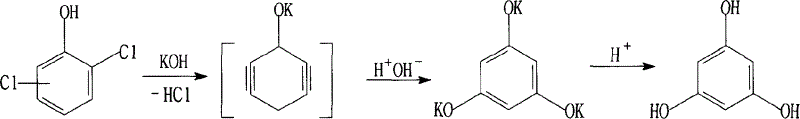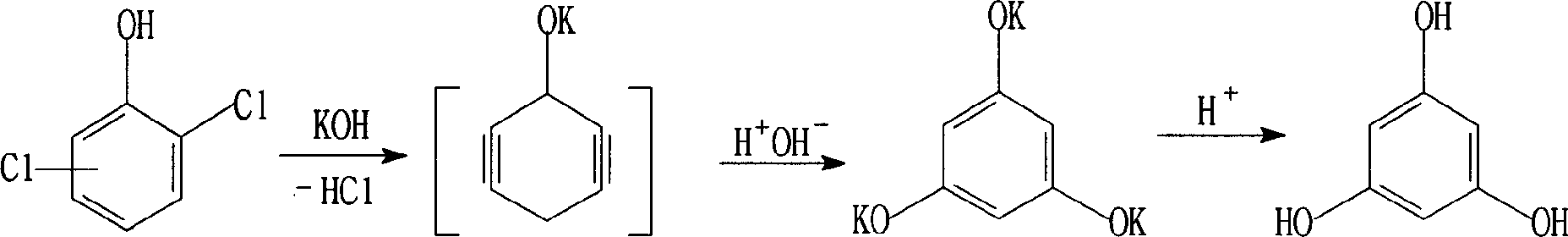Improved preparation technology of phloroglucinol
A technology of phloroglucinol and dichlorophenol, which is applied in the field of improved preparation process, can solve the problems of lower yield and quality, many influencing factors, harsh reaction conditions, etc., so as to reduce the cost of raw materials, shorten the process, and obtain raw materials easily Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a 500ml three-necked flask (equipped with stirring and azeotropic water separator), 90g (1.62mol) of potassium hydroxide and 180ml of tetramethylbenzene were mixed, heated to slight reflux, and filled with nitrogen, so that the reactor was filled with nitrogen. A solution made of 90ml tetramethylbenzene and 32.6g (0.2mol) of 2,4-dichlorophenol was added dropwise, heated to 180-190°C and azeotropically refluxed to divide 6.6-7.2ml of water, and the reaction time was 2 hours. Cool to 140°C, add 100ml of water dropwise to the reaction solution, then cool to 40°C, separate tetramethylbenzene (which can be used in the next batch), acidify with 30% sulfuric acid to pH 3.5-4.0, a large amount of potassium sulfate is formed. Extract with 200ml×3 ethyl acetate, dry at 110°C, and remove crystal water to obtain about 28.0g of off-white solid phloroglucinol with a yield of 55.6% and a content (HPLC)≥98.8%.
Embodiment 2
[0029] In a 1000ml three-necked flask, mix 200g (4.0mol) of potassium hydroxide and 400ml tetramethylbenzene, reflux slightly, fill with nitrogen, add dropwise 200ml tetramethylbenzene and 65.2g (0.4mol) of 2,6-dichlorophenol to form The solution was heated to 180-190°C, 13-14ml of water was rapidly azeotropically separated, and the reaction time was 2 hours. All the other operations are the same as in Embodiment 1. About 56.7 g of the product phloroglucinol was obtained, the yield was 56.2%, and the content (HPLC) was greater than or equal to 98.0%.
Embodiment 3
[0031] In a 1000ml three-necked bottle, put 180g (3.24mol) of potassium hydroxide into it, mix it with 360ml of cumene and fill it with nitrogen, add it to slightly heated reflux, and dropwise add 180ml of cumene and 65.2g of 2,6-dichlorophenol ( 0.4mol) of the prepared solution was heated to 160-170°C, azeotropically refluxed for 10 hours, and 13-14ml of water was divided. All the other operations are the same as in Embodiment 1. 65.8 g of light yellow solid phloroglucinol was obtained, the yield was 65.2%, and the content (HPLC) was ≥98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com