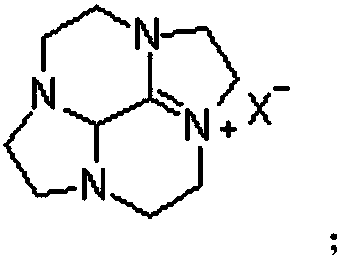
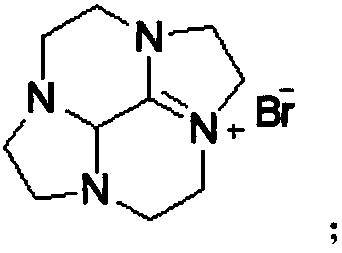
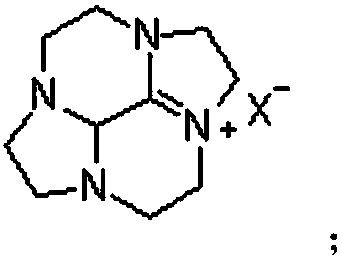
Cycleanine production and purification method
A technology for the purification method of rennetin and its purification method, which is applied in the direction of organic chemistry, and can solve the problems that one-time crystallization cannot meet the product quality requirements of rennetin, many steps in the separation and purification process, and unfavorable industrialization, etc., so as to improve the safety of medication , reduce potential safety hazards, and mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, the preparation of two imidazoline intermediate compounds
[0059] Add 200g of triethylenetetramine, 696g of toluene and 261g of DMF-DMA into the reaction vessel, heat to 85°C-105°C for reaction, separate methanol, continue to add to 120°C, separate 520g of toluene, and cool down to At room temperature, crystallize for 20 hours, filter, rinse the solid with toluene, and then dry it in vacuum at 30°C to obtain 128 g of a bis-imidazoline intermediate compound.
Embodiment 2
[0060] Embodiment 2, the preparation of bromide intermediate compound
[0061] Add 33.3g of potassium carbonate and 200mL of DMF into the reaction flask, stir and heat to 100°C, add 20g of bis-imidazoline intermediate compound, add 22.6g of dibromoethane and 600mL of DMF, and react the reaction solution at 100°C for 2 hours. After cooling down to 65°C, it was filtered, and the filtrate was concentrated under reduced pressure at 65-70°C and dried in vacuo to obtain a bromide salt intermediate compound (yellow semi-solid, 35.5 g).
Embodiment 3
[0062] Embodiment 3, the production and purification of the ring ring Teng Ning
[0063] Add 87g of potassium hydroxide and 100g of water to the reaction flask to obtain an aqueous potassium hydroxide solution, add 35.5g of the bromide salt intermediate compound to 140g of water to obtain an aqueous solution of the bromide salt intermediate compound, heat the potassium hydroxide aqueous solution to 105°C, and add bromine After the aqueous solution of the salt intermediate compound is kept warm for 1 to 2 hours, the reaction solution is cooled to 20°C for the first time, filtered, and the filtrate is cooled to 10°C for the second time to stand for crystallization for 24 hours, filtered and separated by filter cloth, and the solid is vacuum-dried at 30°C , to obtain Luhuan Tengning product (19.3g, yield 86.9%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com