Synthesis method of rosuvastatin calcium intermediate
A technology for rosuvastatin calcium and a synthesis method, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, and organic chemistry, etc., can solve the problem of easily generating side reactions, affecting product purity and product yield Low problems, to achieve good reaction yield and purity, reduce production costs, simplify the effect of synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
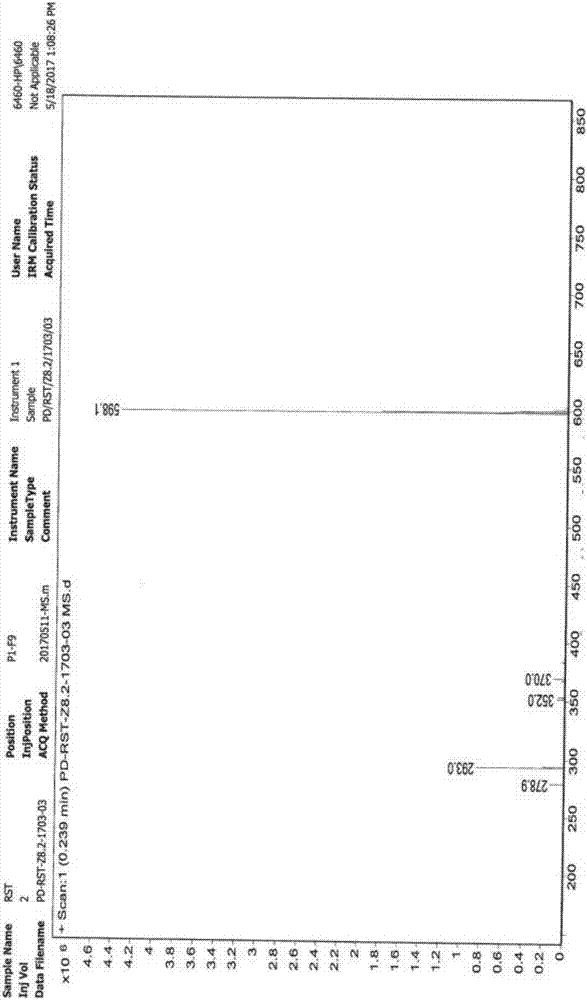
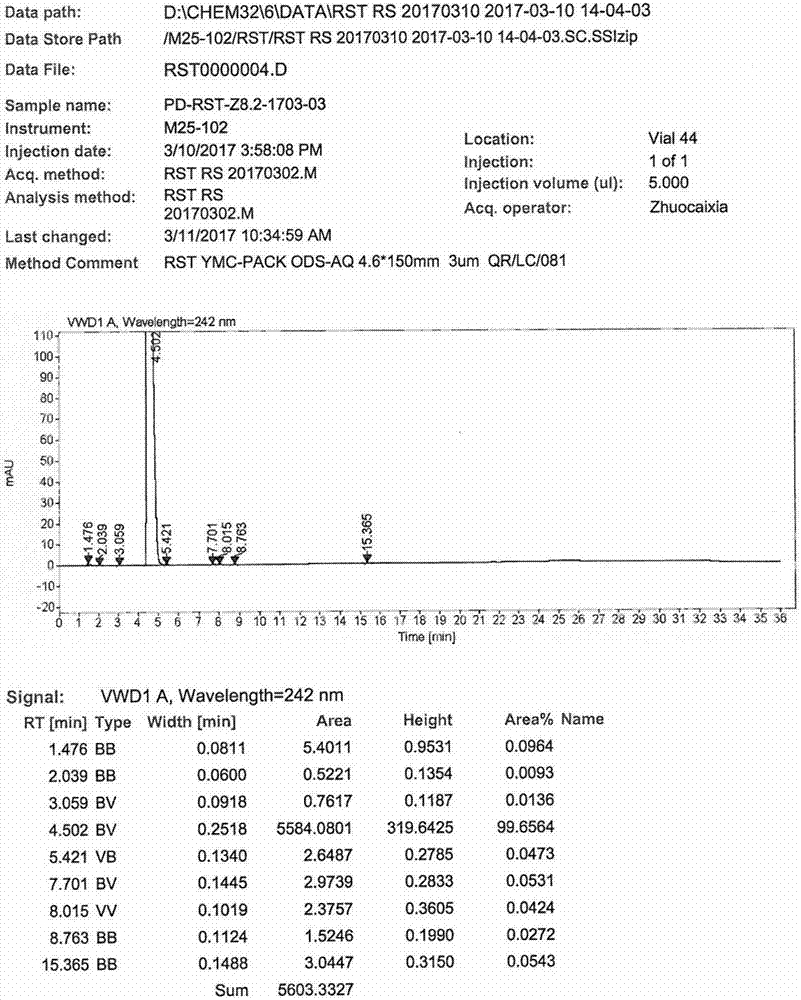
Image
Examples
Embodiment 1
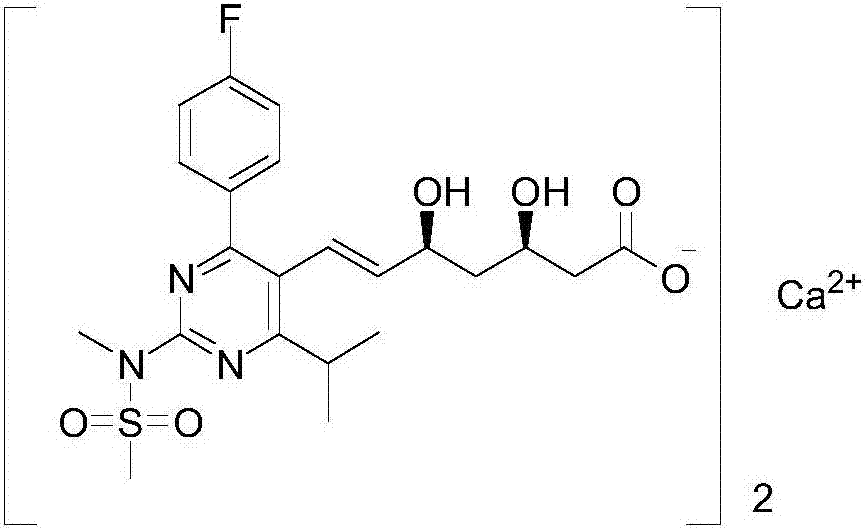
[0030] The synthetic method of rosuvastatin calcium intermediate in this embodiment, that is, the rosuvastatin calcium intermediate is [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N -methylsulfonyl amido)-5-pyrimidinyl] the synthetic method of triphenylphosphine bromide comprises the following steps:
[0031] (1) Under nitrogen protection, add 400mL tetrahydrofuran to a 1000mL dry four-necked reaction flask, add 40g anhydrous magnesium chloride and 40g potassium borohydride, heat and reflux for 1 hour; distill at atmospheric pressure, remove 300mL tetrahydrofuran, add 750mL toluene and 100g of compound 2 was refluxed for 7 hours; after the reaction was completed, cool to room temperature, filter, add dropwise 145mL of 1N hydrochloric acid to the filtrate to quench the reaction, stir and separate layers, dry the organic layer with anhydrous magnesium sulfate, filter, and transfer the obtained filtrate to 2000mL standby in the reaction flask;
[0032] (2) Add 27 mL of phosphorus...
Embodiment 2
[0035] The synthetic method of rosuvastatin calcium intermediate in this embodiment, that is, the rosuvastatin calcium intermediate is [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N -methylsulfonyl amido)-5-pyrimidinyl] the synthetic method of triphenylphosphine bromide comprises the following steps:
[0036] (1) Under nitrogen protection, add 400mL tetrahydrofuran to a 1000mL dry four-necked reaction flask, add 40g anhydrous magnesium chloride and 40g potassium borohydride, heat and reflux for 1 hour; distill at atmospheric pressure, remove 300mL tetrahydrofuran, add 750mL toluene and 100g of compound 2 was refluxed for 6 hours; after the reaction was completed, cool to room temperature, filter, add dropwise 145mL of 1N hydrochloric acid to the filtrate to quench the reaction, stir and separate layers, dry the organic layer with anhydrous magnesium sulfate, filter, and transfer the obtained filtrate to 2000mL standby in the reaction flask;
[0037] (2) Add 27 mL of phosphorus...
Embodiment 3
[0040] The synthetic method of rosuvastatin calcium intermediate in this embodiment, that is, the rosuvastatin calcium intermediate is [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N -methylsulfonyl amido)-5-pyrimidinyl] the synthetic method of triphenylphosphine bromide comprises the following steps:
[0041] (1) Under nitrogen protection, add 400mL tetrahydrofuran to a 1000mL dry four-necked reaction flask, add 40g anhydrous magnesium chloride and 40g potassium borohydride, heat and reflux for 1 hour; distill at atmospheric pressure, remove 300mL tetrahydrofuran, add 750mL toluene and 100g of compound 2 was refluxed for 8 hours; after the reaction was completed, cool to room temperature, filter, add dropwise 145mL of 1N hydrochloric acid to the filtrate to quench the reaction, stir and separate layers, dry the organic layer with anhydrous magnesium sulfate, filter, and transfer the obtained filtrate to 2000mL standby in the reaction flask;
[0042](2) Add 27 mL of phosphorus ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com