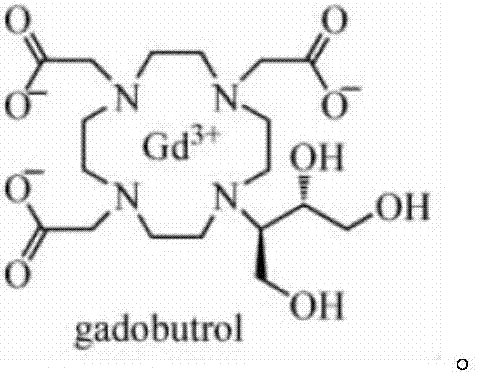
Preparation process method of gadobutrol epoxy side chain intermediate
A preparation process, alcohol epoxy technology, applied in the direction of organic chemistry, etc., can solve the problems of insufficient product purity, reduce production costs, and cannot realize green and pollution-free promotion of mutual benefit, so as to improve product purity and product quality, improve purity and quality , Reduce the effect of equipment anti-corrosion level and process equipment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
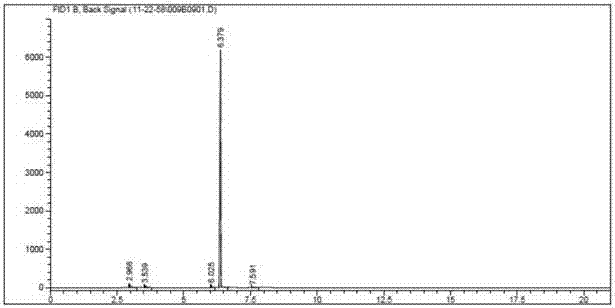
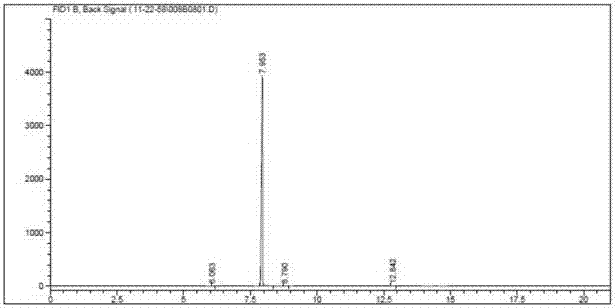
Image
Examples
Embodiment 1
[0071] Embodiment 1, the preparation process of gadobutrol epoxy side chain intermediate
[0072] step 1:
[0073] (1) Add 300g of compound A-1 in the reaction flask;
[0074] (2) Add 450g of compound A-2;
[0075] (3) Add the p-toluenesulfonic acid monohydrate of 0.9g;
[0076] (4) Stir and heat to 70°C, distill 219g of methanol, and heat and distill methanol for 3 hours at the same time;
[0077] (5) heating up to 120°C, distilling off excess compound A-2;
[0078] (6) be heated up to 170 degrees centigrade, and atmospheric distillation separates the fraction impurity before separating, and collection distillation range is 80-142 ℃;
[0079] (7) Compound A-3 (354 g, purity 96.2%) of components with a distillation range of 142-147° C. was collected.
[0080] Step 2:
[0081] (1) Add 338g of compound A-3, 315g of acetonitrile, 270g of methanol and 330g of water in the reaction flask, then add 2.4g of disodium hydrogen phosphate;
[0082] (2) stirring and heating the rea...
Embodiment 2
[0090] Embodiment 2, the preparation process of gadobutrol epoxy side chain intermediate
[0091] step 1:
[0092] (1) Add 180g of compound A-1 in the reaction flask;
[0093] (2) adding 319g of compound A-2;
[0094] (3) Add 0.4g of p-toluenesulfonic acid monohydrate;
[0095] (4) Stir and heat to 70°C, distill 128g of methanol, and heat and distill methanol for 2 hours at the same time;
[0096] (5) heating up to 120°C, distilling off excess compound A-2;
[0097] (6) be warming up to 170 degrees centigrade, the cut impurity before atmospheric pressure distillation is separated,;
[0098] (7) Compound A-3 (212 g, purity 96.3%) was collected with a distillation range of 142-147°C.
[0099] Step 2:
[0100] (1) Add 180g of compound A-3, 190g of acetonitrile, 157g of methanol and 198g of water in the reaction flask; then add 1.4g of disodium hydrogen phosphate;
[0101] (2) stirring and heating the reaction solution;
Embodiment 3
[0110] Example 3, workshop scale-up preparation process of gadobutrol epoxy side chain intermediate
[0111] step 1:
[0112] (1) Add 186kg of compound A-1 in the reactor;
[0113] (2) Add 330kg of compound A-2 again;
[0114] (3) Add the p-toluenesulfonic acid monohydrate of 372g;
[0115] (4) Stir and heat to 80° C., distill methanol; heating and separating methanol continue for 3 hours at the same time;
[0116] (5) heating up to 120° C., distilling off excess compound A-2 to obtain crude compound A-3;
[0117] (6) The crude compound A-3 was distilled under reduced pressure to obtain compound A-3 (216 kg, purity 96.2%).
[0118] Step 2:
[0119] (1) Add 215kg of compound A-3, 208kg of acetonitrile, 178kg of methyl alcohol and 217kg of water in the reactor, then add 1.6kg of disodium hydrogen phosphate;
[0120] (2) stirring and heating the reaction solution;
[0121] (3) Add 257kg of 27% hydrogen peroxide and 103kg of 1M sodium hydroxide aqueous solution into the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com