Recombinant pig alpha-interferon as well as preparation method and application thereof
A technology of interferon and restriction endonuclease, applied in the field of preparation of recombinant porcine α-interferon, can solve the problems of no cytotoxicity found, achieve good market potential, low endotoxin content, and improve safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Select the porcine alpha-interferon gene sequence
[0026] The porcine interferon alpha gene sequence with GenBank accession number 28623 was selected, and its nucleotide sequence is shown in SEQ ID NO.1. The nucleotide is translated into an amino acid sequence, as shown in SEQ ID NO.2.
[0027] 2. Optimize according to E. coli codons
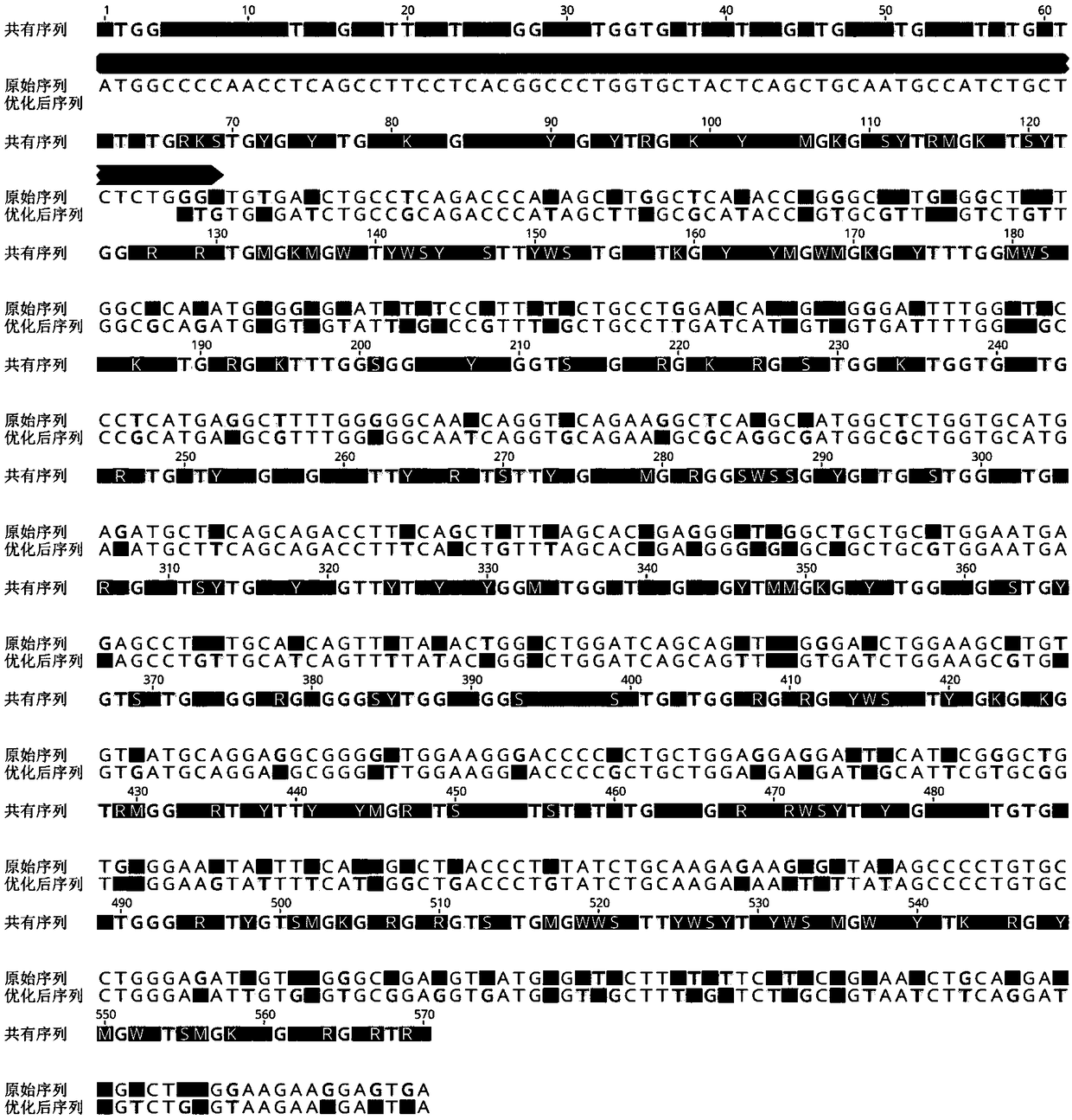
[0028] The above-mentioned expressed sequence was optimized on the DNAworks online software according to the E. coli codons to eliminate conventional restriction endonuclease cleavage sites, and the RNA secondary structure was optimized. The optimized nucleotide sequence is as SEQ IDNO. As shown in 3, the last three GAA are stop codons and no protein is expressed. Among them, conventional restriction endonucleases are those well-known to those skilled in the art, such as BamHI, EcoRI, NdeI, XhoI and the like.
[0029] 3. Optimized sequence confirmation
[0030] 1) Nucleotide sequence alignment
[0031] The nucleotide sequence shown in SEQ ID ...
Embodiment 2
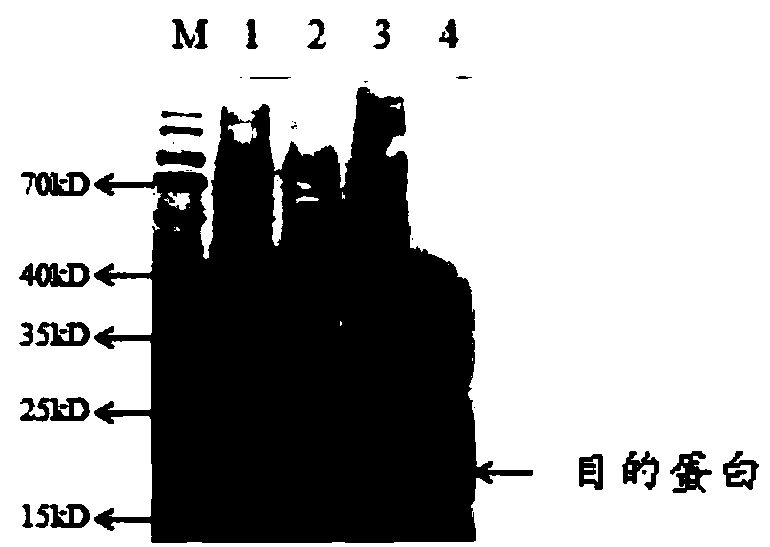
[0048] The embodiment of the present invention also provides a medicine containing the above-mentioned recombinant porcine alpha-interferon. Due to the high titer of the recombinant porcine interferon alpha provided by the above examples, the antiviral activity can reach 10 9 U / mg, and the content of endotoxin is extremely low, which creates prerequisites for the transformation into drugs, greatly improves the safety of drug use, and has good market potential and strong market competitiveness. The drug can be an anti-virus, anti-cell proliferation and immune-enhancing drug, and can be used alone or in combination to prevent and treat porcine viral diseases, and has potential application value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com