Continuous low-cost preparation method of ibrutinib
A low-cost, phenoxyphenyl technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of high cost, excessive use of triphenylphosphine, and large residual amount of raw materials, etc., to achieve low production cost, Solvent recovery is easy to apply, and the effect of high comprehensive yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
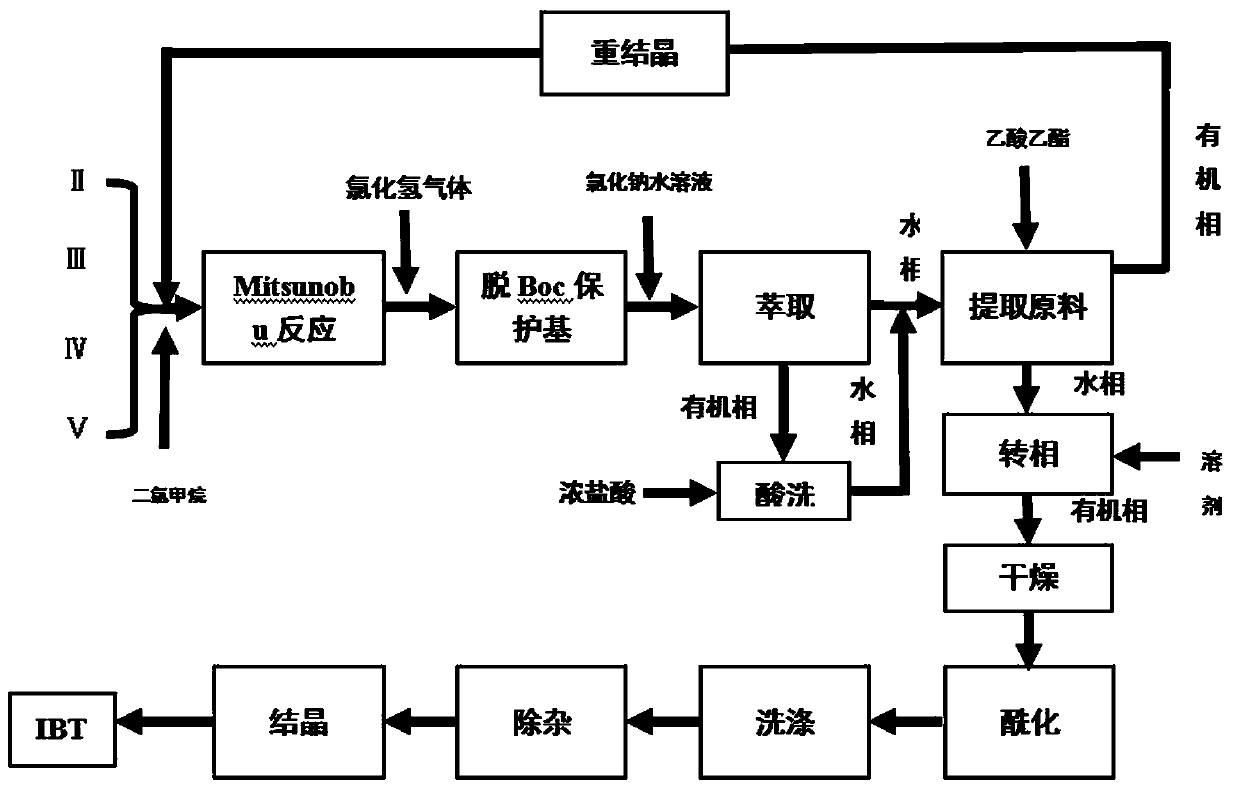
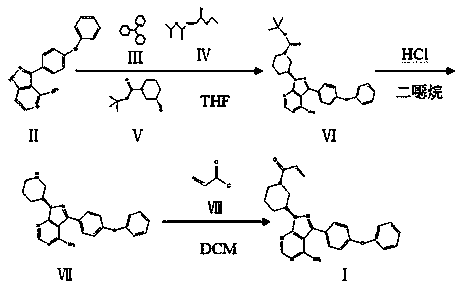
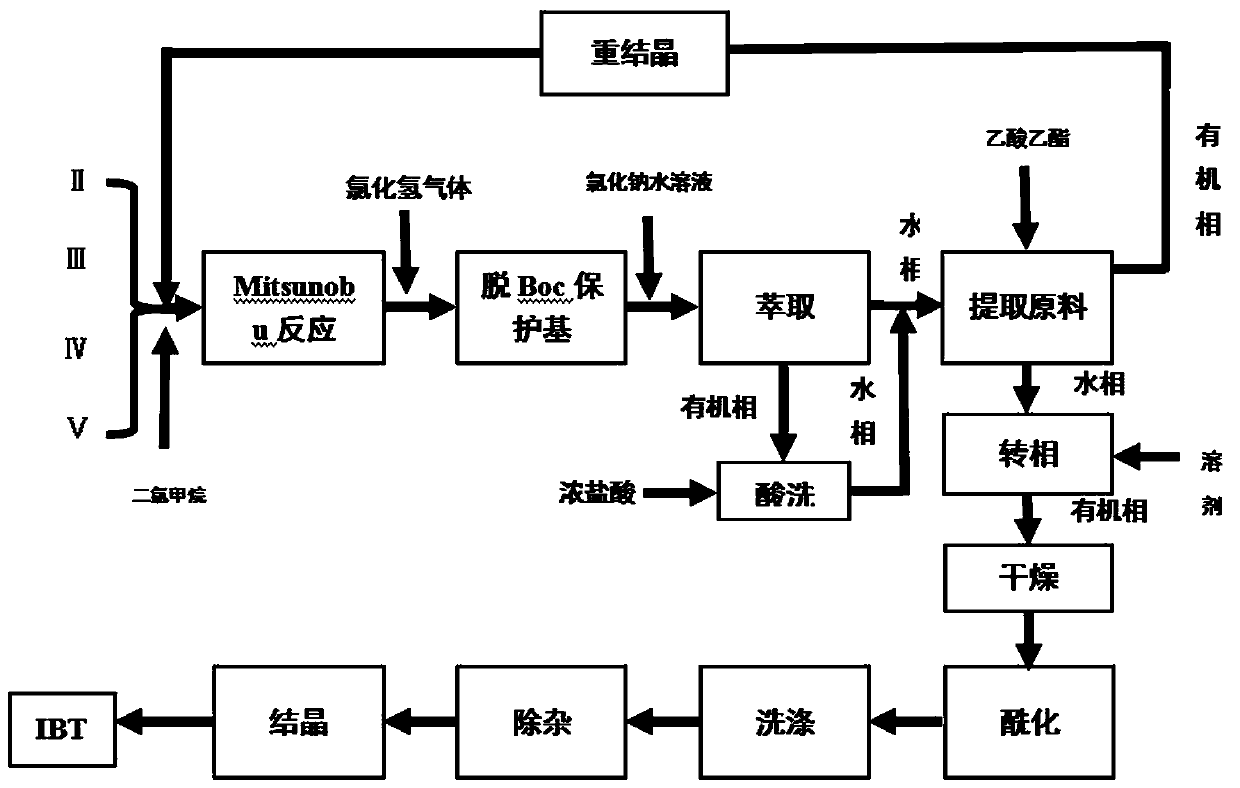
[0038] Add 40ml of dichloromethane to a 100ml reaction flask, and add (2.875g, 0.0095mol) 3-(4-phenoxyphenyl)-1H-pyrazol[3,4-d]pyrimidine-4- Amine, (2.479 g, 0.0123 mol) (s)-1-tert-butoxycarbonyl-3-hydroxypiperidine, (2.479 g, 0.0123 mol) triphenylphosphine. Under temperature control at 25°C, diisopropyl azodicarboxylate dichloromethane solution (dissolve 2.492g, 0.0123mol DIAD in 15ml dichloromethane) was added dropwise, and the dripping was completed in 35min, and the reaction was kept for 5h.
[0039]Control the temperature at 0°C, add HCl gas into the reaction solution under stirring, continue for 4 hours, then add sodium chloride aqueous solution for extraction after 3 hours of heat preservation, and add 30ml concentrated hydrochloric acid under stirring for the dichloro phase (organic phase obtained after extraction), and stir After 30 min, the aqueous phase was separated and the two aqueous phases were combined, and the pH was adjusted to 2.8 with 10% aqueous sodium hyd...
Embodiment 2
[0043] Add 40ml of dichloromethane into a 100ml reaction flask, and add (2.875g, 0.0095mol) compound formula II (including 0.358g of compound formula II recovered in Example 1), (1.907g, 0.0095mol) compound formula V, (1.989g, 0.0076mol) compound of formula III. Under temperature control at 25°C, dichloromethane solution of compound formula IV (dissolve 1.533 g, 0.0076 mol of DIAD in 15 ml of dichloromethane) was added dropwise, the dripping was completed in 35 minutes, and the reaction was kept for 5 hours.
[0044] The temperature was controlled at 0°C, and HCl gas was passed into the reaction solution under stirring for 4 hours. Insulation reaction 3h. Add aqueous sodium chloride for extraction. Add 30ml of concentrated hydrochloric acid to the dichloro phase while stirring, stir for 30min, separate the water phase and combine the two water phases, adjust the pH to 3.1 with 10% aqueous sodium hydroxide solution while stirring, add 60ml of ethyl acetate, and extract compou...
Embodiment 3
[0048] Add 40ml of dichloromethane into a 100ml reaction flask, and add (2.875g, 0.0095mol) compound formula II (including 1.014g of compound formula II recovered in Example 2), (2.479g, 0.0123mol) compound formula V, (3.232g, 0.0123mol) compound of formula III. Under temperature control at 20°C, dichloromethane solution of compound formula IV (dissolve 2.492 g, 0.0123 mol of DIAD in 15 ml of dichloromethane) was added dropwise, the dripping was completed in 35 minutes, and the reaction was kept for 5 hours.
[0049] The temperature was controlled at 0°C, and HCl gas was passed into the reaction solution under stirring for 4 hours. Insulation reaction 3h. Add sodium chloride aqueous solution for extraction, add 30ml of concentrated hydrochloric acid while stirring the dichloro phase, separate the water phase after stirring for 30min and combine the two water phases, adjust the pH to 2.5 with 10% sodium hydroxide aqueous solution while stirring, add 60ml of ethyl acetate Este...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com