AFP recombinant protein, and in vitro high-efficiency recombination expression method
A recombinant protein and in vitro expression technology, applied in chemical instruments and methods, recombinant DNA technology, medical preparations of non-active ingredients, etc., can solve problems such as dysfunction and reduced ability of allogeneic lymphocyte proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
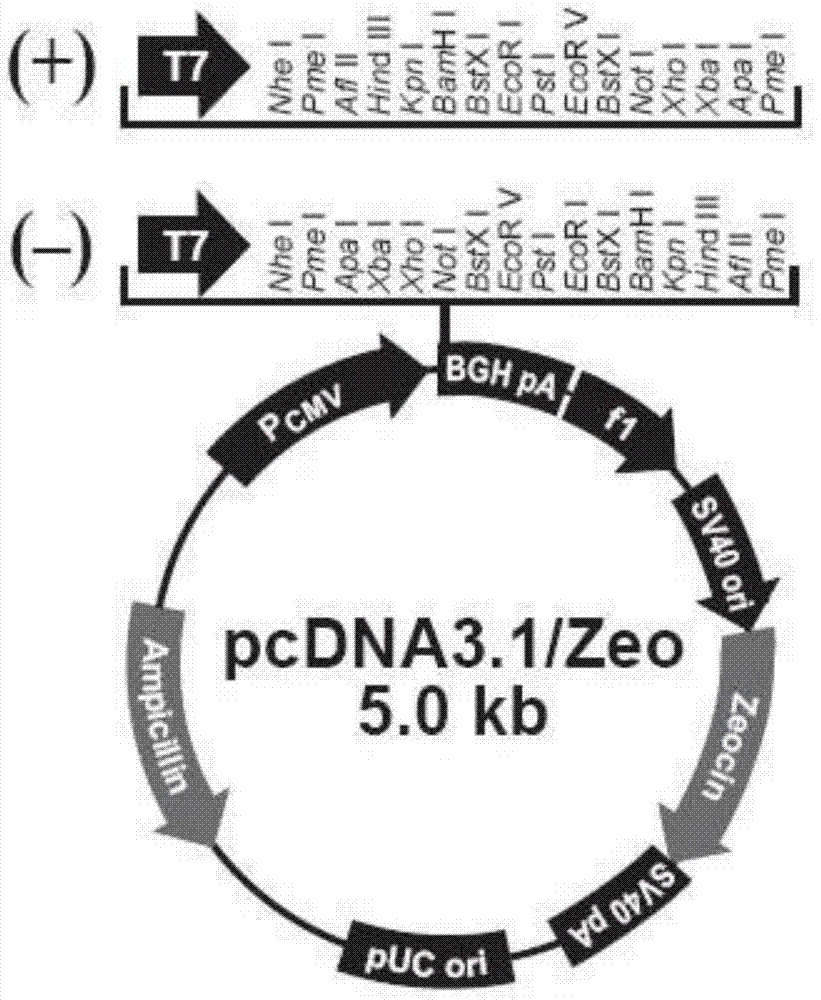
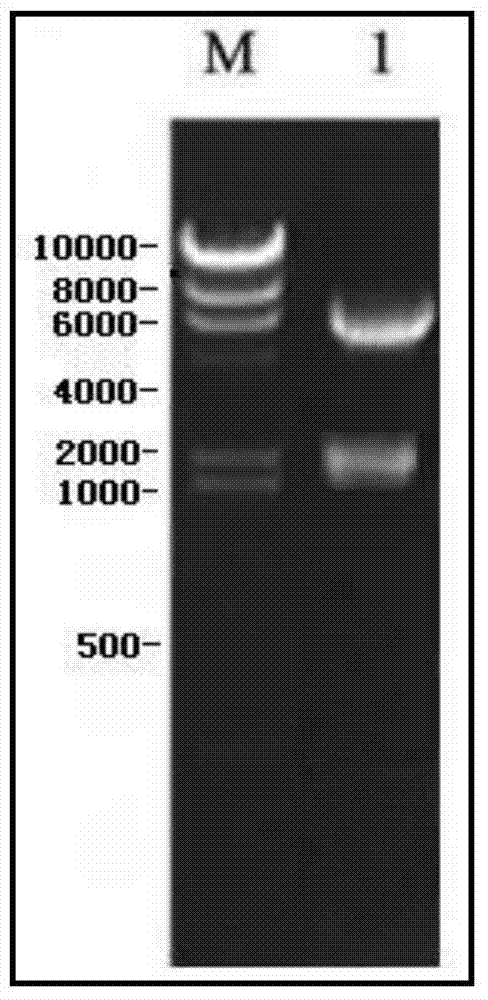
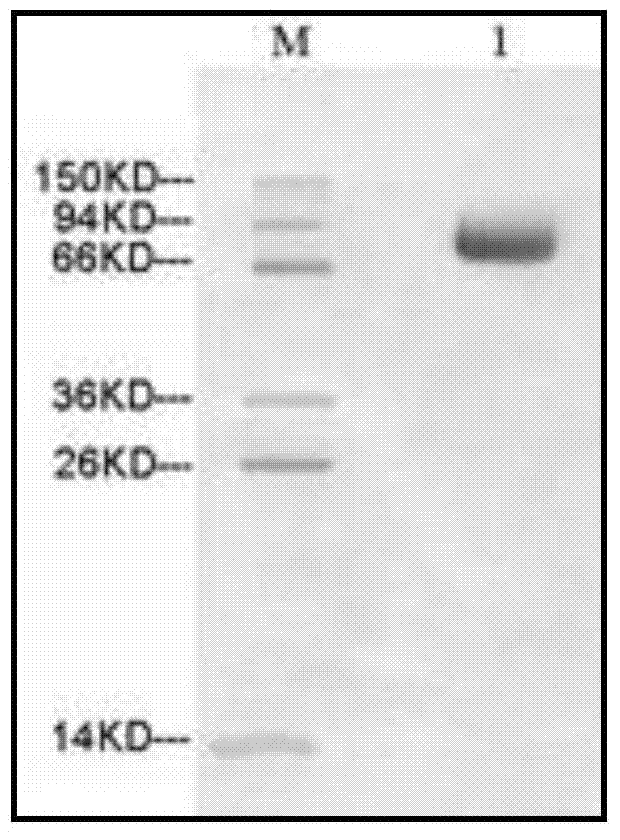
[0038] 1. Research materials: The recombinant plasmid pUC57-AFP was provided by an outsourcing service company; DNA markers, restriction endonucleases, T4 ligase, etc. were all purchased from Takara; pcDNA3.1+, CHO-S and Ni-NTA resins were all from Invitrogen The company's products; CHO-S cell culture medium FreeStyle CHO are all products of Gibco. PEI is a product of Polysciences.
[0039] 2. Workflow:
[0040](1) Optimize the DNA expression sequence of recombinant AFP in CHO: mainly optimize in three aspects: transcription, translation and protein folding. Transcription efficiency optimization: optimize GC content; SD sequence; TATA box; termination signal; CpG dinucleotide content. Translation efficiency optimization: optimize codon preference in CHO; mRNA secondary structure; PolyA early signal; repression site; RNA instability motif. Protein folding efficiency optimization: optimize RNA secondary structure; codon context correlation; codon-anticodon interaction.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com