Method for producing isopropanolamine by using fixed bed tubular reactor
A tubular reactor and isopropanolamine technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of difficult process control, low production efficiency, and many by-products, and achieve uniform distribution , reduce production efficiency and save total energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
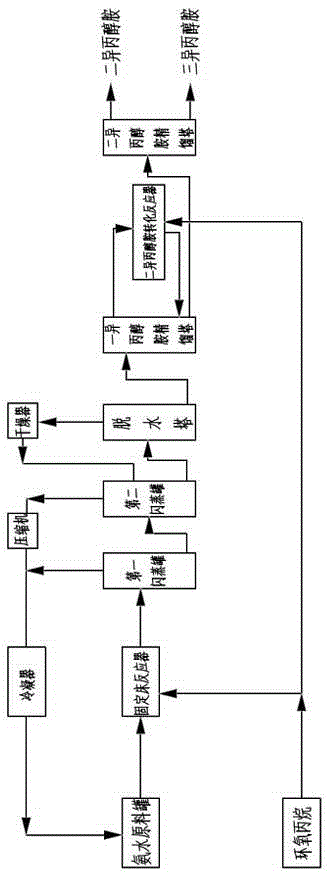
[0035] Such as figure 1 A kind of method that utilizes fixed-bed tubular reactor to produce isopropanolamine is shown, and its steps are:
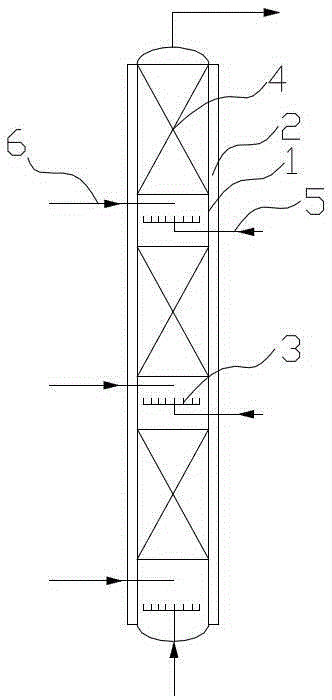
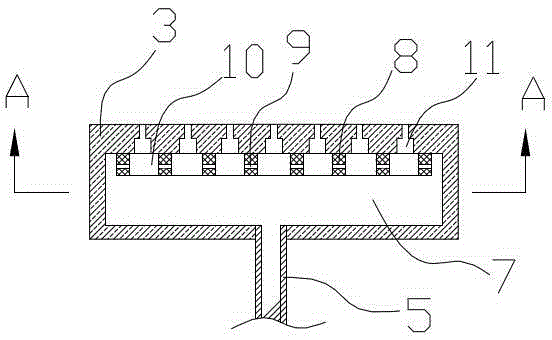
[0036] (a) Using water as a catalyst, mix ammonia and water to form ammonia water with a concentration of 90% and store it in the ammonia water raw material tank, put ammonia water and propylene oxide into a fixed bed tubular reactor for reaction at the same time, ammonia water and propylene oxide The feeding molar ratio of the fixed bed tubular reactor is 3.5:1, the operating pressure of the fixed bed tubular reactor is 8MPa, and the operating temperature of the fixed bed tubular reactor is 160°C. The fixed bed tubular reactor includes the reaction tube body 1, the Jacket 2 (see figure 2), the reaction tube body is provided with a number of propylene oxide feed liquid phase distribution plates 3 at longitudinal intervals, and the propylene oxide feed liquid phase distribution plate divides the inside of the reaction tube body into sever...
Embodiment 2
[0042] The technological process of the present embodiment and embodiment 1 figure 1 , fixed-bed tubular reactor structure and embodiment 1 Figure 2~4 They are all identical, so they will not be repeated here. The difference lies in the process parameters of each step:
[0043] (a) Using water as a catalyst, mix ammonia and water to prepare ammonia water with a concentration of 99.5% and store it in the ammonia water raw material tank. Put ammonia water and propylene oxide into a fixed-bed tubular reactor for reaction at the same time. Ammonia water and propylene oxide The molar ratio of feed to material is 15:1, the operating pressure of the fixed bed tubular reactor is 25MPa, and the operating temperature of the fixed bed tubular reactor is 220°C.
[0044] (b) The operating pressure of the first flash tank is 18 bar, and the operating pressure of the second flash tank is 1.0 bar.
[0045] (c) Pass the bottom discharge of the second flash tank into the dehydration tower, a...
Embodiment 3
[0048] The technological process of the present embodiment and embodiment 1 figure 1 , fixed-bed tubular reactor structure and embodiment 1 Figure 2~4 They are all identical, so they will not be repeated here. The difference lies in the process parameters of each step:
[0049] (a) Using water as a catalyst, mix ammonia and water to form ammonia water with a concentration of 95% and store it in the ammonia water raw material tank, put ammonia water and propylene oxide into a fixed bed tubular reactor for reaction at the same time, ammonia water and propylene oxide The molar ratio of feed to material is 5:1, the operating pressure of the fixed bed tubular reactor is 10MPa, and the operating temperature of the fixed bed tubular reactor is 180°C.
[0050] (b) The operating pressure of the first flash tank is 12 bar, and the operating pressure of the second flash tank is 0.5 bar.
[0051] (c) Pass the bottom discharge of the second flash tank into the dehydration tower, and sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com