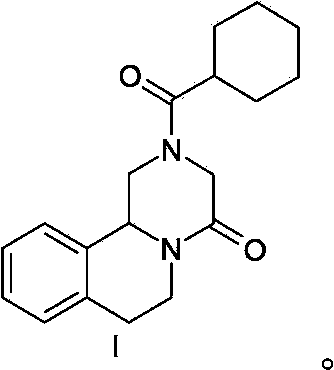
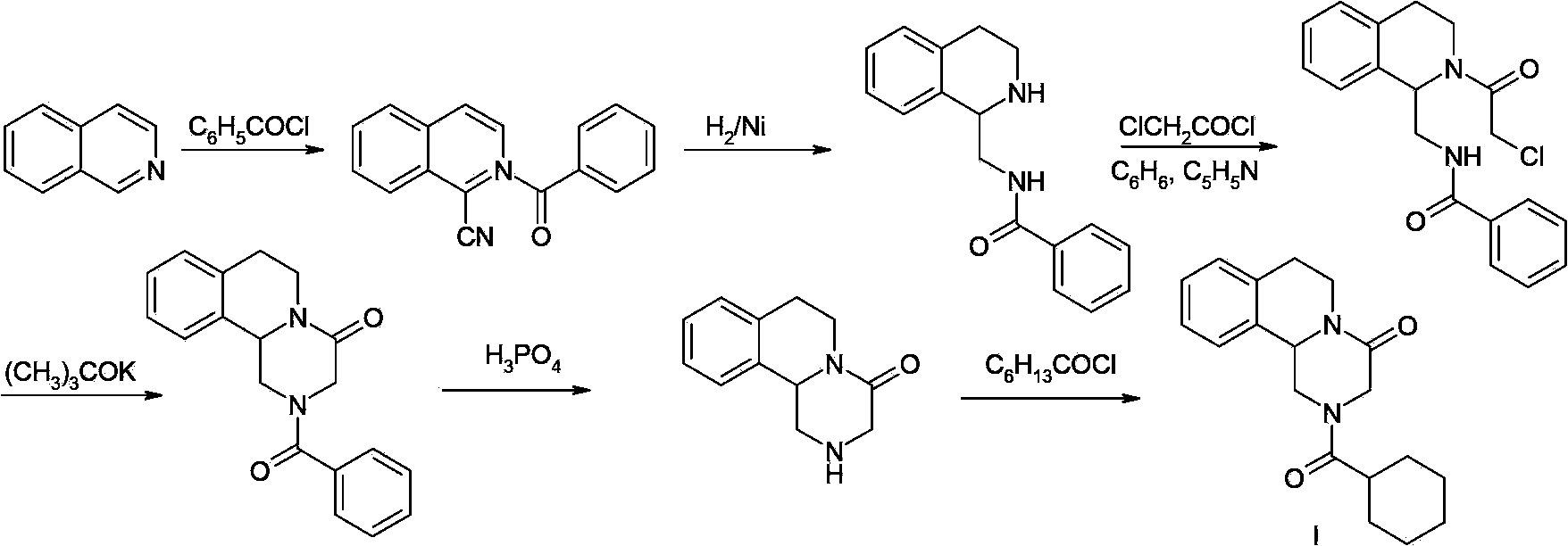
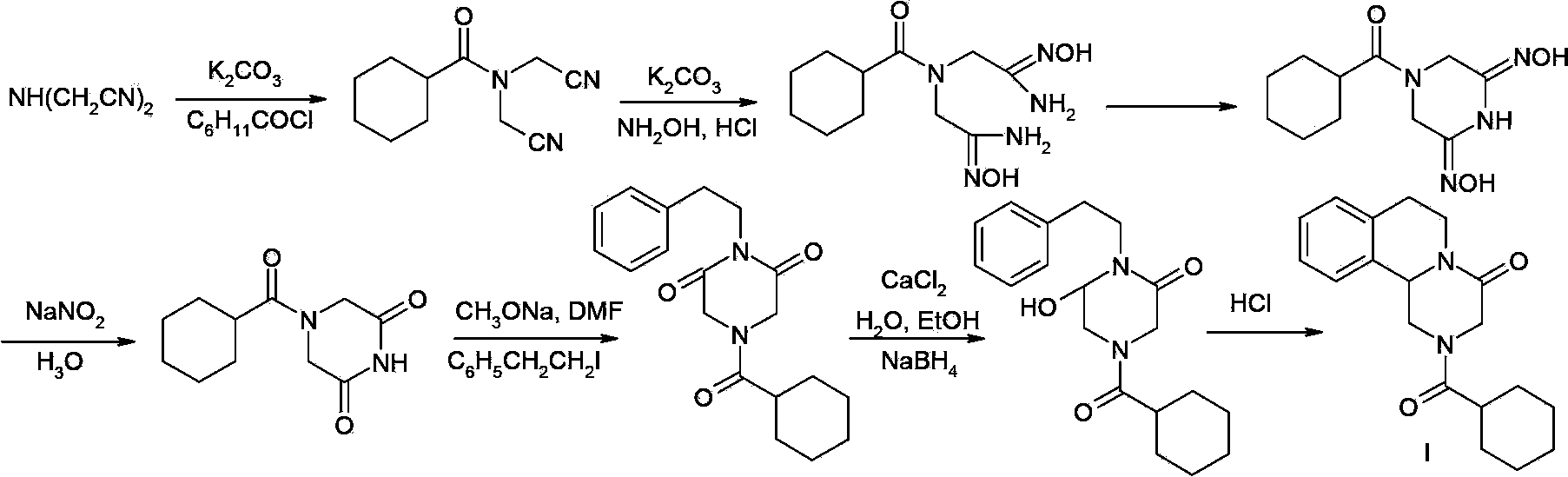
Praziquantel preparation process
A praziquantel and process technology, applied in the field of drug synthesis, can solve the problems that praziquantel is not suitable for large-scale industrial production, does not meet industrial production requirements, affects the quality of final products, etc., achieves low risk, simplifies operations, and avoids side effects. effect of reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the preparation of formula II compound
[0062]
[0063] Method 1: Add (83g, 1.1mol) glycine and (88g, 2.2mol) sodium hydroxide to 750mL water, under the protection of argon, cool down to 5-10°C, add dropwise (146g, 1.0mol) cyclohexanecarbonyl chloride After the addition, the reaction at room temperature was complete; the pH of the reaction system was adjusted to 1-2 with hydrochloric acid, and a large amount of solid was precipitated, filtered and dried to obtain 178 g of the compound of formula II (96.2% yield).
[0064] Method 2: Add (83g, 1.1mol) glycine and (201g, 2.0mol) triethylamine into 750mL water, under the protection of argon, cool down to -10~-5°C, add dropwise (146g, 1.0mol) cyclohexane After the addition of formyl chloride, the reaction was complete at room temperature; the pH of the reaction system was adjusted to 1-2 with hydrochloric acid, and a large amount of solid precipitated, filtered and dried to obtain 156 g of the compound of f...
Embodiment 2
[0065] Embodiment 2: the preparation of formula IV compound
[0066]
[0067] Method 1: Add (242.36g, 2.0mol) phenethylamine and (44.0g, 1.1mol) sodium hydroxide into the reactor, raise the temperature to 120-125°C, add dropwise (124.57g, 1.0mol) 2-chloroethyl Dimethyl acetal, after dripping, keep warm and continue to react for 6 to 8 hours; finish the reaction, filter, and distill the filtrate under reduced pressure to remove phenethylamine, and the distillation residue is the compound of formula IV (200g), which is directly used for the next reaction.
[0068] Method 2: Add (242.36g, 2.0mol) phenethylamine and (152g, 1.1mol) potassium hydroxide into the reactor, raise the temperature to 130-140°C, and add (152.0g, 1.0mol) 2-chloroacetaldehyde dropwise Diethyl ether, after dropping, keep warm and continue to react for 6 to 8 hours; finish the reaction, filter, and distill the filtrate under reduced pressure to remove phenethylamine, and the distillation residue is the comp...
Embodiment 3
[0069] Embodiment 3: the preparation of formula V compound
[0070]
[0071] Method 1: Add (18.5g, 0.1mol) compound of formula II and (10.0g, 0.157mol) methyl chloroformate (compound of formula III) into 150mL of dichloromethane, and cool down to 0-5°C under the protection of argon , add (10.5g, 0.1mol) triethylamine dropwise, after dropping, continue the reaction for 1h; add (20.9g, 0.1mol) 2,2-dimethoxy-N-phenethylethylamine (compound of formula IV) After the addition, warm up to room temperature and react for 6 to 8 hours; at room temperature, add 4 mL of concentrated sulfuric acid with a mass fraction of 98%, after the addition, continue to react for 16 to 20 hours at room temperature; to end the reaction, add 100 mL of water, separate the liquid, and concentrate Dichloromethane in the dry organic phase; refined with methyl tert-butyl ether; 30.0 g of the compound of formula V was obtained (yield: 96.3%).
[0072] Method 2: Add (18.5g, 0.1mol) compound of formula II an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com