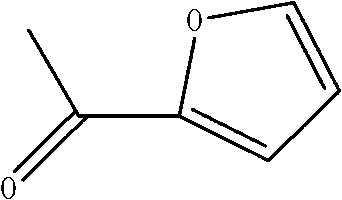
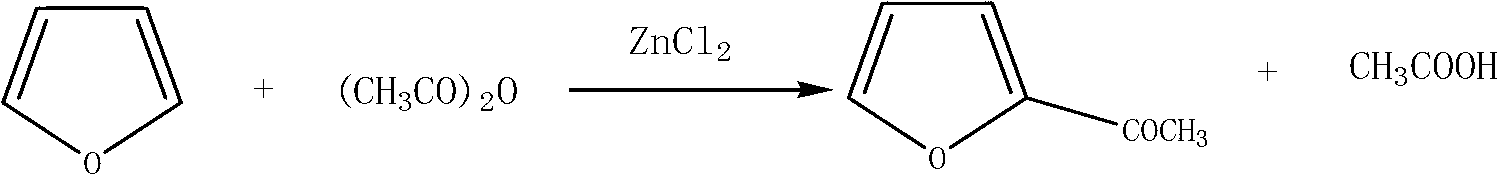Method for preparing 2-acetylfuran
A technology of acetyl furan and acetylation, which is applied in the direction of organic chemistry, can solve the problems of harsh operating conditions, cumbersome process, and large amount of acetic anhydride, and achieve the effects of reducing energy consumption, reducing process flow, and eliminating environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 53.6g (0.53mol) of acetic anhydride, 12.0g (0.20mol) of acetic acid, and 1.0g of zinc chloride in sequence into a 250mL three-necked flask equipped with a stirring and condenser, start stirring at 25°C, and drop at this temperature Add 34.0 g (0.50 mol) of furan, drop it in about 1 hour, slowly raise the temperature to 50°C, and keep it warm for 3 hours. After gas chromatography detects that there is no raw material furan, cool down to 30°C. Under the vacuum condition of 50mbar, acetic acid was recovered at 44±2°C, and the fraction at 80~110°C was collected as 2-acetylfuran to obtain 59.3g (0.463mol) of the product with a yield of 92.7% and a purity of 99.8%.
Embodiment 2
[0029] Add 53.6g (0.53mol) of acetic anhydride, 3.0g (0.05mol) of acetic acid, and 1.0g of zinc chloride in sequence into a 250mL three-necked flask equipped with a stirring and condenser, start stirring at 25°C, and drop at this temperature Add 34.0 g (0.50 mol) of furan, drop it in about 1 hour, slowly raise the temperature to 80°C, and keep it warm for 3 hours. After gas chromatography detects that there is no furan raw material, cool down to 30°C. Under the vacuum condition of 50mbar, acetic acid was recovered at 44±2°C, and the fraction at 80~110°C was collected as 2-acetylfuran to obtain 50.0g (0.391mol) of the product with a yield of 78.2% and a purity of 99.6%.
Embodiment 3
[0031] Add 53.6g (0.53mol) of acetic anhydride, 33.0g (0.55mol) of acetic acid, and 1.0g of zinc chloride in sequence into a 250mL three-necked flask equipped with a stirring and condenser, start stirring at 25°C, and drop at this temperature Add 34.0 g (0.50 mol) of furan, drop it in about 1 hour, slowly raise the temperature to 110°C, and keep it warm for 3 hours. After gas chromatography detects that there is no furan raw material, cool down to 30°C. Under the vacuum condition of 50mbar, acetic acid was recovered at 44±2°C, and the fraction at 80~110°C was collected as 2-acetylfuran to obtain 59.0g (0.460mol) of the product, with a yield of 92.0% and a purity of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com