, pH7.5, 0.05%v / v Tween 20), and concentrate the antibody concentration to 20mg / ml;
[0084] (2) Preparation of SMCC-DM1 mother solution: Weigh SMCC-DM1 with a molar ratio of 5.5 times that of trastuzumab in step (1), and fully dissolve it with dimethylacetamide (DMA) to prepare 10 mg / ml of SMCC-DM1 Mother liquor;
[0085] (3) Coupling reaction:
[0086] Add the SMCC-DM1 mother solution prepared in step (2) to the trastuzumab prepared in step (1) to start the coupling reaction, the reaction temperature is 25°C, and the reaction time is 1.5h;
...
Embodiment 3
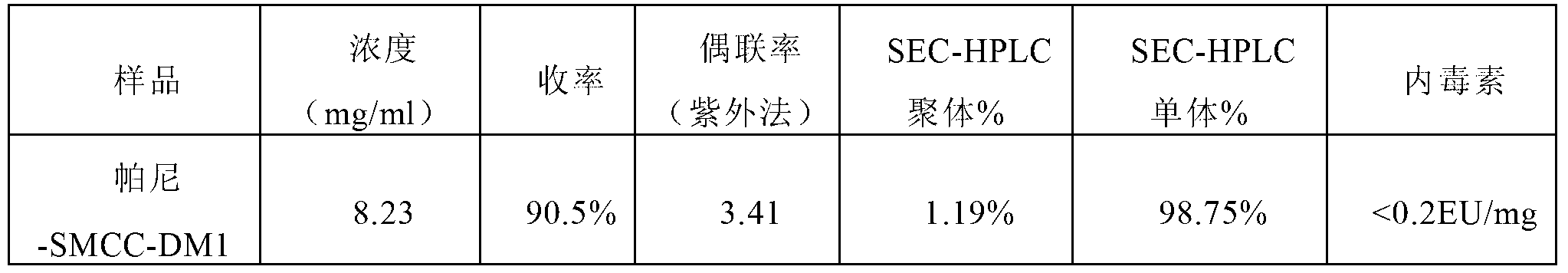
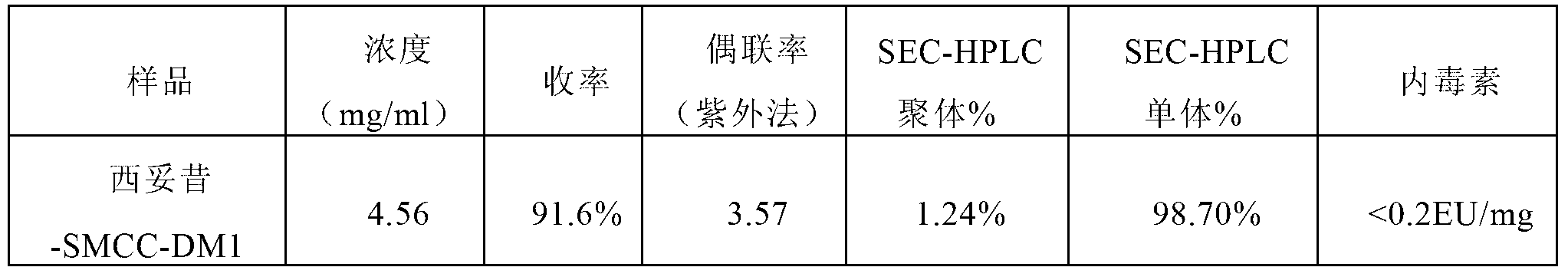
[0096] Embodiment 3, preparation of Pani-SMCC-DM1 conjugates, the steps are as follows:
[0097] (1) Antibody replacement buffer
[0098] First, use ultrafiltration concentration (pellicon system) to replace the Panitum monoantigen solution into the reaction buffer (20mM potassium phosphate buffer, 0.02% v / v Tween 20, pH 6.0), and concentrate the antibody concentration to 30mg / ml.
[0099] (2) Preparation of SMCC-DM1 mother liquor
[0100] Weigh SMCC-DM1 that is 5.0 times the molar ratio of panitumumab in step (1), and fully dissolve it with dimethylacetamide (DMA) to prepare a 10 mg / ml SMCC-DM1 mother solution.
[0101] (3) Coupling reaction
[0102] Add the SMCC-DM1 mother solution prepared in step (2) to the panitumumab prepared in step (1) to start the coupling reaction, the reaction temperature is 25°C, and the reaction time is 2.0h;
[0103] (4) Anion exchange chromatography
[0104] The above reaction solution was loaded on Capto Adhere anion exchange chromatograp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com