Man's serum albumin with man's parathormone (1-34) fusion protein and its application
A technology of human serum albumin and parathyroid hormone, applied in the field of genetic engineering, can solve the problems of high price and limited long-term application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
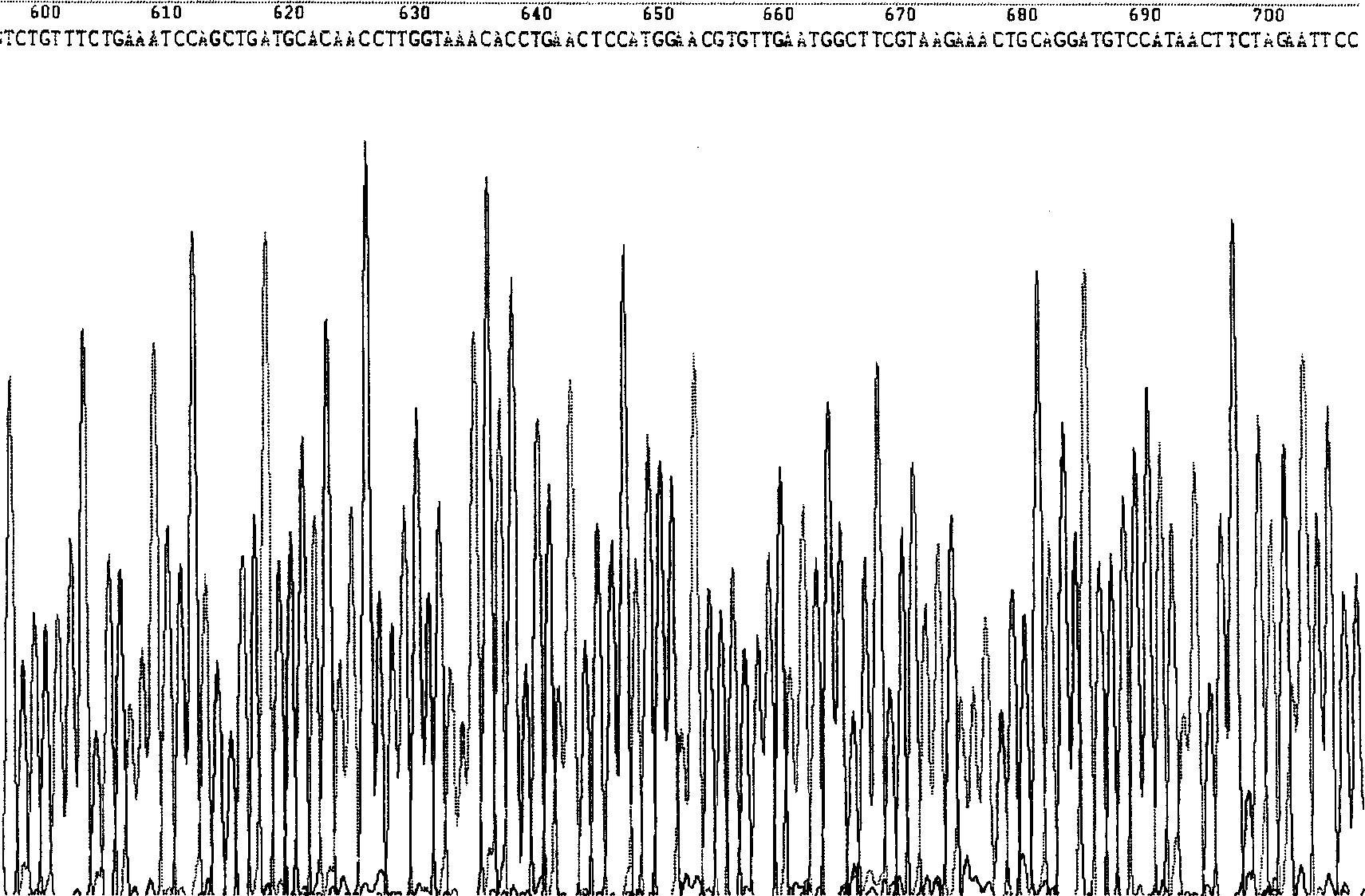
[0158] Example 1 Production of HSA and hPTH (1-34) fusion protein construction of yeast engineering bacteria
[0159] 1. Materials and methods
[0160] (1) Experimental materials
[0161] Yeast Pichia Pastoris GS115 (his4 Mut + ), the expression plasmid pPIC9 is a product of Invitrogen Company. The cloning vector pGEM-T is a product of Promega Company.
[0162] TRIzol reagent, TaqDNA polymerase, restriction endonuclease, PCR product recovery kit, plasmid extraction kit, and agarose gel recovery kit were all purchased from Shanghai Sangon Bioengineering Technology Service Co., Ltd. Engineering Technology Service Co., Ltd. synthesized it on its behalf, and the sequencing service was completed by Shanghai United Gene Biotechnology Service Company.
[0163] Yeast medium YPD, BMGY, BMMY, RDB, MM, MD components are the same as Invitrogen Pichia experiment manual. The goat anti-human serum albumin polyclonal antibody is the product of Beckman Company, and the goat anti-human hPT...
Embodiment 2
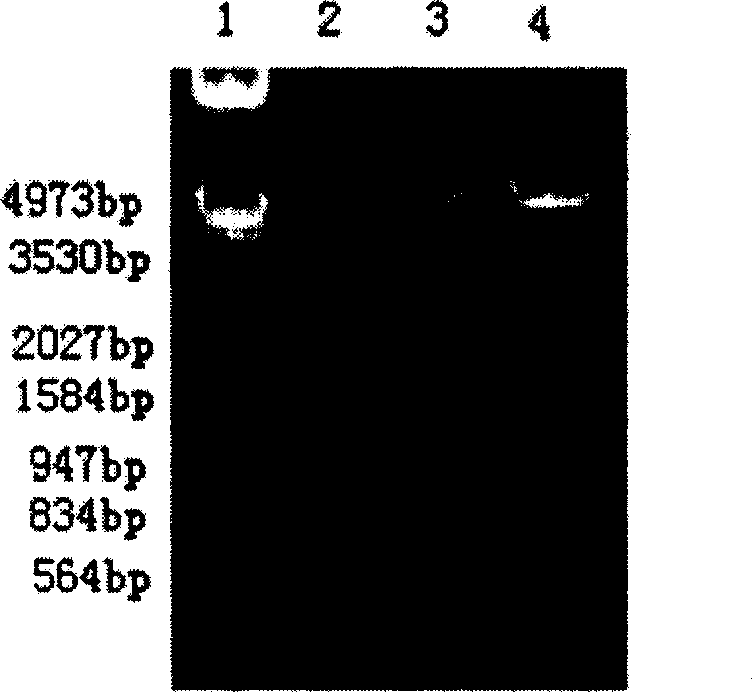
[0186] Example 2 Construction of pPIC9-HSA-hPTH(1-34) recombinant yeast expression plasmid
[0187] For the secreted expression of HSA and hPTH(1-34) as a fusion protein from Pichia pastoris, see figure 2 , select the pPIC9 plasmid as the vector, and insert the fusion protein gene at the EcoRI and NotI sites downstream of the alcohol oxidase AOX promoter of the vector. The vector pPIC9 was double digested with EcoRI and NotI overnight, and the gel was recovered for use. The aforementioned pGEM-HSA plasmid was double-digested with EcoRI and BamHI, and a fragment of about 1.8 kb was recovered. Put the linearized pPIC9 vector after enzyme digestion and the cDNA fragments containing HSA cDNA and hPTH(1-34) into the ligation system according to the appropriate molar ratio, catalyze with T4 ligase, react overnight at 16°C, and transform the freshly prepared DH5α sensory State cells were spread on LB agar plates containing ampicillin to pick positive clones. After identification ...
Embodiment 3
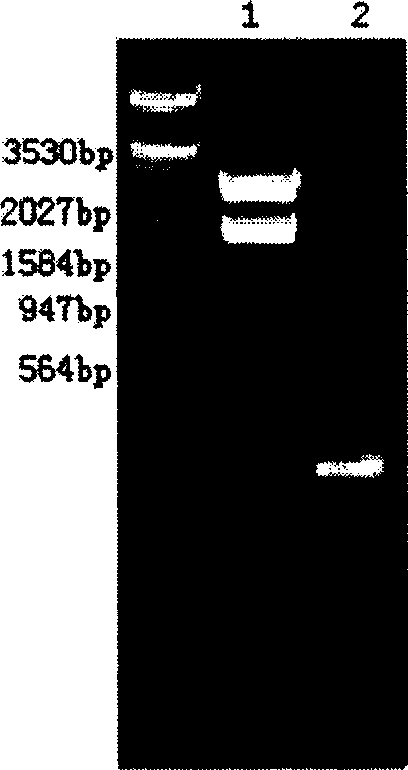
[0188] Example 3 Construction of other types of yeast expression plasmids for HSA and hPTH(1-34) fusion genes
[0189] Enzymes based on other yeast expression vectors such as pPIC9, pPIC9K, pPICZα, pHIL-S1, pPIC6α, pGAPZα and other yeast secretion vectors and other yeast intracellular expression vectors such as pPIC3.5K, pPAO815, pPICZ, pHIL-D2, pPIC6, pGAPZ Cut the site, modify the 5' and (or) 3' ends of the fusion gene of HSA-hPTH (1-34), and use conventional methods of molecular biology such as PCR, blunt end filling, blunt end connection, etc., to insert expression The multiple cloning site region of the vector. Construction work is carried out according to ligation, transformation, enzyme digestion and identification, and the sequencing steps are the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com