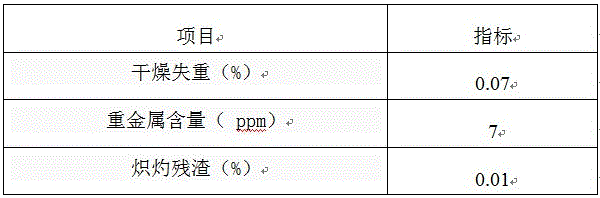
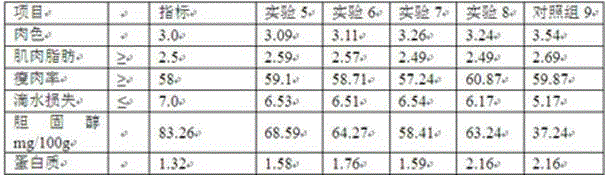
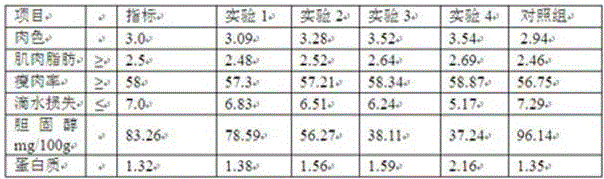
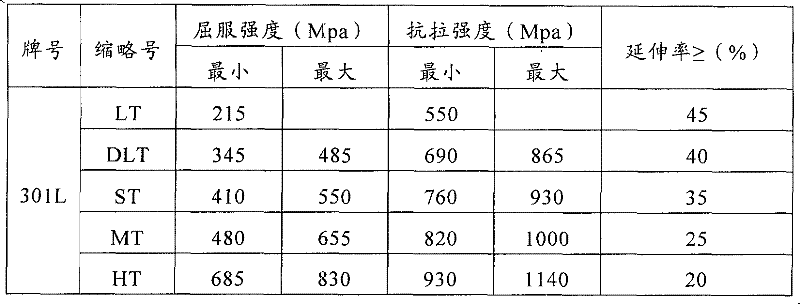
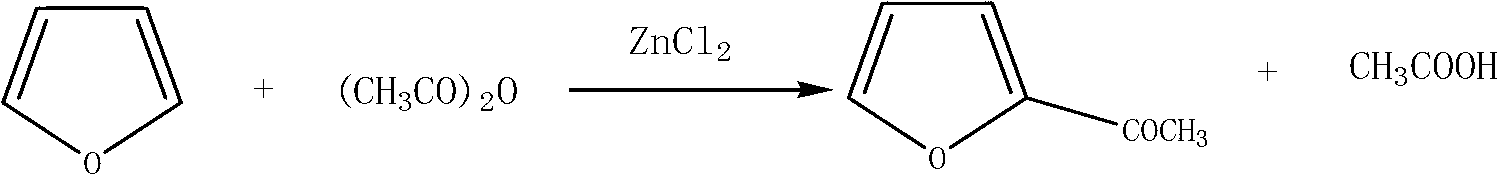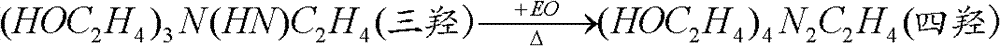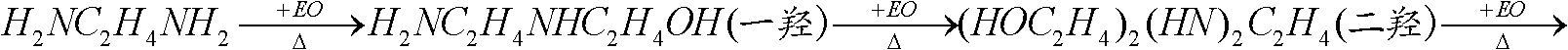Patents
Literature
78results about How to "Reduce the feed ratio" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
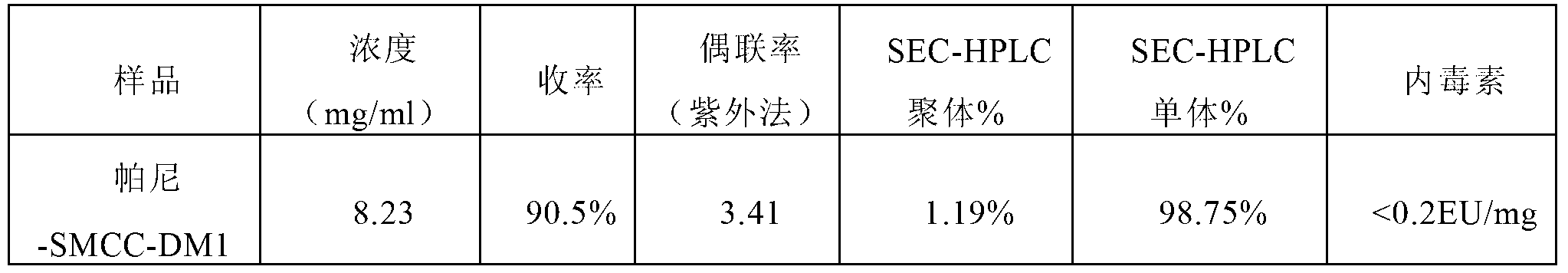
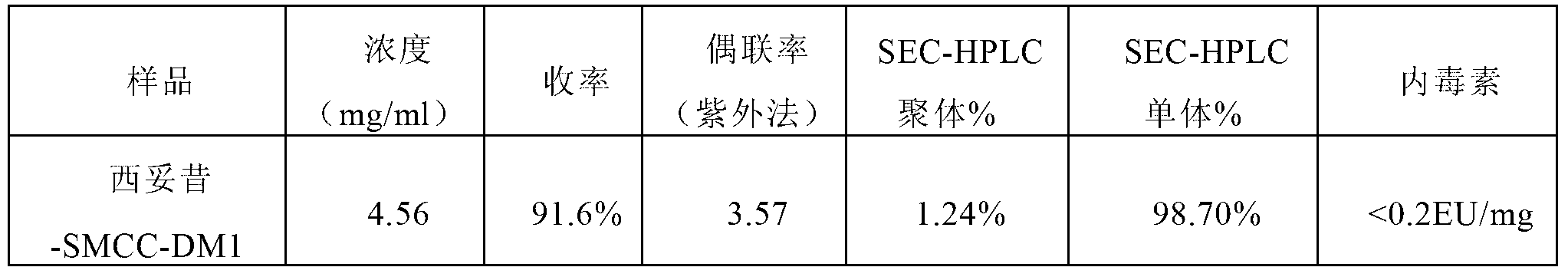
Method for preparing antibody-maytansine alkaloid medicine conjugate
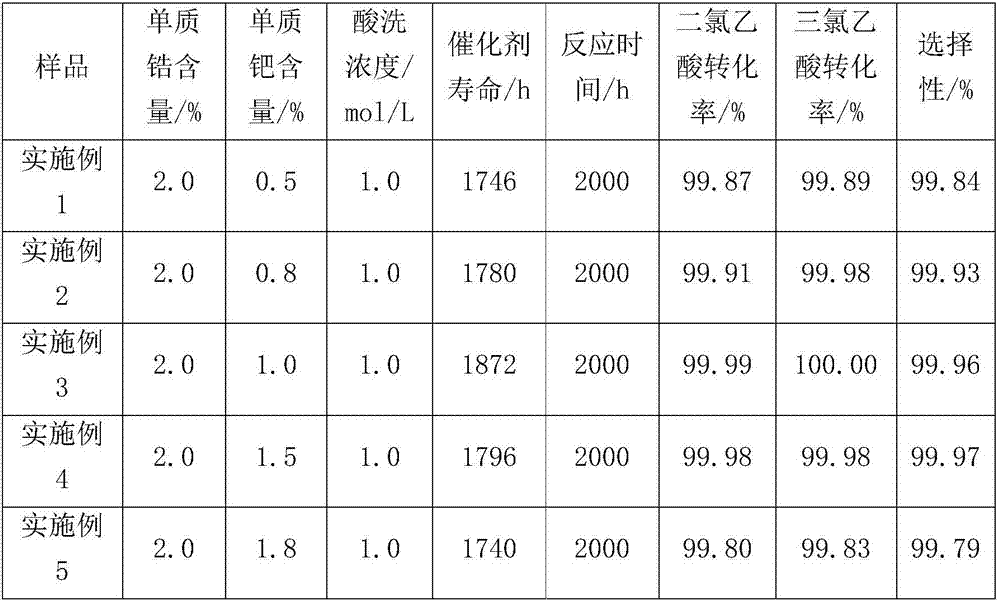
ActiveCN103254311AReduce hidden dangersImprove efficiencyImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsAnion-exchange chromatographyAlkaloid
The invention relates to a method for preparing antibody-maytansine alkaloid medicine conjugate. The method comprises the following steps of replacing the antibody into a reaction buffer solution; dissolving a dual-function bridging agent-maytansine alkaloid with an organic solvent so as to prepare the mother liquor of maytansine alkaloid medicine; mixing the replaced antibody with the mother liquor of maytansine alkaloid medicine for coupled reaction for 1-4 hours at 20-30 DEG C; and carrying out anion exchange chromatography on the reaction liquid, conducting Sephadex TMG25 desalination chromatographic column purification on collected flow liquid, and collecting a first peak sample as the prepared antibody-maytansine alkaloid medicine conjugate. The prepared antibody-maytansine alkaloid medicine conjugate is proper in coupling rate, high in purity and low in endotoxin content.
Owner:QILU PHARMA CO LTD
Condensation production process and special device of N-long-chain acyl amino acid salt
ActiveCN102126984AReduce contentSolve the inhibition problem of acid chloride hydrolysisOrganic compound preparationSulfonic acids salts preparationCondensation processN acylaminoacid
The invention relates to a production process and special device of the condensation process of N-long-chain acyl amino acid salt. By adopting the process and special device, the hydrolysis of acyl chloride can be inhibited and the content of the by-product fatty acid salt can be reduced in the condensation process of acyl amino acid type surfactant. Therefore, the weight of the surfactant can be increased, the content of fatty acid salt can be reduced by 50%-75% compared with the single-pot reaction, the conversion rate can be increased to more than 99%, the feeding ratio can be reduced, the condensation time can be shortened and the production efficiency can be increased.
Owner:SHANGHAI OLI ENTERPRISES CO LTD
Method for synthesizing dimethyl isophthalate-5-sodium sulfonate
ActiveCN102633693AReduce usageImprove conversion rateSulfonic acids salts preparationSulfonateCadmium sulfate
The invention discloses a method for synthesizing dimethyl isophthalate-5-sodium sulfonate. Dimethyl isophthalate-5-sodium sulfonate is prepared by steps of sulfonation reaction, esterification, neutralization reaction and aftertreatment with isophthalic acid and fuming sulfuric acid serving as raw materials, wherein the sulfonation reaction is completed in three reaction temperature zones, different catalysts are used in different reaction temperature zones, cadmium sulfate is used as the catalyst at the temperature ranging from 150 DEG C to 159 DEG C, SiO2 is used as the catalyst at the temperature ranging from 160 DEG C to 169 DEG C, and mercuric sulfate is used as the catalyst temperature ranging from 170 DEG C to 181 DEG C. By the method, sulfonation reaction effect is improved, conversion rate of isophthalic acid is increased, sulfonation reaction temperature is lowered while sulfonation reaction time is shortened, reaction by-products including disulfonate, polymer and sulfone compounds are greatly reduced, product purity is improved while product yield is increased, and further, usage of fuming sulfuric acid and usage of sodium carbonate in a subsequent neutralization procedure are reduced, so that production cost is reduced.
Owner:WEIFANG WORLD CHEM
Reactor and method for preparing epoxypropane by reactor
ActiveCN104907009AIntensified interphase mixingHigh selectivityOrganic chemistryChemical/physical processesSpray nozzleFixed bed
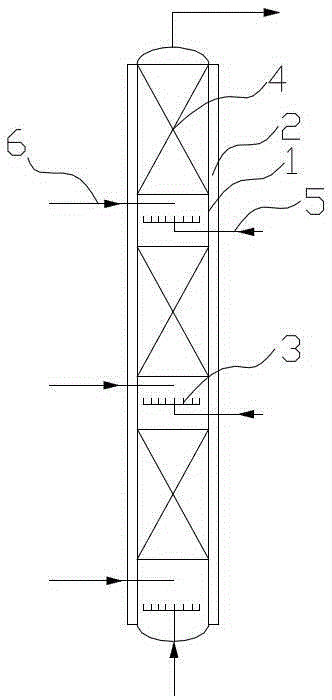
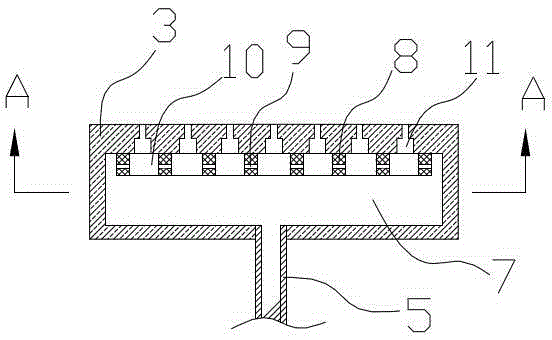
The invention discloses a reactor. The middle of the reactor is filled with an epoxidation catalyst, the top wall of the reactor is provided with a gas outlet, the upper side wall of the reactor is provided with a liquid outlet, the lower side wall of the reactor is provided with one or two groups of injectors, each group of the injectors comprises two injectors symmetrically distributed on the same plane, the injector comprises a jet pipe and a nozzle connected to the jet pipe, the nozzle is frustoconical, the diameter of one end of the nozzle connected to the jet pipe is greater than that of the other end, the nozzle stretches into a fixed bed reactor, the jet pipe comprises two sections, the section of the jet pipe far away from the nozzle is cylindrical, the other section is frustoconical, and the diameter of one end of the jet pipe far away from the nozzle is greater than the diameter of one end of the jet pipe connected to the nozzle. The invention discloses a method for preparing epoxypropane by the reactor and a reaction system composed of the reactors connected in series. The reactor realizes reactant high-speed clashing mixing, improves reactant mixing effects, improves heat and mass transfer effects and improves a cumene hydroperoxide conversion rate and epoxypropane selectivity.
Owner:HONGBAOLI GRP CO LTD +1
Low-silicon composite molecular sieve, and synthetic method and application thereof
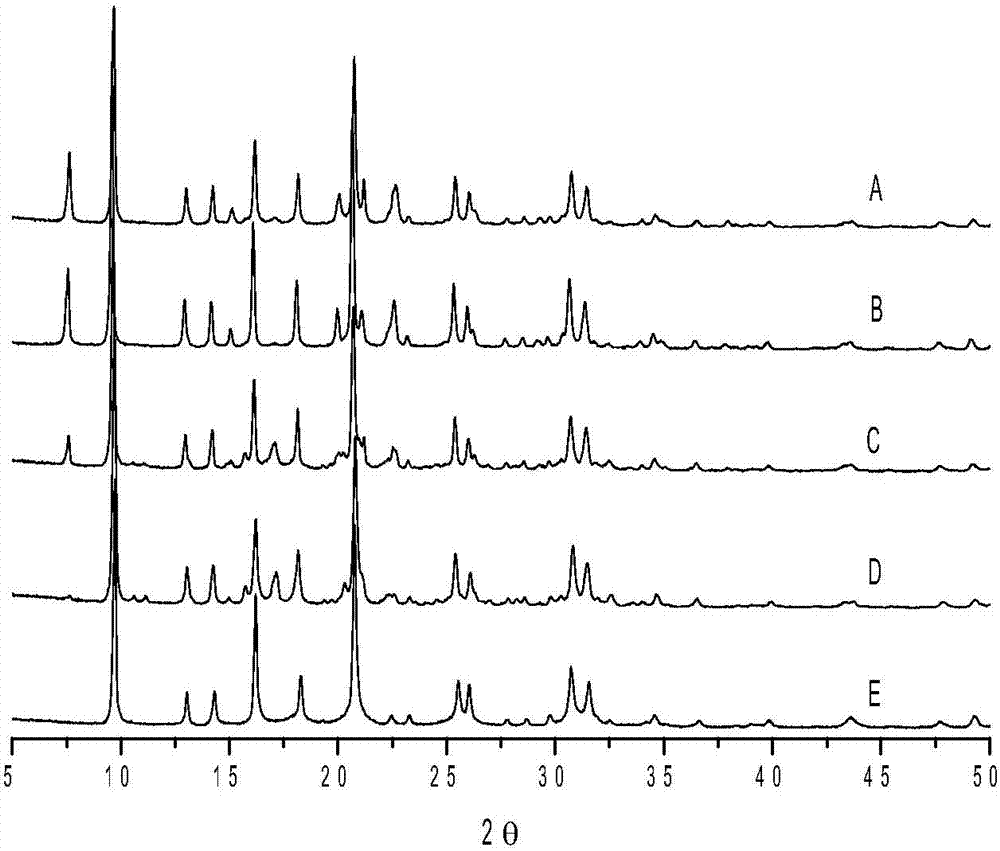
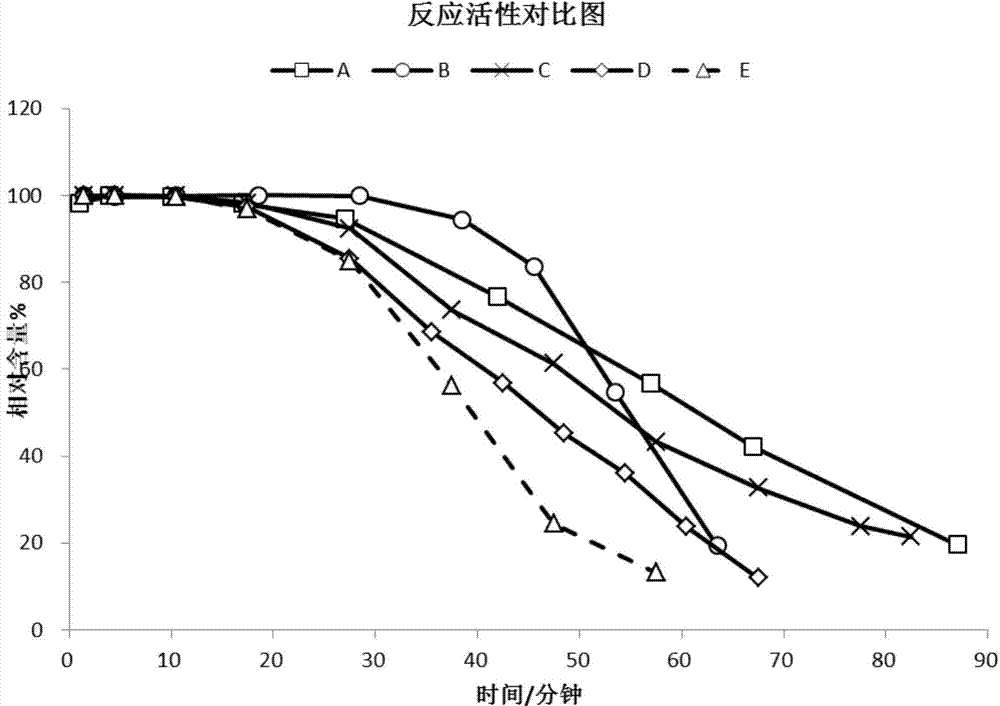
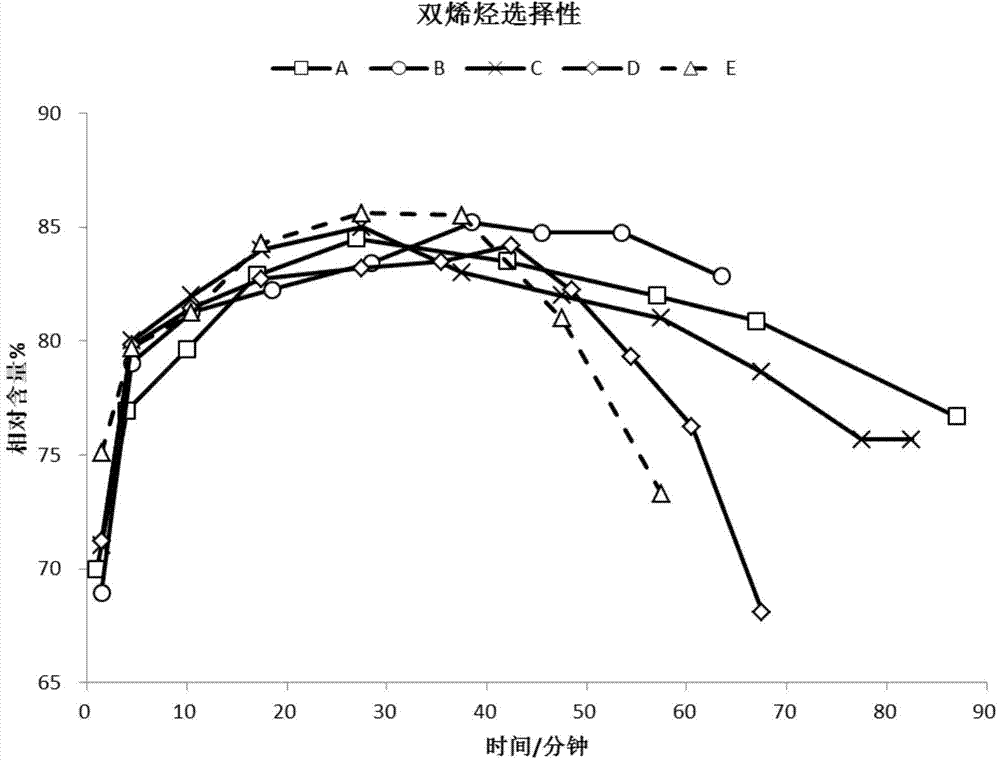
ActiveCN104326483AIncrease profitReduce the feed ratioMolecular sieve catalystsHydrocarbon from oxygen organic compoundsMolecular sieveComputational chemistry
A low-silicon composite molecular sieve comprises, by mass, 2-25% of an SAPO-5 molecular sieve and 75-98% of an SAPO-34 molecular sieve, and a molar ratio of SiO2 / Al2O3 is 0.07-0.15. The low-silicon composite molecular sieve has the advantages of low silicon, low cost, simple synthesis technology, high average diolefin selectivity in a methanol-to-olefin reaction, and long life.
Owner:天津众智科技有限公司
Solid-phase preparation method of efficient prostate-specific membrane antigen ligand PSMA-617
ActiveCN109134602AReduce the feed ratioReduce usagePeptide preparation methodsBulk chemical productionAntigenProstate specific membrane
The invention discloses a solid-phase preparation method of an efficient prostate-specific membrane antigen ligand PSMA-617. By a solid-phase polypeptide synthesis method of amino acid protected by Fmoc, hydroxyl resin serves a starting point, and PSMA-617 sequences are sequentially synthesized. According to the method, high-risk reagent triphosgene used in the prior art is avoided, Pd(Ph3P)4 is omitted, synthesis safety is improved, the purity of an end-product is higher than 98.5%, the method has the advantages of low cost, less waste liquid, high efficiency and safety and easiness in purification, and large-scale and industrial production is facilitated.
Owner:LANZHOU UNIVERSITY
Praziquantel preparation process
ActiveCN103570710AReduced post-processingReduce processingOrganic compound preparationCarboxylic acid amides preparationAlkaneLower risk
The present invention discloses a praziquantel preparation process, which comprises the following one-pot reaction, wherein R1 and R2 in the reaction formula are respectively and independently selected from C1-C4 alkane. According to the present invention, the existing multi-step reaction is combined into the one-pot reaction without any separation and purification operations so as to reduce the material feeding ratio during the reaction process, save the raw materials, reduce the cost, substantially simplify the operations and reduce the three-waste treatment, the high purity product can be obtained with the simple post-treatment, the yield can be up to more than 95%, and the important significance is provided for large-scale praziquantel preparation; and with the process technology, synthesis of the high purity praziquantel by using inexpensive and easily available raw materials, simple operations and mild reaction conditions in the low toxicity, low risk and low cost manner can be achieved, and the industrial production requirements are met.
Owner:SHANGHAI DESANO CHEM PHARMA
Method for preparing 4,4'-dicyclohexyl methane diisocyanate
InactiveCN102093259AAccurate measurementControl the feeding ratioIsocyanic acid derivatives preparationOrganic compound preparationSolventMethane
The invention belongs to the technical field of organic compounds and in particular relates to a method for preparing dicyclohexyl methane diisocyanate by a non-phosgene method. In the method, 4,4'-diamino dicyclohexyl methane or an isomer mixture thereof or salt thereof or a mixture of amine and salt thereof, serving as a raw material, reacts with di(trichloromethyl)carbonic ester or trichloromethyl chloroformate or a mixture of the di(trichloromethyl)carbonic ester and the trichloromethyl chloroformate in an inert solvent. By the method, defects of long process route, complex technology, low safety and the like in the preparation of the 4,4'-dicyclohexyl methane diisocyanate by the traditional phosgene method are overcome. The method has the advantages of convenience of operation, high safety and environmental friendliness. In the method, reactants are charged and metered accurately and rate of charge is reduced, so production safety coefficient is improved. Tail gas is simple in treatment; hydrogen chloride gas generated by the reaction is absorbed by water; high-purity hydrochloric acid can be prepared; chloride ions can be utilized completely; and environmental friendliness is contributed.
Owner:UPCHEM CHINA
Method for preparing methylcyclohexyl diisocyanate
InactiveCN102086162AAccurate measurementControl the feeding ratioOrganic compound preparationPreparation from carbamatesSimple Organic CompoundsSolvent
The invention belongs to the technical field of organic compounds and in particular relates to a method for preparing methylcyclohexyl diisocyanate by non-phosgene. The method comprises the following step of: reacting methylcyclohexyl diamine or an isomer mixture thereof or salt thereof or a mixture of amine and salt thereof as a raw material with bis(trichloromethyl)carbonate or trichloromethyl chloroformate or a mixture of the bis(trichloromethyl)carbonate and the trichloromethyl chloroformate in an inert solvent. In the method, the phosgene is replaced by the bis(trichloromethyl)carbonate or the trichloromethyl chloroformate or the mixture of the bis(trichloromethyl)carbonate and the trichloromethyl chloroformate; and the bis(trichloromethyl)carbonate or the trichloromethyl chloroformate or the mixture of the bis(trichloromethyl)carbonate and the trichloromethyl chloroformate reacts with methylcyclohexyl diamine or salt thereof to prepare the methylcyclohexyl diisocyanate. Compared with the traditional phosgene method, the method provided by the invention has convenience for operation, high safety, environment friendliness and mild reaction condition; and because the charging and the reaction are performed in normal pressure, the requirements on production equipment are reduced, and the process is simplified.
Owner:UPCHEM CHINA
Modified hydrodechlorination catalyst for producing high-purity chloroacetic acid and preparation method of modified hydrodechlorination catalyst
ActiveCN107413333AHigh hydrogenation reducibilityGood choiceOrganic compound preparationCarboxylic compound preparationActivated carbonPalladium
Owner:QINGDAO UNIV OF SCI & TECH
Method for producing isopropanolamine by using fixed bed tubular reactor
ActiveCN105348118AIncrease one-time synthesis rateAvoid side effectsOrganic compound preparationAmino-hyroxy compound preparationSide productChemistry
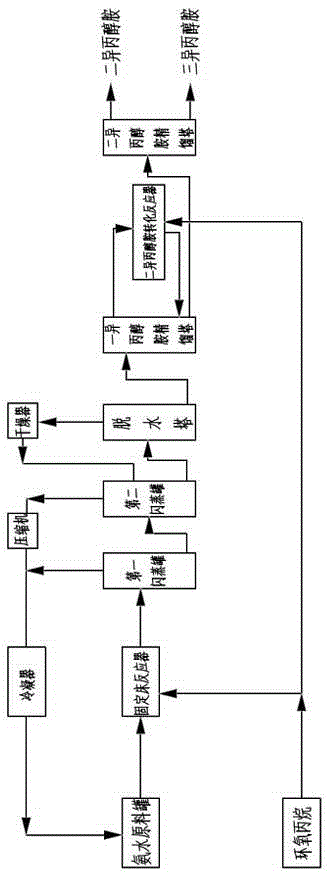
The present invention discloses a method for producing isopropanolamine by using a fixed bed tubular reactor. The method comprises: by using water as a catalyst, putting ammonia and epoxy propane with a molar ratio of (3.5-15):1 into a fixed bed reactor to perform a reaction, wherein the operation pressure of the reactor is 8-25 MPa, and the operation temperature is 160-220 DEG C; pumping a reaction product sequentially into a first flash evaporation tank and a second flash evaporation tank, performing two-stage flash evaporation and ammonia removal on the reaction product, and then introducing the processed reaction product into a dehydration tower for primary dehydration; introducing dehydration materials at the bottom of the dehydration tower into a monoisopropanolamine rectification tower, introducing discharged materials at the top of the monoisopropanolamine rectification tower into a monoisopropanolamine conversion reactor, and returning the materials to the bottom of the monoisopropanolamine rectification tower after reaction; introducing discharged materials at the top of the monoisopropanolamine rectification tower into a diisopropanolamine rectification tower, and after low pressure rectification, obtaining diisopropanolamine at the top of the diisopropanolamine rectification tower, and obtaining triisopropanolamine at the bottom of the diisopropanolamine rectification tower. The method provided by the present invention is capable of reducing the energy consumption of the whole process, effectively avoids reaction and side product generation, and improves the product purity.
Owner:ZHEJIANG JINGGONG NEW MATERIAL TECH
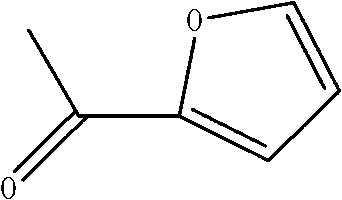
Method for preparing 2-acetylfuran
ActiveCN102702143AReduce the feed ratioEliminate pollutionOrganic chemistryAcetic anhydrideDistillation
The invention relates to a method for preparing 2-acetylfuran, which comprises the reaction step of furan acetylation. In the acetylation reaction, in the presence of acetic acid, zinc chloride is used as a catalyst, and acetic anhydride and furan are acylated. The anhydrous zinc chloride is used as the catalyst, so that the acylation reaction temperature and the acylation time are reduced, conditions for furan polymerization are reduced, and the yield is increased. The addition of the acetic acid overcomes the defect that furan autopolymer is produced easily when lewis acid is used as the catalyst for the acylation reaction. Because the zinc chloride and the acetic acid are used, the feed ratio of the reaction raw materials furan and the acetic anhydride is reduced from 1:1.5 in the prior art to 1:1.06, i.e. the reaction is complete, and the difficulty in recycling finished products at the later period is reduced. After the acetic acid is recycled, a finished product can be obtained through direct distillation, complex postprocessing steps such as neutralization, extraction, solvent distillation and the like are avoided, the process flows are greatly reduced, the energy consumption is reduced, and environment pollution increased by unorganized emission is eliminated.
Owner:四平市精细化学品有限公司
Method for producing beta-hydroxyethyl ethylenediamine
ActiveCN102786425AReduce the feed ratioEvenly dispersedOrganic compound preparationAmino-hyroxy compound preparationEthylenediamineMicroreactor
The invention relates to a method for producing beta-hydroxyethyl ethylenediamine. The method comprises the following steps that an ethylenediamine liquid and ethylene oxide gas are continuously and synchronously input into a microreactor and undergo a reaction; and an effluent from the microreactor is subjected to heat preservation at a temperature of 50 to 140 DEG C for 2 to 30min, wherein a reaction temperature in the microreactor is in a range of 10 to 115 DEG C; a mole ratio of ethylenediamine to ethylene oxide is (5 to 15): 1; and an ethylene oxide airspeed is 4 to 400000kg.h<-1>.m<-3>. Under the optimal conditions, ethylene oxide is fully transformed; beta-hydroxyethyl ethylenediamin discharged from the microreactor has a concentration of 5 to 20%; and the content of beta-hydroxyethyl ethylenediamin in products obtained by ethanediamine separation is above 95%. Compared with the prior art, the method has the advantages that a ratio of ethanediamine to ethylene oxide is lower; a production capacity is improved; a reaction period is short; and by-products are reduced.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Calcined anionic clay visible-light catalyst and preparation method and purposes thereof
ActiveCN103055872ASimple preparation routeNo strict requirement for airtightnessWater/sewage treatment by irradiationMetal/metal-oxides/metal-hydroxide catalystsDivalent metalWastewater
The invention discloses a calcined anionic clay visible-light catalyst and a preparation method and purposes thereof. The catalyst is prepared by the following steps of preparing mixed solution of metal salts and urea; heating the mixed solution to the temperature of between 100 and 120 DEG, reacting for 36 to 54 h, and obtaining acidic turbid liquid of which the pH value is between 6.0 and 6.5; centrifuging the acidic turbid liquid, taking precipitation out, washing the precipitation by deionized water until the pH value is neutral, and dewatering and drying; and grinding the dried precipitation, calcining the precipitation at the temperature of between 400 and 900 DEG C for 4 to 6 h, and thus obtaining the calcined anionic clay visible light catalyst, wherein the metal salts comprise divalent metal salts and trivalent metal salts. The prepared calcined anionic clay visible light catalyst can catalytically degrade organic wastewater in visible light, ultraviolet light equipment is not required, a feeding ratio is low, the degradation time is short, and the calcined anionic clay visible-light catalyst has low energy consumption and high efficiency and is suitable for the treatment of medium and low-concentration industrial wastewater.
Owner:SOUTH CHINA UNIV OF TECH
Cycleanine production and purification method
ActiveCN108794416AEasy to operateEasy to control temperatureOrganic chemistryPurification methodsCycleanine
The invention discloses a cycleanine production and purification method which includes the steps: cooling cycleanine production reaction liquid for the first time; filtering the cycleanine productionreaction liquid; cooling filtrate for the second time and then performing standing, crystallization, filtering and drying to obtain a cycleanine product. According to the cycleanine production and purification method, cycleanine product post-treatment purification operation steps are simplified, hot filtration is omitted, the potential safety hazards of operators are reduced, production operationsafety level is reduced, and the method is green and environmentally friendly. The obtained cycleanine product is high in purity and good in quality, medication safety is improved, and industrialization is facilitated.
Owner:成都药云科技有限公司
Method for preparing flonicamid
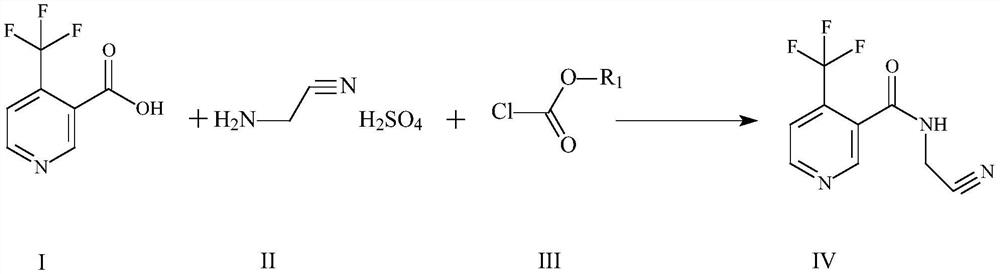
The invention discloses a method for preparing flonicamid. The method comprises the following steps: S1, condensation reaction: in an organic solvent A, carrying out condensation reaction on a compound in a formula I and a compound in a formula III under the action of an acid-binding agent; wherein the organic solvent A is halogenated hydrocarbon or aromatic hydrocarbon; and S2, amidation reaction: after the condensation reaction in the step S1 is completed, directly adding a compound shown in a formula II without separation to carry out amidation reaction so as to obtain a compound shown in aformula IV. The intermediate one-pot method is adopted for continuous reaction to prepare flonicamid, the intermediate does not need to be purified, a target compound can be obtained only through simple operation, the feeding ratio is reduced, raw materials are saved, cost is saved, and operation is simplified. The method has the advantages of mild operation and adjustment, low risk, cheap and easily available raw materials, reduction of the generation and treatment of three wastes, realization of the high-purity flonicamid from the final product, realization of the total yield reaching 90% or above, great significance in the large-scale preparation of flonicamid, and meeting of the requirements of industrial large-scale production.
Owner:厦门优孚利生物医药科技有限公司
A kind of production method of high-purity metformin hydrochloride
InactiveCN104788345BLower the temperature of the addition reactionLow impurity contentOrganic chemistryOrganic compound preparationMetformin HydrochlorideHydrochloride
The invention discloses a high-purity metformin hydrochloride preparation method. N-methyl pyrrolidone is used as a solvent, dicyandiamide and dimethylamine hydrochloride are taken as a solute, the solvent and the solute are added into a synthesis kettle for reaction, and metformin hydrochloride is prepared through steps of stirring, cooling, spin-drying, washing, crystallizing and drying. The metformin hydrochloride prepared through the preparation method provided by the invention has the advantages that the yield is higher than 96.4 percent, and the product purity is higher than 99.93 percent.
Owner:TAISHAN MEDICAL UNIV
Feed added with protein-mulberry leaf additive for reducing cholesterol contents of livestock meat, poultry meat and eggs as well as preparation method thereof
InactiveCN105876141AImprove qualityImprove antioxidant capacityFood processingAnimal feeding stuffMuscle tissueCholesterol
The invention relates to the field of animal feeds for livestock and poultry, and especially relates to a feed added with a protein-mulberry leaf additive for reducing cholesterol contents of livestock meat, poultry meat and eggs as well as a preparation method thereof. The feed added with the protein-mulberry leaf additive for reducing the cholesterol contents of the livestock meat, the poultry meat and the eggs comprises the following raw-material ingredients in parts by weight: 35-60 parts of protein-mulberry leaves, 15-20 parts of pine needle powder, 1.2-2.0 parts of dendranthema indicum, 2.0-2.7 parts of wormwood, 1.0-1.5 parts of eucommia ulmoides leaves, 1.0-1.5 parts of lotus leaves, 10-20 parts of rice bran, 10-20 parts of corn powder and 10-30 parts of fermentation broth of live bacteria. The protein-mulberry leaves, the dendranthema indicum, the wormwood, the pine needle powder, the eucommia ulmoides leaves, the lotus leaves and so on are mixed according to a certain ratio in the preparation method, and the mixture is then fermented; thus, the fermented materials are enabled to exert synergistic effects, and the feed is capable of improving qualities of the livestock meat, the poultry meat and the eggs. Moreover, the feed is capable of enhancing antioxidant capabilities of living bodies and slaughtered muscle tissues of the livestock and the poultry, so as to reduce oxidation products and prevent fatty acid formation in the meat; thus, the feed greatly reduces the low-density cholesterol contents in the livestock bodies and the poultry bodies.
Owner:LUOYANG YIBAI AGRI & HUSBANDRY TECH CO LTD
Preparation method of 4,4'-dicyclohexylmethane diisocyanate
ActiveCN103553969ALower requirementAccurate feeding and meteringIsocyanic acid derivatives preparationOrganic compound preparationSimple Organic CompoundsSolvent
The invention belongs to the technical field of an organic compound, and particularly relates to a method for preparing 4,4'-dicyclohexylmethane diisocyanate by utilizing a non-phosgene process. The method comprises the following step: reacting 4,4'-dicyclohexylmethane diisocyanate or an isomer mixture or salt thereof, or a mixture of amine and salt thereof which are as raw materials with bis(trichloromethyl) carbonate or trichloromethyl chlorocarbonate or a mixture thereof in an inert solvent. By adopting the method disclosed by the invention, the defects that preparation of the 4,4'-dicyclohexylmethane diisocyanate by utilizing the traditional phosgene process is long in process route, complicated in technique, poor in safety and the like are overcome. The method disclosed by the invention has the advantages of being convenient to operate, high in safety, environment-friendly, and accurate in reactant feeding and metering, and the feeding ratio is reduced, so that the safety coefficient of production is improved. In addition, exhaust treatment is simple; high-purity hydrochloric acid can be prepared from a hydrogen chloride gas generated by water absorption reaction; chloridion can be fully utilized; environmental conservation is facilitated.
Owner:UPCHEM CHINA
Stainless steel for passenger cars and method for producing same
InactiveCN102650022AMeets range requirements for strength classesAvoid the hassle of changeHeating timeChemical composition
The invention relates to an austenitic stainless steel for passenger cars, which comprises chemical compositions in mass percent: not more than 0.03% of C, 0.3-0.9% of Si, 0.6-1.5% of Mn, 16.0-18.0% of Cr, 5.5-7.5% of Ni, 0.1-0.22% of N, 0.1-0.6% of Cu, not more than 0.040% of P, no more than 0.03% of S, and the balance of Fe and unavoidable impurity elements; and moreover, Md is between 20-45 DEG C. A method for producing the stainless steel comprises the steps that a steel ingot or a slab is produced; then, the steel ingot or the slab is heated by a heating furnace to 1210-1270 DEG C, and the heating time is between 1-2 minute / mm; the steel ingot or the slab is rolled into a strip steel or a plate after being thermally deformed within the scope of 900-1200 DEG C; solid solution treatment and acid washing are carried out within the temperature scope of 1050-1150 DEG C, and the thermal treatment time is determined every 3-5 minutes according to the thickness of each millimeter; and next, cold rolling is carried out, 80% of austenite is enabled to be converted into deformed martensite after the cold rolling, the total rolling reduction of cold rolling is generally 60-80%, and the temperature of a material is controlled not to be higher than 45 DEG C during the cold rolling process.
Owner:BAOSHAN IRON & STEEL CO LTD
Method for producing tert-butanol by hydration of isobutene in mixed n-butane
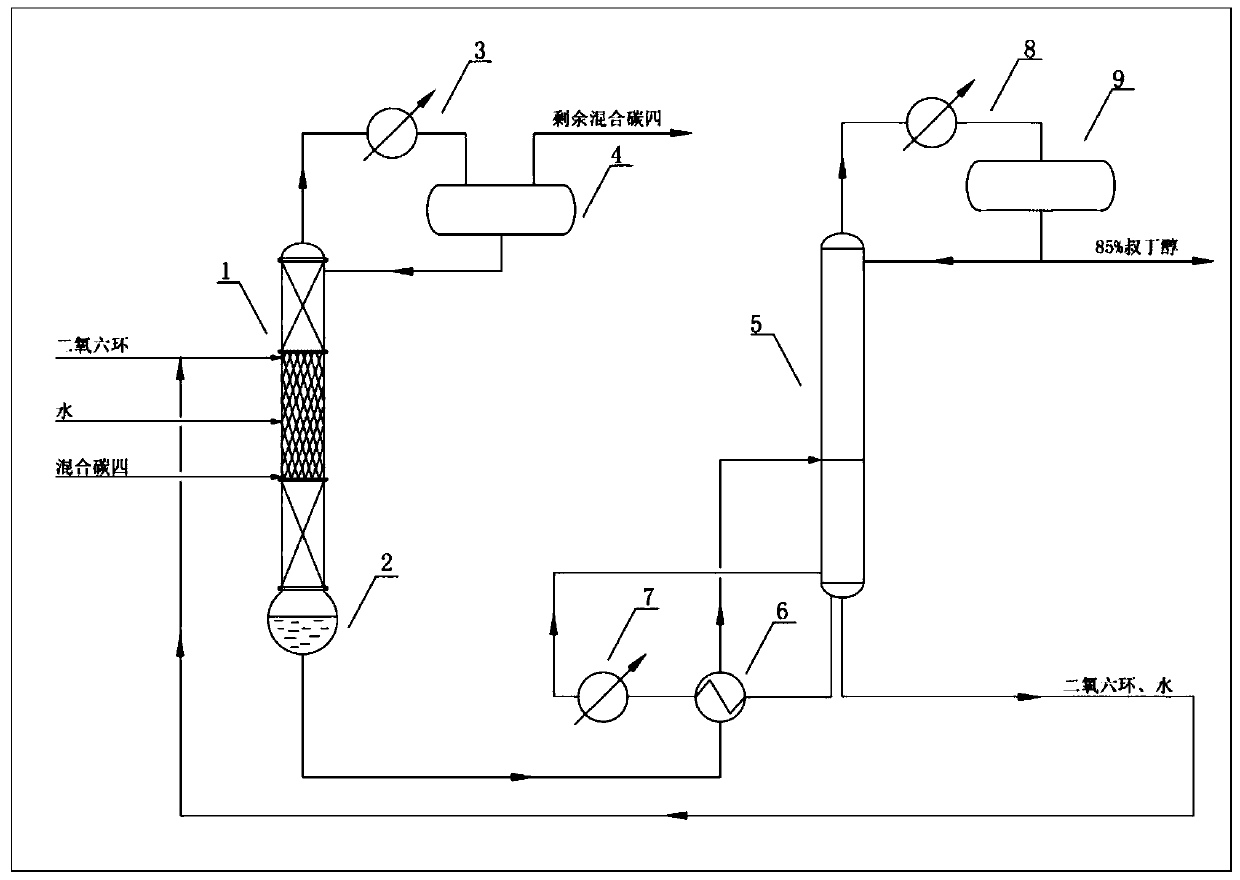
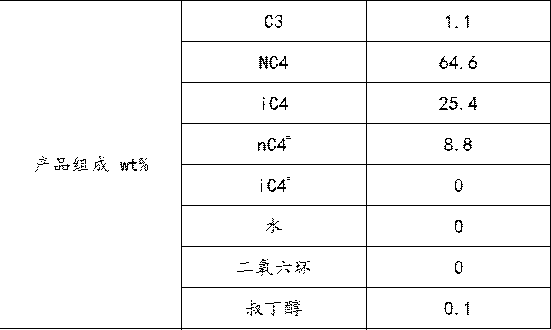
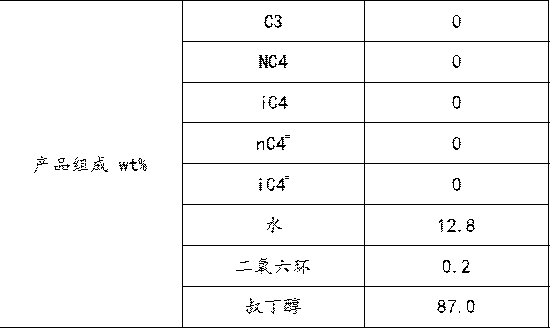
ActiveCN109824475AImprove solubilityImprove conversion rateOrganic compound preparationChemical industryChemical industryHydration reaction
The invention relates to the field of chemical industry, in particular to a method for producing tert-butanol by hydration of isobutene in mixed n-butane. Under the action of dioxane as a co-solvent,mixed n-butane reacts with water in a reaction section of a reactive distillation column. The method adopts a double-effect distillation model, tert-butanol generated in the reaction, remaining waterand dioxane are taken out from a tower kettle of the reactive distillation column, and the materials discharged from the tower kettle of the reactive distillation column serve as heat sources of reboilers I and II of a tert-butanol refining tower and enter the tert-butanol refining tower for further separation after heat exchange. The catalytic reactive distillation process with dioxane as the co-solvent is introduced, the conversion of isobutene is improved, the problems are solved that an original reaction and distillation process is complex, high in energy consumption and low in yield, andthe method has broad market application prospects.
Owner:YANTAI UNIV
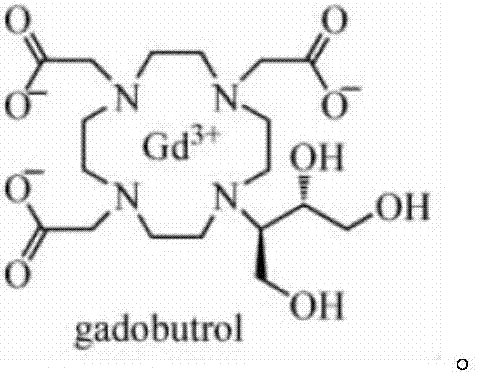
Preparation process method of gadobutrol epoxy side chain intermediate
ActiveCN106967083ASuitable for industrial scale-up productionReduce the potential threat level of life hazardOrganic chemistryEpoxyEnvironmental resistance
The invention disclose a 4,4-dimethyl 3,5,8-trioxabic [5,1,0]ycloctane and particularly relates to a preparation process method of a gadobutrol epoxy side chain intermediate. According to the preparation process method disclosed by the invention, by adjusting the weight ratio of feeding materials, optimizing reaction conditions and improving post treatment and purification methods, the impurity content of an obtained gadobutrol epoxy side chain intermediate product is low; the quality of the intermediate product is greatly improved while the yield is increased, so that the difficulty of process control of the gadobutrol crude drugs during the production process is reduced, and the quality and the qualification rate of the gadobutrol crude drugs are improved. The preparation process method disclosed by the invention has the advantages of simple operation methods in all process steps, safe and feasible solvent and technological conditions, realization of green and environmental-friendly production and wide application prospect.
Owner:穆云
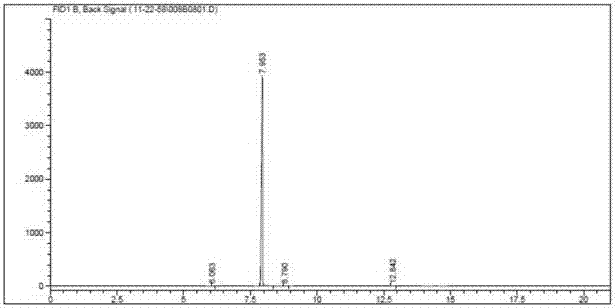
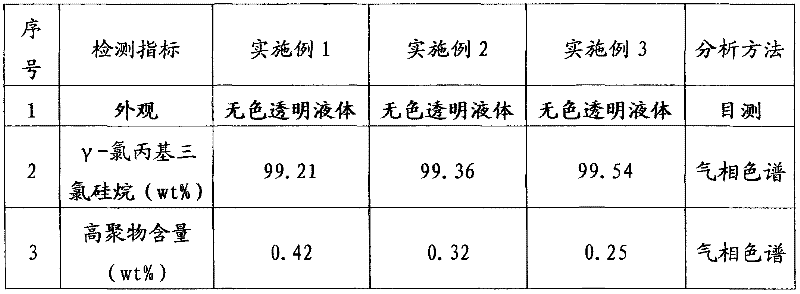
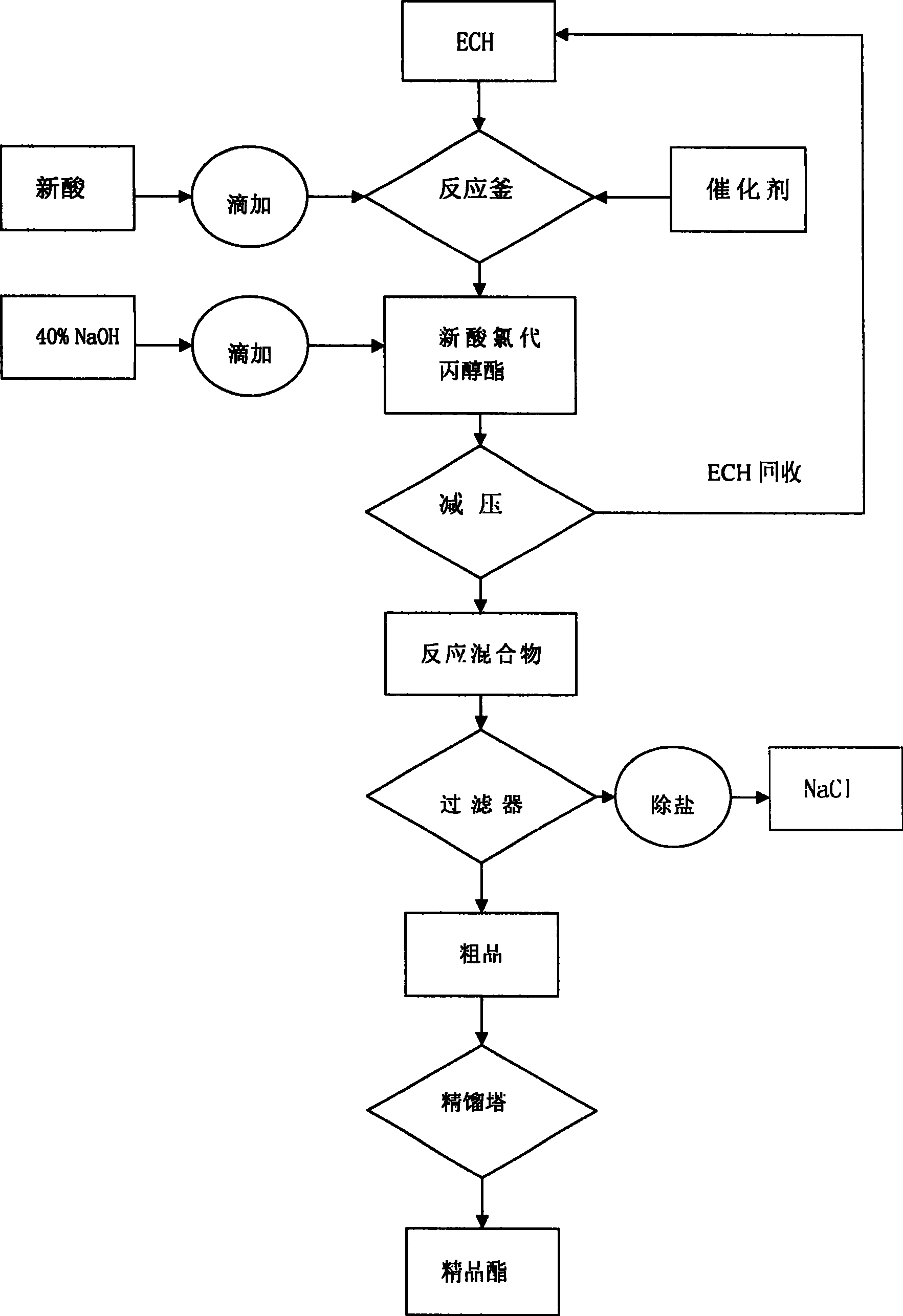
Method for producing gamma-chloropropyl trichlorosilane
InactiveCN102127104ATake full advantage of the characteristicsGive full play to the catalytic activityGroup 4/14 element organic compoundsMetal/metal-oxides/metal-hydroxide catalystsGas phaseN-Butylamine
The invention discloses a method for producing gamma-chloropropyl trichlorosilane. The method comprises the following process steps of: forming mixed solution by using chloropropene and trichlorosilane; adding part of platinum catalyst solution into part of the mixed solution; reacting for a period of time, and uniformly adding the residual mixed solution dropwise; reacting the mixed solution with the residual platinum catalyst solution continuously; adding part of chloroplatinic acid catalyst solution and all n-butylamine; reacting for a period of time, and adding the residual chloroplatinicacid catalyst solution; and under the conditions of vacuum degree of more than or equal to 0.096 MPa and gaseous phase temperature on the top of more than or equal to 95 DEG C after the dropping of the residual mixed solution and the reaction are finished, distilling materials to obtain the product gamma-chloropropyl trichlorosilane. In the method, the mixed solution of the raw materials are added into a reaction system uniformly; the reaction catalyst at the early stage and the reaction catalyst at the later stage are added segmentally; initiation and the reaction are performed by stages; the reaction is performed completely; a small number of reaction byproducts are obtained; a reaction conversion rate is high; the purity of the product is improved; and reaction time is shortened.
Owner:郭学阳
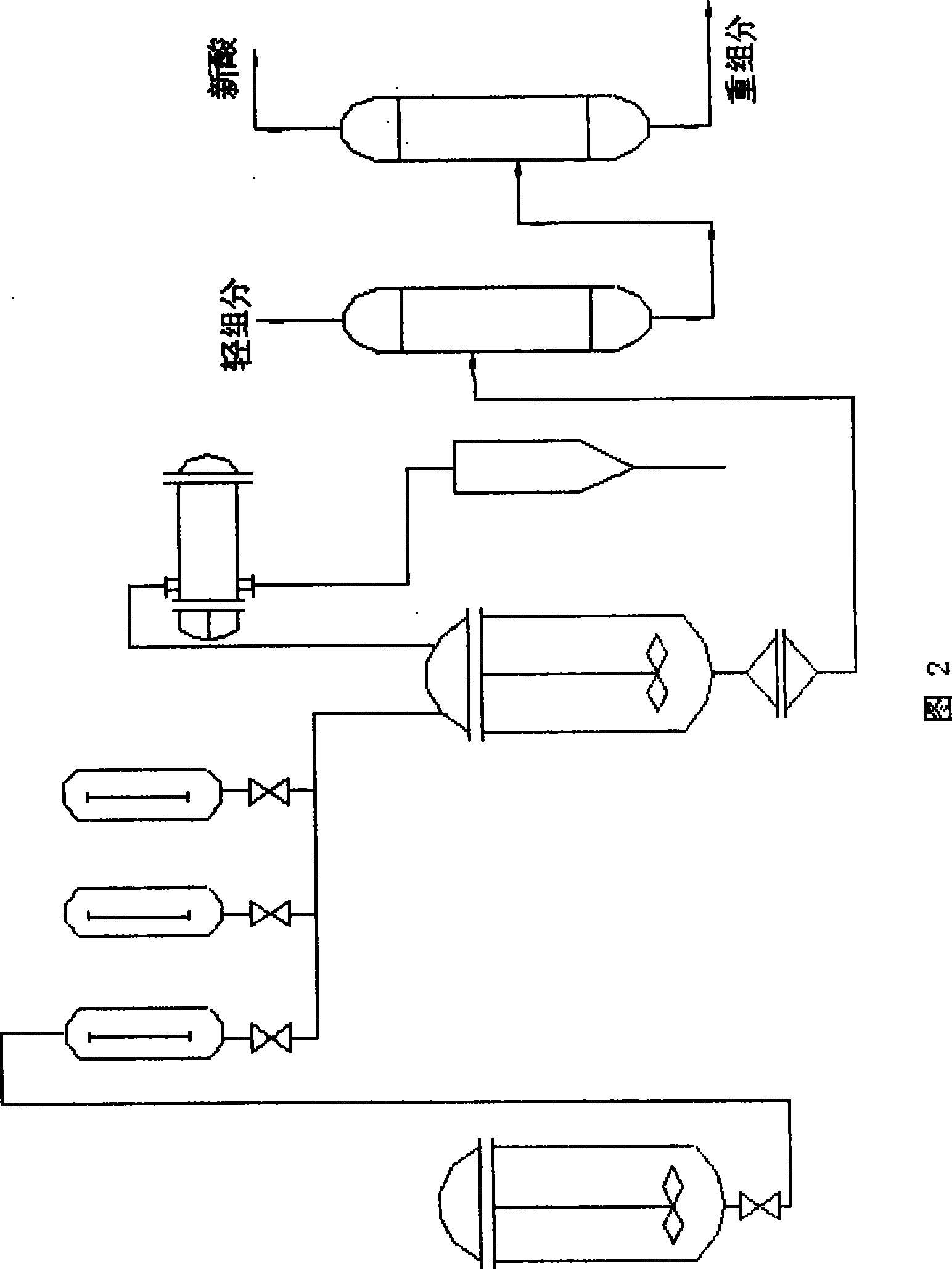
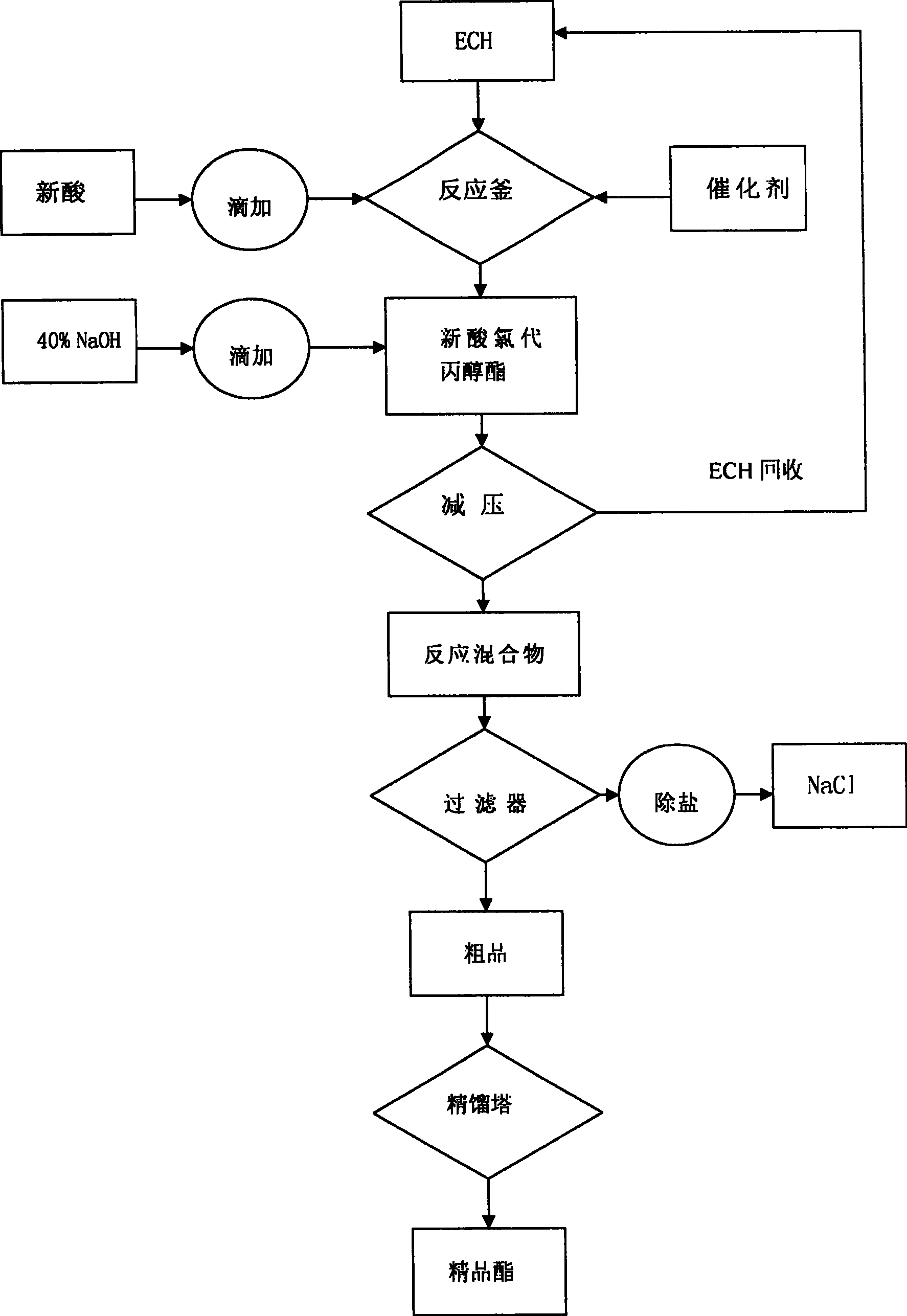
Process for producing tertiary carbonic acid glycidyl ester
A preparation method of neodecanoic acid glycidyl ester has the main raw materials of neodecanoic acid C10, epichlorohydrin, sodium hydroxide and catalyst; the epichlorohydrin and the catalyst are heated to 90 DEG C in a flask with four standard necks of 1 liter with the mechanical stirring, the neodecanoic acid is dripped, the dripping speed is controlled to maintain the temperature at 90 DEG C,the dripping is completed after about half an hour, the esterification reaction is completed after the continual reaction for half an hour; the acid value is less than 0.1 by measurement; the prepared solution containing NaOH is dripped in a reaction bottle for producing a white solid, at this time, a water pump is opened to slowly increase vacuum degree, the residual epichlorohydrin and water inthe reaction bottle are commonly boiled and evaporated, the process needs about one hour; the products are depressurized and vacuum filtrated to remove the NaCl solid substances, the filtrate is subject to vacuum distillation; and the neodecanoic acid glycidyl ester which takes the epoxy as the benchmark and has a content of 96 percent is obtained under the conditions of 90 to 92 DEG C / 140Pa. Thereaction cycle is short, the reaction yield is high, and the yield is above 86 percent. The recovery rate of epichlorohydrin is high. The reaction is smooth, the product quality is good, the operation is simple; and the preparation method is beneficial to the improvement of the product quality.
Owner:TIANJIN SHIELD SPECIALTY CHEM
Method for producing beta-hydroxyethyl ethylenediamine
ActiveCN102786425BReduce the feed ratioEvenly dispersedOrganic compound preparationAmino-hyroxy compound preparationEthylenediamineMicroreactor
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Synthetic method of cis-4-methoxycyclohexyl-1-carbamic acid
InactiveCN109796373AReduce the feed ratioLow costCarbamic acid derivatives preparationOrganic compound preparationMicroreactorWastewater
The invention provides a synthetic method of cis-4-methoxycyclohexyl-1-carbamic acid. The synthetic method includes the steps of catalytic hydrogenation, oxidative reaction, replacement reaction and hydrolytic reaction; the hydrolytic reaction is carried out in a microreactor, with a Jones reagent acting as an oxidant; barium hydroxide octahydrate is used as an alkaline material in the hydrolyticreaction. The synthetic method has few steps and shorter reaction time; the salt content in reaction wastewater is low, the content of heavy metals is low, and discharged waste is little; the finishedproduct has the content of 98% and above; the total reaction yield is 45% and above.
Owner:NANTONG JIANGSHAN AGROCHEM & CHEM LIMITED LIABILITY
Preparation method of alkali-promoted asymmetric organic persulfide compound
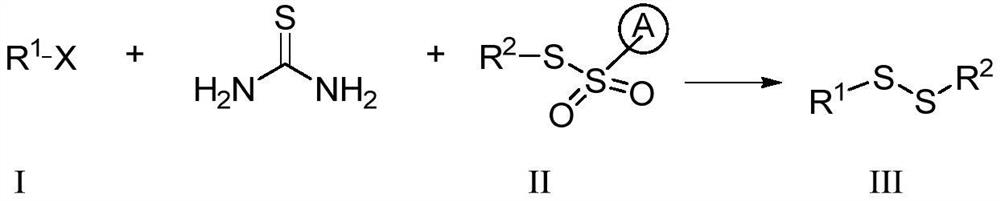
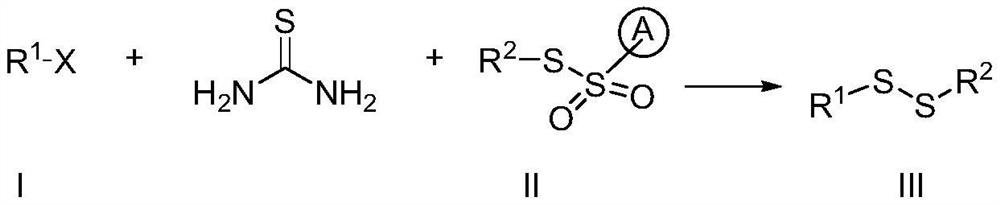
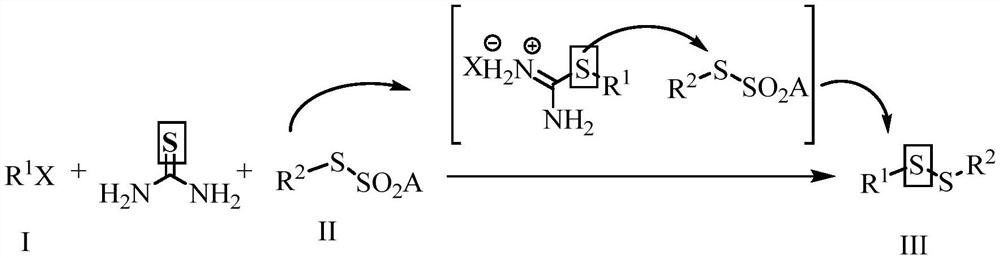
ActiveCN112724054ARaw materials are cheap and easy to getReduce feed ratioSteroidsSulfide preparationSulphur compoundAmino acid
The invention discloses a preparation method of an asymmetric organic persulfide compound. The preparation method comprises the following step: under the promotion of alkali, carrying out one-pot reaction on a compound shown in a formula (I), a compound shown in a formula (II) and thiourea to obtain the asymmetric organic persulfide compound shown in a formula (III). The preparation method is mild in reaction condition, easy to operate and high in yield. The preparation method can be used for preparing organic persulfide compounds and realizing the overvulcanization modification of pharmaceutical molecules such as acetaminophen and estrone and natural product molecules such as zingiberone and amino acid.
Owner:ZHEJIANG UNIV
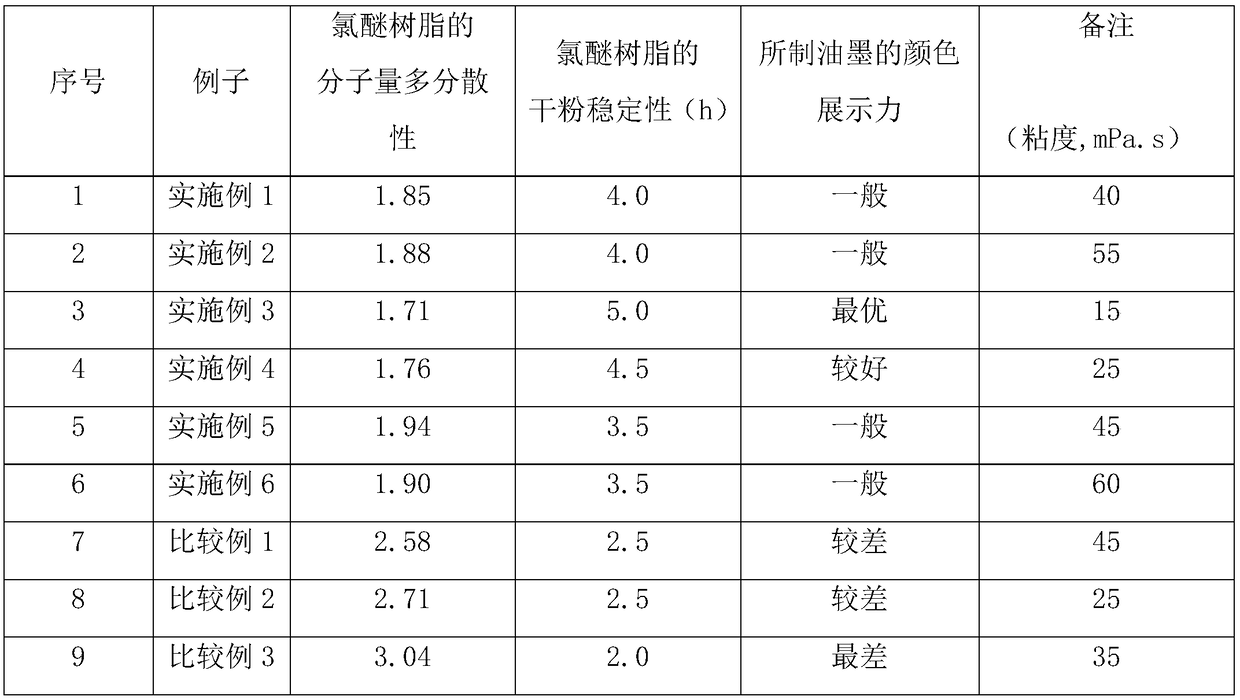
Narrow molecular weight distribution chloroether resin and preparation method thereof
The invention discloses a narrow molecular weight distribution chloroether resin and a preparation method thereof. The narrow molecular weight distribution chloroether resin comprises, by mass, 1.5-3parts of a dodecyl sodium salt, 2.5-7 pats of isobutyl vinyl ether, 0.4-0.9 parts of a persulfate, 2.5 parts of sodium dodecyl sulfate, 1.25-2.15 parts of a buffer agent, 65-80 parts of vinyl chlorideand 17.5-35 parts of isobutyl vinyl ether. The preparation method comprises: adding a part of isobutyl vinyl ether into a polymerization reactor, reducing a use amount of a buffer agent in the polymerization reactor to 0-1 / 3 the total use amount of the buffer agent and carrying out a reaction process under constant temperature and constant pressure control to obtain chloroether resin. The chloroether resin has narrow molecular weight distribution, contains regular particles and has a loose structure and good processing properties. The preparation method has the advantages of stable reaction,easy control, large polymerization rate, short reaction time and low raw material cost.
Owner:杭州电化新材料有限公司
Continuous low-cost preparation method of ibrutinib
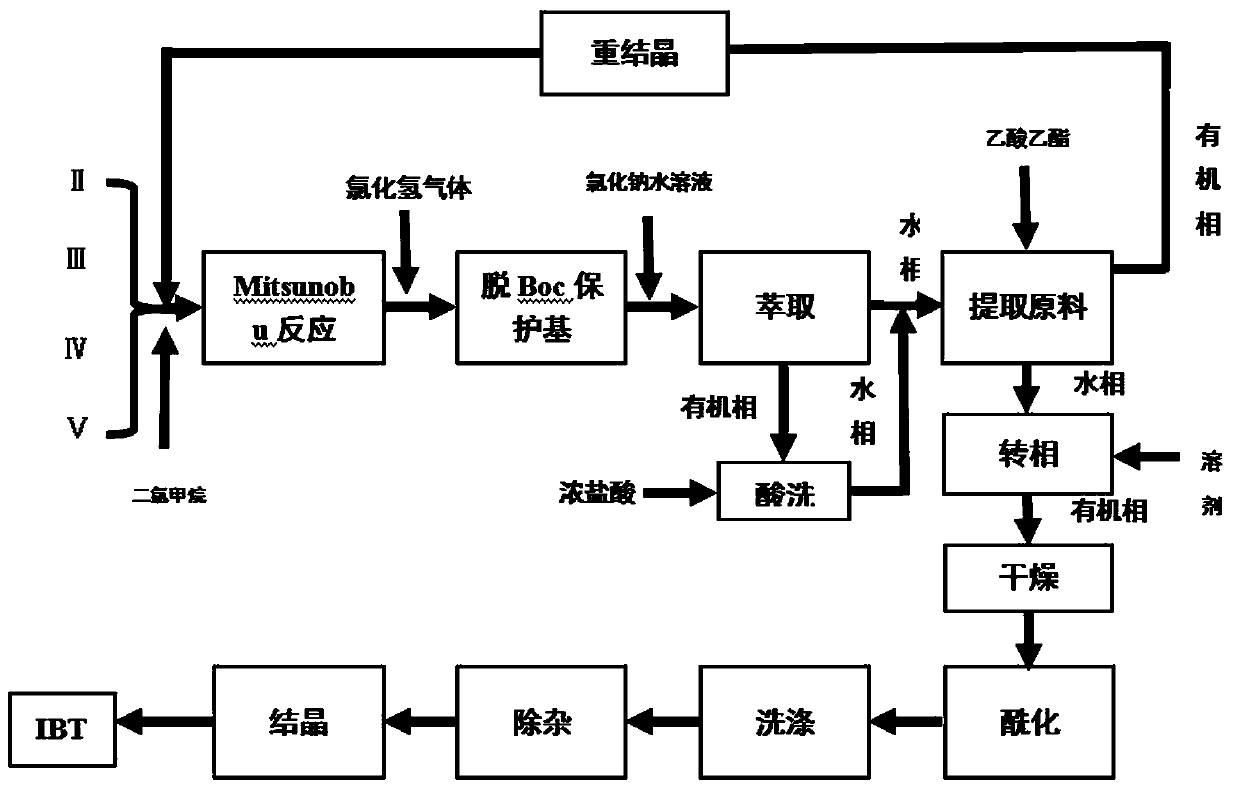
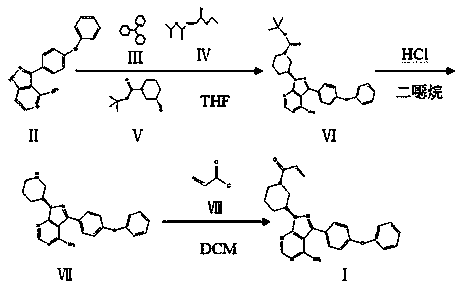
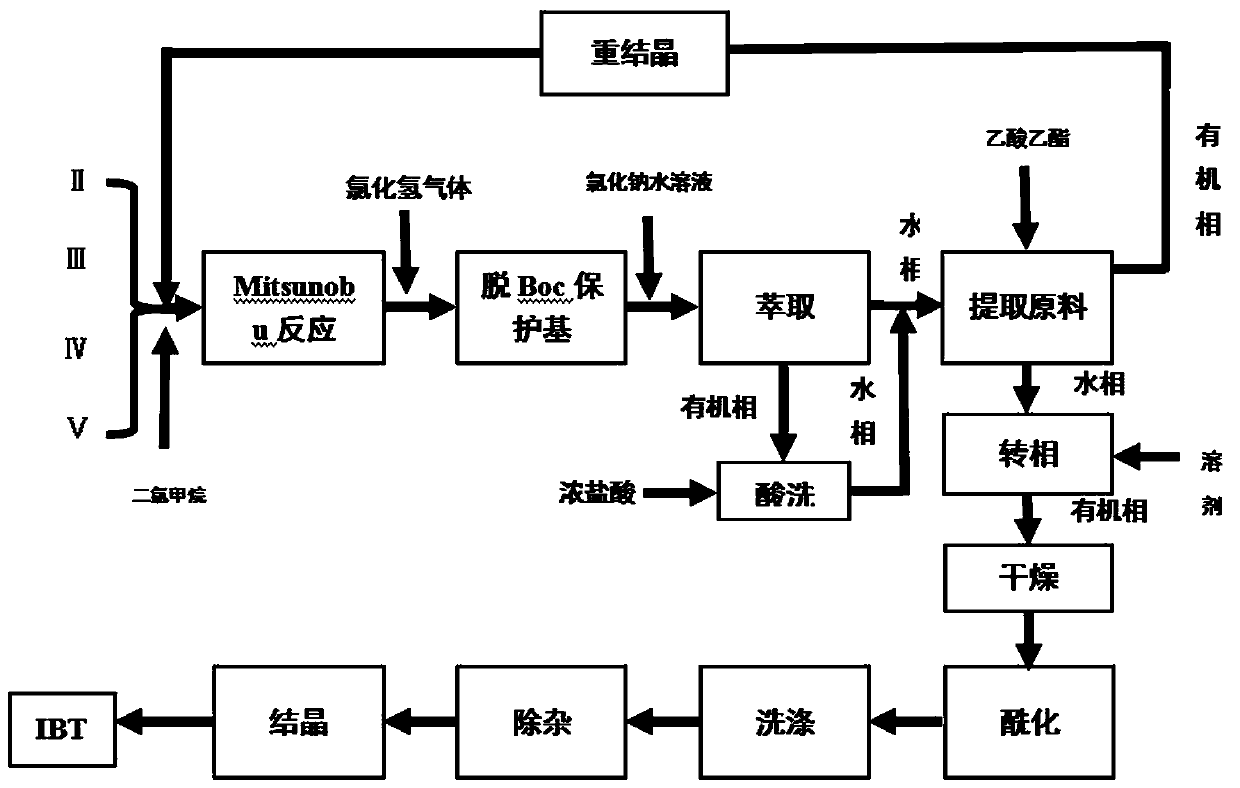
ActiveCN111116593AReduce the feed ratioReduce the difficulty of purificationOrganic chemistryBulk chemical productionPhenyl groupPyrazole
The invention discloses a continuous low-cost preparation method of ibrutinib, belonging to the technical field of medicine synthesis. The method comprises the following steps: 1) carrying out a Mitsunobu reaction on a raw material, DIAD and triphenylphosphine to obtain an intermediate; 2) removing a Boc protecting group from a reaction solution, extracting unreacted 3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, and feeding and applying the unreacted 3-(4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine indiscriminately; 3) adding dichloromethane into a water phase obtained afterraw material extraction in the step 2), adjusting a pH value by using an aqueous sodium hydroxide solution, separating out an organic phase, carrying out drying by using anhydrous sodium sulfate, anddirectly adding triethylamine and acryloyl chloride for an acylation reaction; and 4) conducting washing, removing impurities, and performing evaporating to remove solvents to obtain ibrutinib. The preparation method of the invention overcomes the defects of large residual quantity of raw materials, excessive use of triphenylphosphine oxide, high cost and the like in the prior art, has the advantages of high comprehensive yield, low production cost, environment friendliness and continuity, and is suitable for industrial production.
Owner:河北合佳医药科技集团股份有限公司
Synthesis method of rosuvastatin calcium intermediate
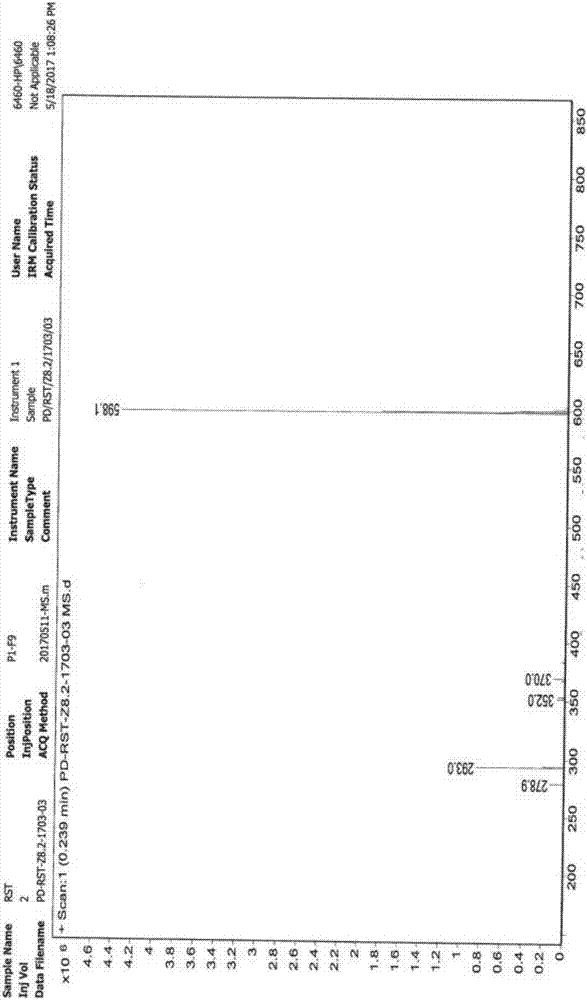
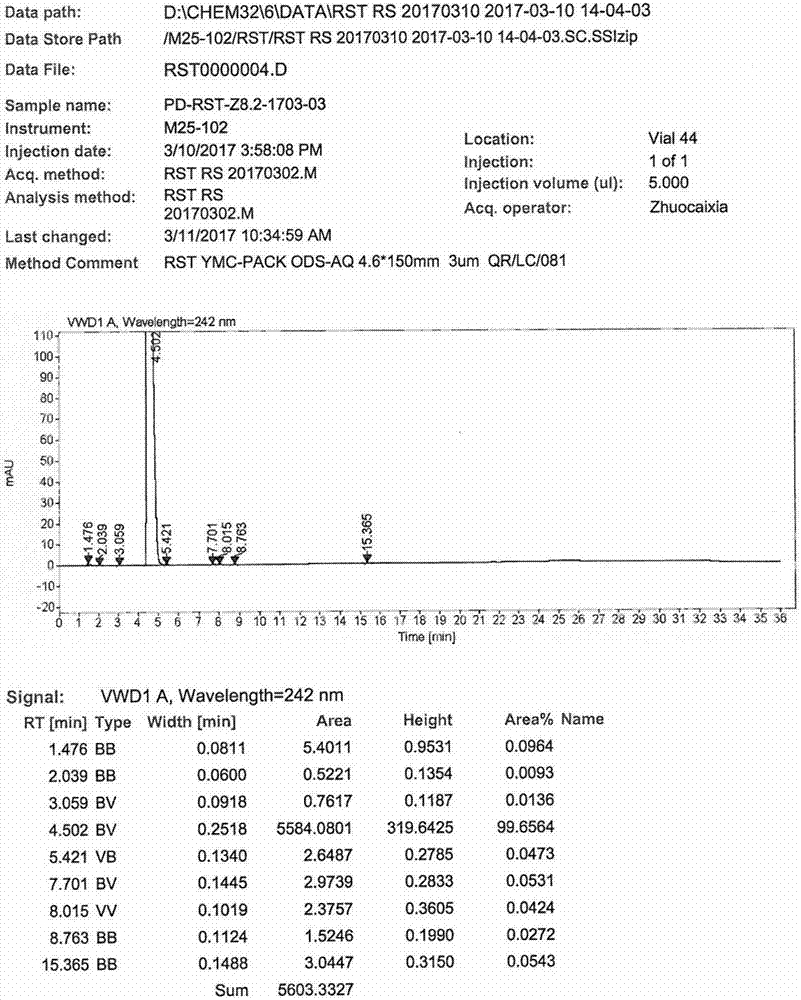
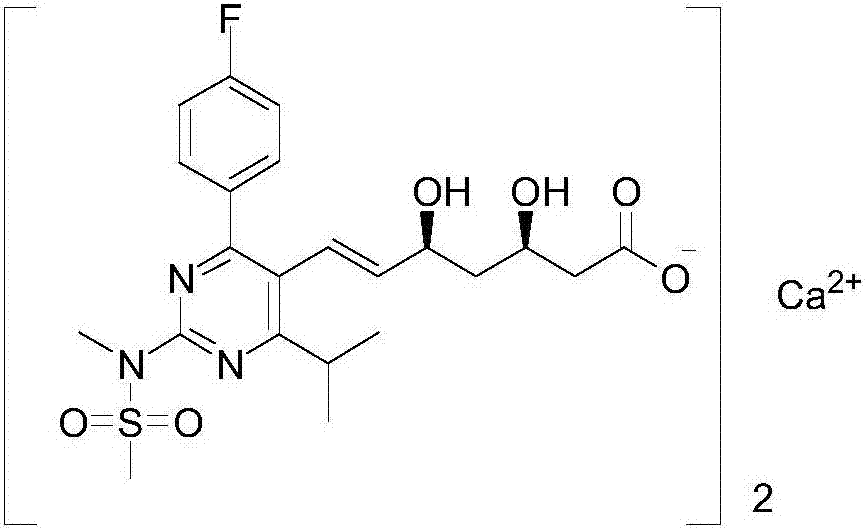
InactiveCN107445992AThe synthesis steps are simpleReduce the feed ratioGroup 5/15 element organic compoundsTolueneOrganic solvent
The invention relates to a synthesis method of a rosuvastatin calcium intermediate. The rosuvastatin calcium intermediate is [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonamide)-5-pyrimidyl] triphenylphosphonium bromide.[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonamide)-5-pyrimidyl] triphenylphosphonium bromide is successfully prepared by a one-pot method by using a mixed organic solvent of tetrahydrofuran and methylbenzene to unify a reaction medium for a three-step reaction. Any separation and purification operation is not required in a reaction process; the synthesis steps are simplified; a feed ratio in the reaction process is reduced; raw materials are saved; a better reaction yield and purity are achieved; the total yield reaches above 80%; the purity is above 99.6%; the operating cost is saved; the production cost is lowered.
Owner:ZHEJIANG MENOVO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com