Freeze-dried live attenuated hepatitis A vaccine not containing gelatin or human albumin protective agent and preparation method for freeze-dried live attenuated hepatitis A vaccine
A technology of human serum albumin and live attenuated vaccine is applied in the field of freeze-dried hepatitis A live attenuated vaccine and its preparation, and can solve the problems of carrying mad cow disease virus and human serum albumin prone to allergic reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1. According to the volume of 0.6ml per person, the virus content is 7.00-7.38lgCCID 50 / ml, calculate the amount of hepatitis A virus live virus stock solution.
[0019] 2. The formula of vaccine stock solution and protective agent (16-3) is calculated according to the volume ratio of 1:0.7-1.2. Weigh 40-120 grams of trehalose, 40 6-18 grams of dextran, and 1-5 grams of L-cysteine , 2-8 grams of arginine, 4-10 grams of glutamic acid, 5-20 grams of glycine, 1-5 grams of magnesium chloride, 1-5 grams of magnesium sulfate, 10-40 grams of sorbitol, 10-40 grams of mannitol, Appropriate amount of trishydroxymethyl aminomethane.
[0020] 3. Mix the above-mentioned trehalose, dextran 40, L-cysteine, arginine, glutamic acid, glycine, magnesium chloride, magnesium sulfate, sorbitol, mannitol, and trishydroxymethylaminomethane. Add 1000 ml of water for injection in turn, stir and mix well, adjust the pH to 6.8-7.4, sterilize by 0.1um filter, and set aside at 4°C.
[0021] 4. I...
Embodiment 2
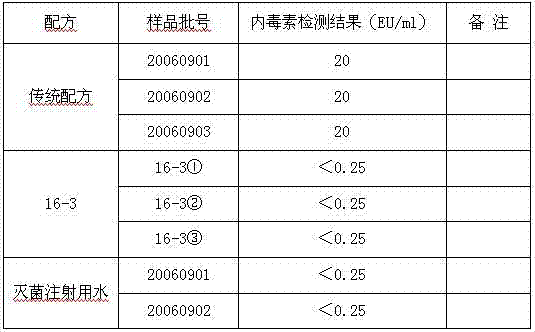
[0027] Protectant (16-3) formulation of gelatin-free, human albumin-free freeze-dried live attenuated hepatitis A vaccine, with three batches of vaccine containing gelatin (traditional) formulation, and two batches of sterile water for injection, It can be seen from the test results that the endotoxin content of the 16-3 formula without gelatin is significantly reduced, and its detection value is the same as that of sterile water for injection. see table 2
[0028] Table 2. Comparison of endotoxin content between 16-3 formula and the original production formula containing gelatin
[0029]
Embodiment 3
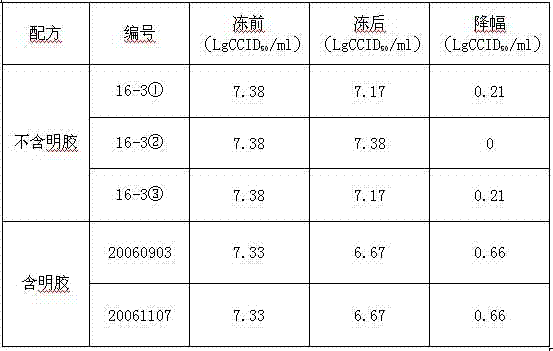
[0031] Vaccines with the same batch number containing the protective agent (16-3) formula of freeze-dried hepatitis A live attenuated live attenuated vaccine without gelatin and human serum albumin, stored in the refrigerator at 2-8°C, and randomly sampled regularly to determine the infectivity of the vaccine Titer, the result proves that according to the above-mentioned preparation method, freeze-drying makes freeze-dried hepatitis A live vaccine, after 2-8 ℃ of storing 21 months, the virus infectivity titer of vaccine remains unchanged, shows that the stability of vaccine is good. See Table 3.
[0032] Table 3. Stability of 16-3 freeze-dried hepatitis A vaccine stored at 2-8°C
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com