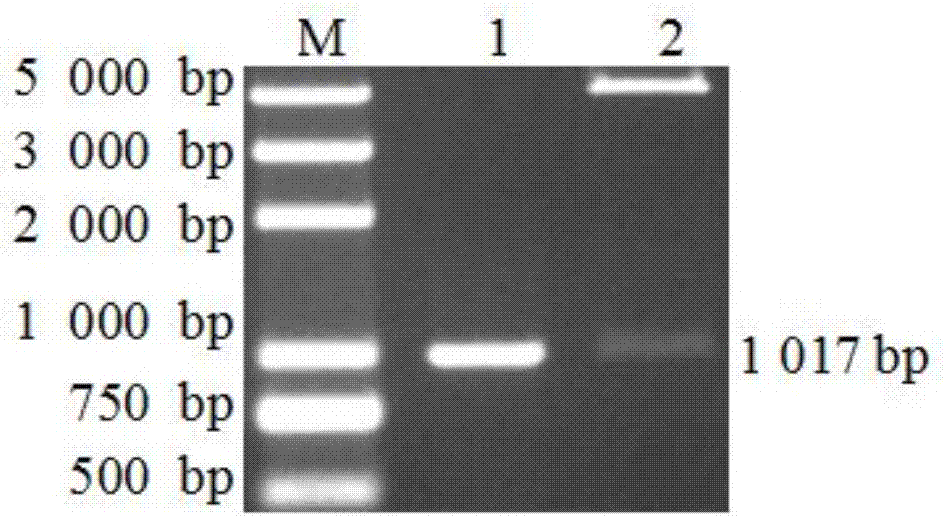
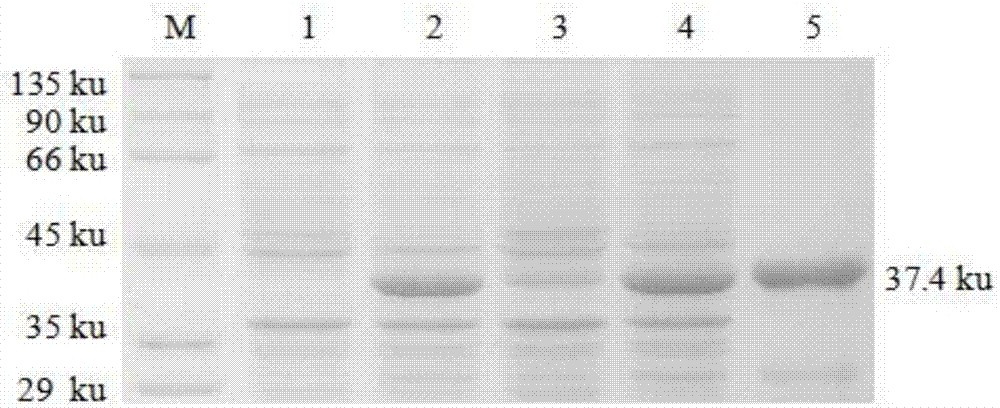
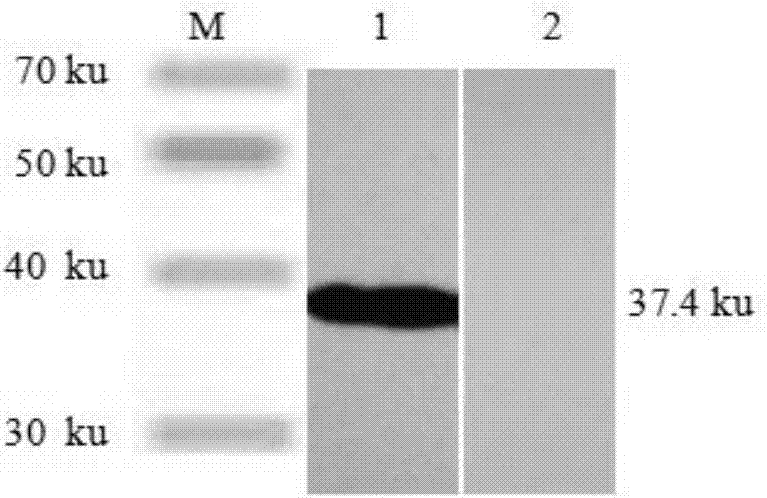
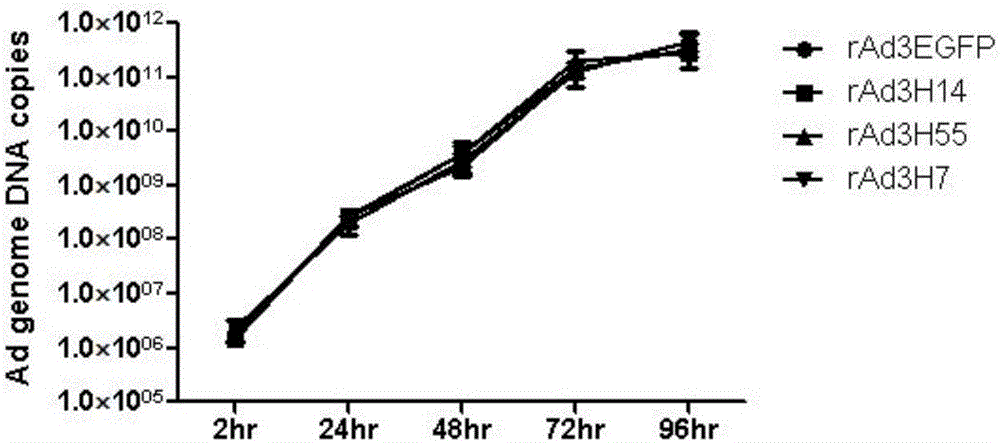
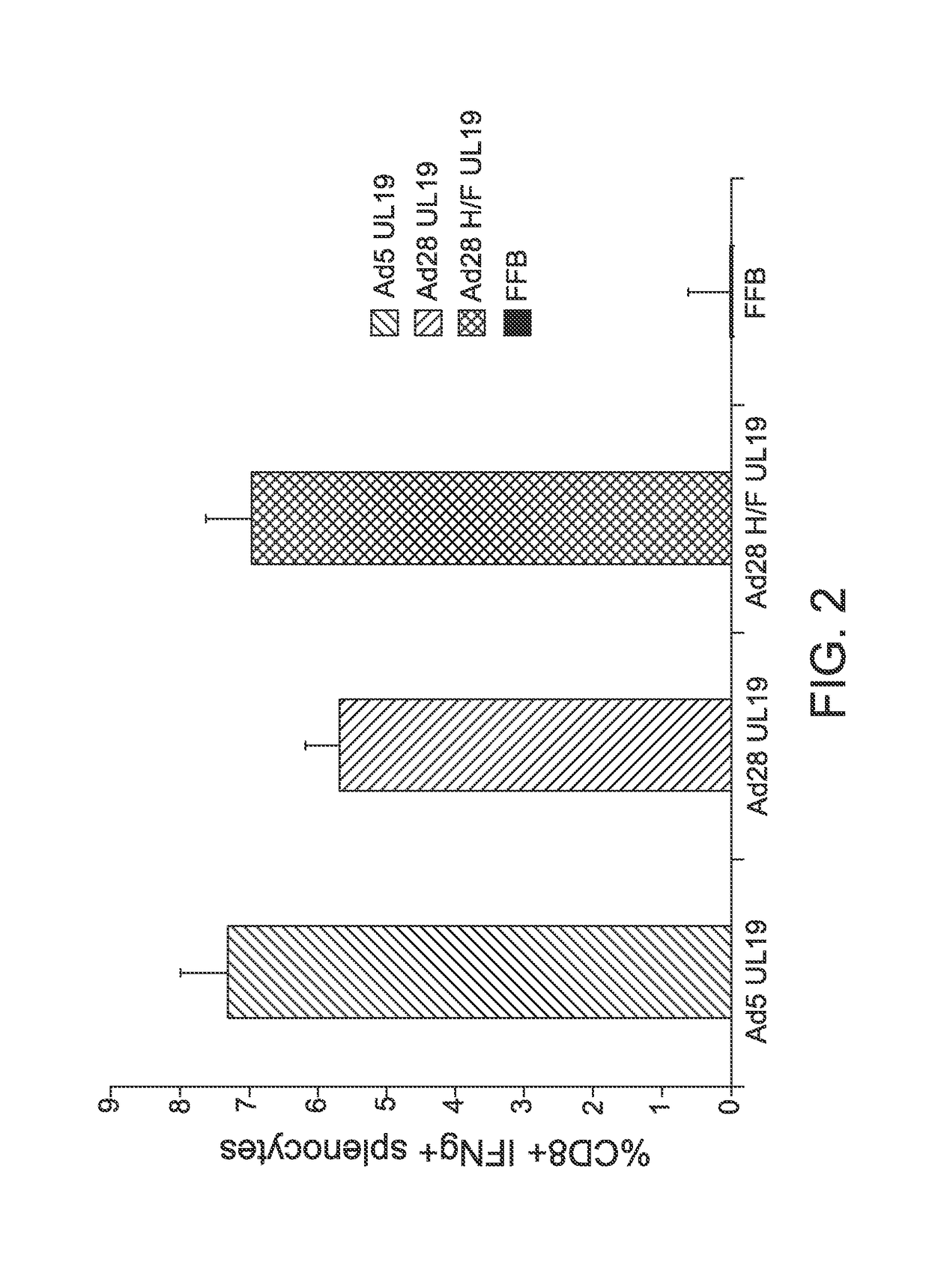
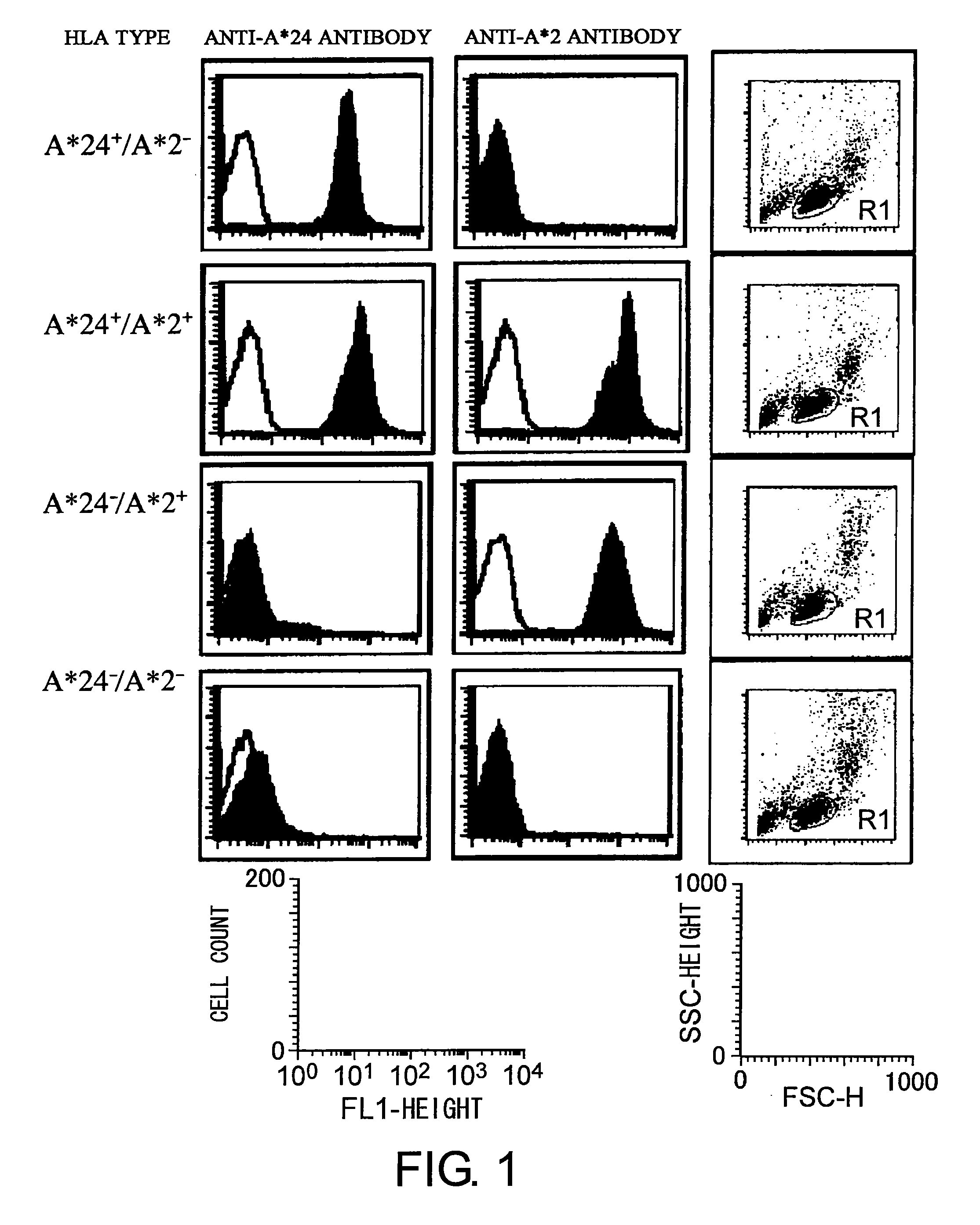
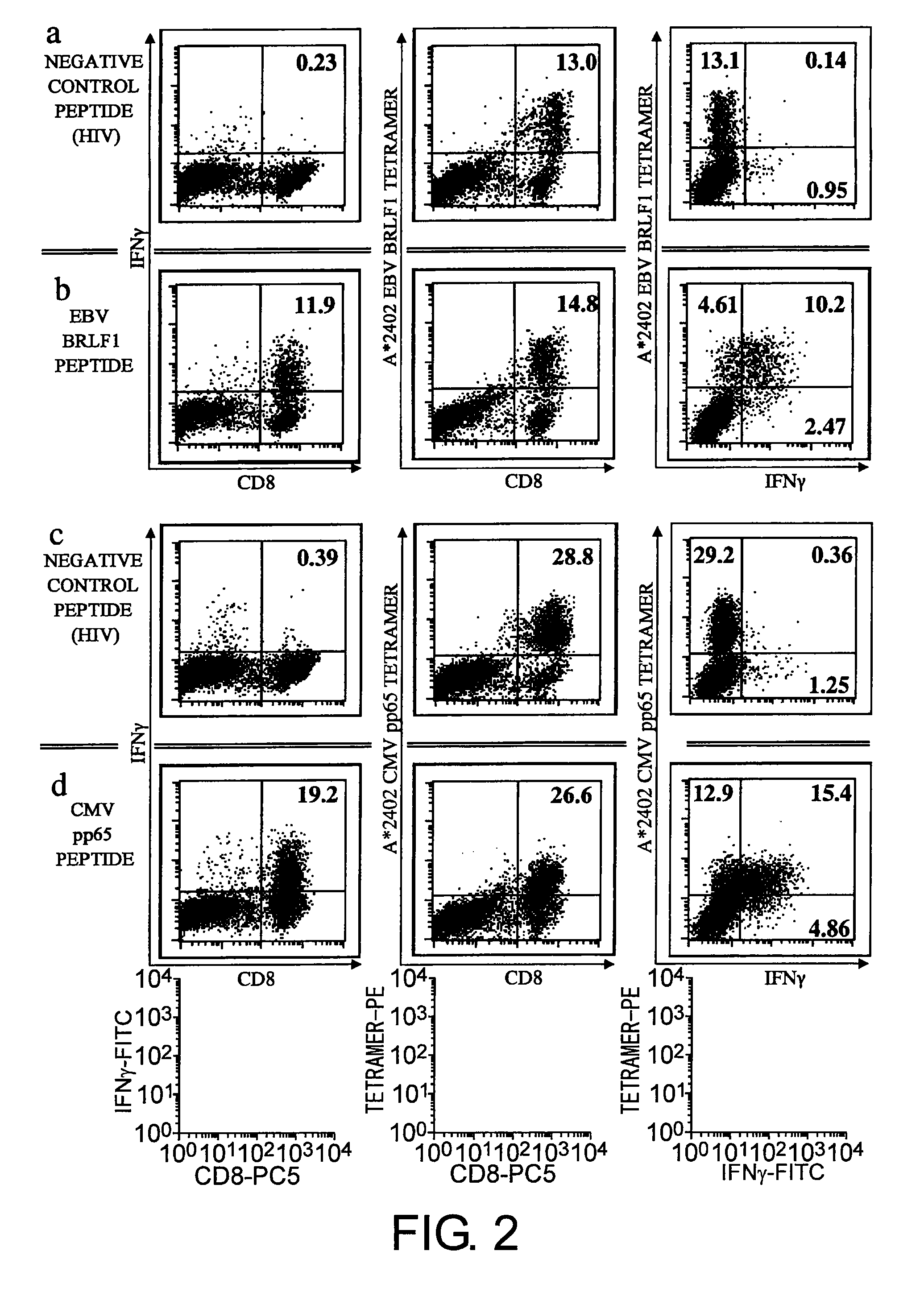
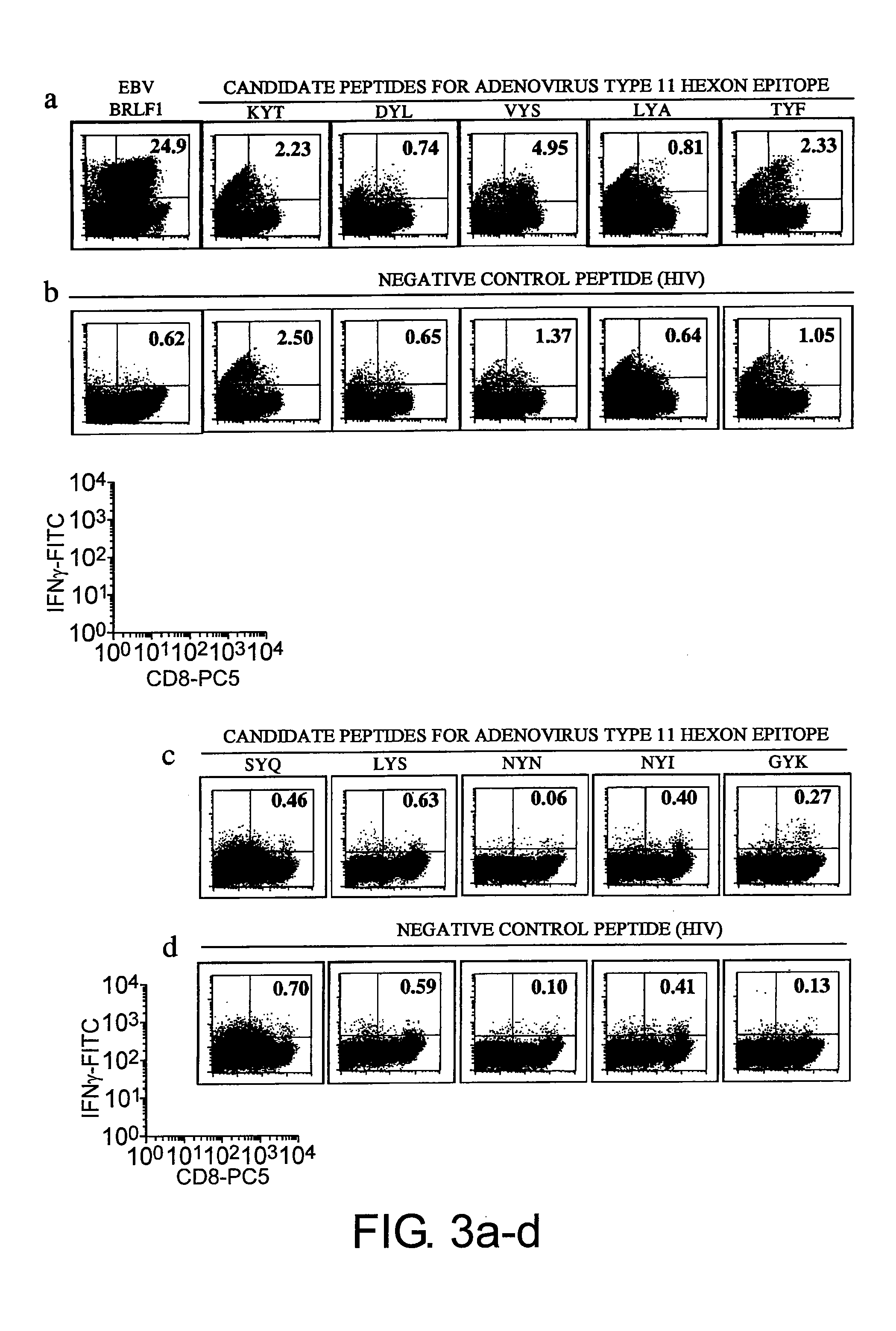
Patents
Literature
61 results about "Hexon protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In molecular biology, the hexon protein is a major coat protein found in Adenoviruses. Hexon coat proteins are synthesised during late infection and form homo-trimers. The 240 copies of the hexon trimer that are produced are organised so that 12 lie on each of the 20 facets. The central 9 hexons in a facet are cemented together by 12 copies of polypeptide IX. The penton complex, formed by the peripentonal hexons and penton base (holding in place a fibre), lie at each of the 12 vertices. The hexon coat protein is a duplication consisting of two domains with a similar fold packed together like the nucleoplasmin subunits. Within a hexon trimer, the domains are arranged around a pseudo 6-fold axis. The domains have a beta-sandwich structure consisting of 8 strands in two sheets with a jelly-roll topology; each domain is heavily decorated with many insertions. Some hexon proteins contain a distinct C-terminal domain.
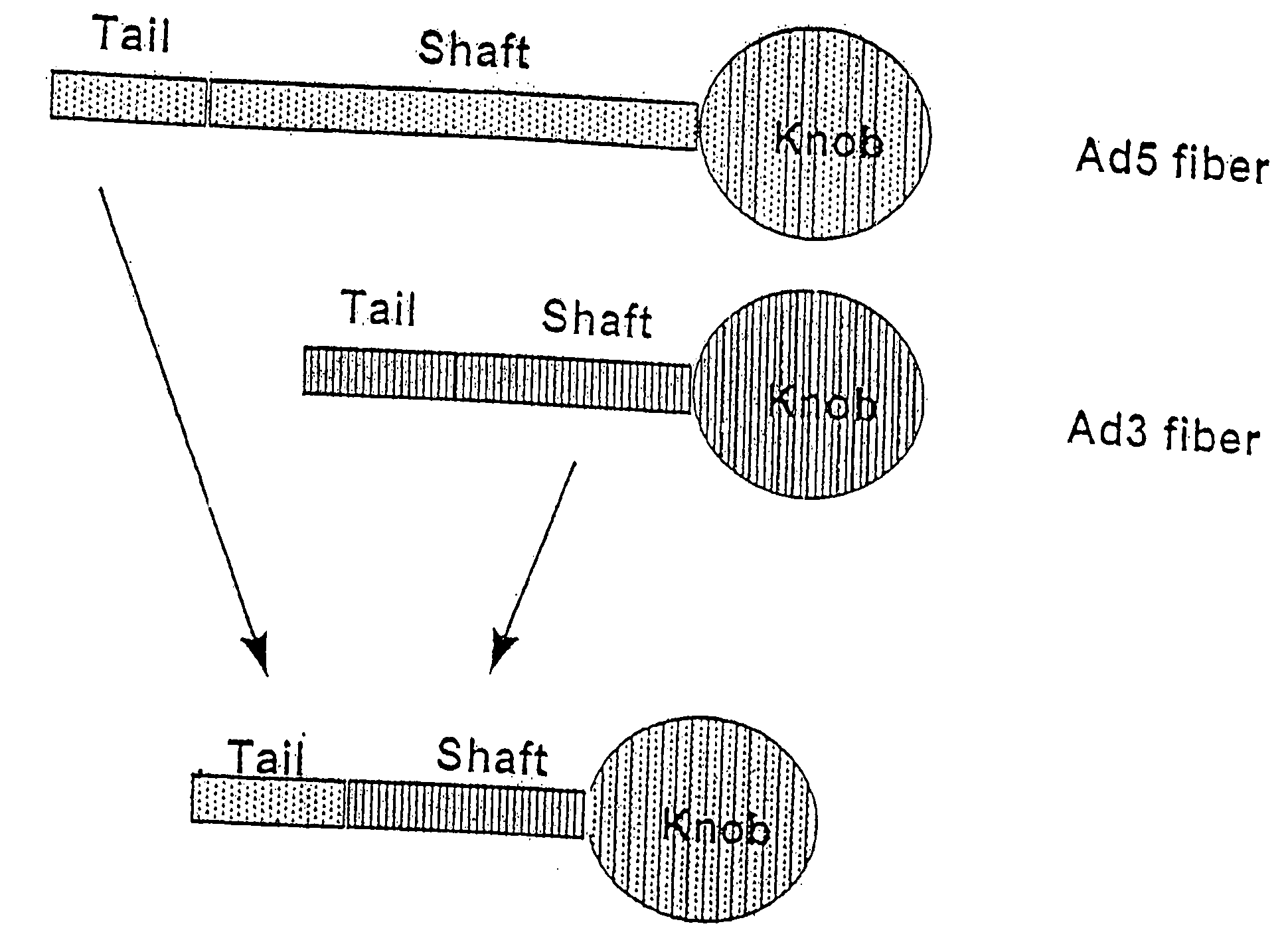
Targeted adenovirus vectors for delivery of heterologous genes
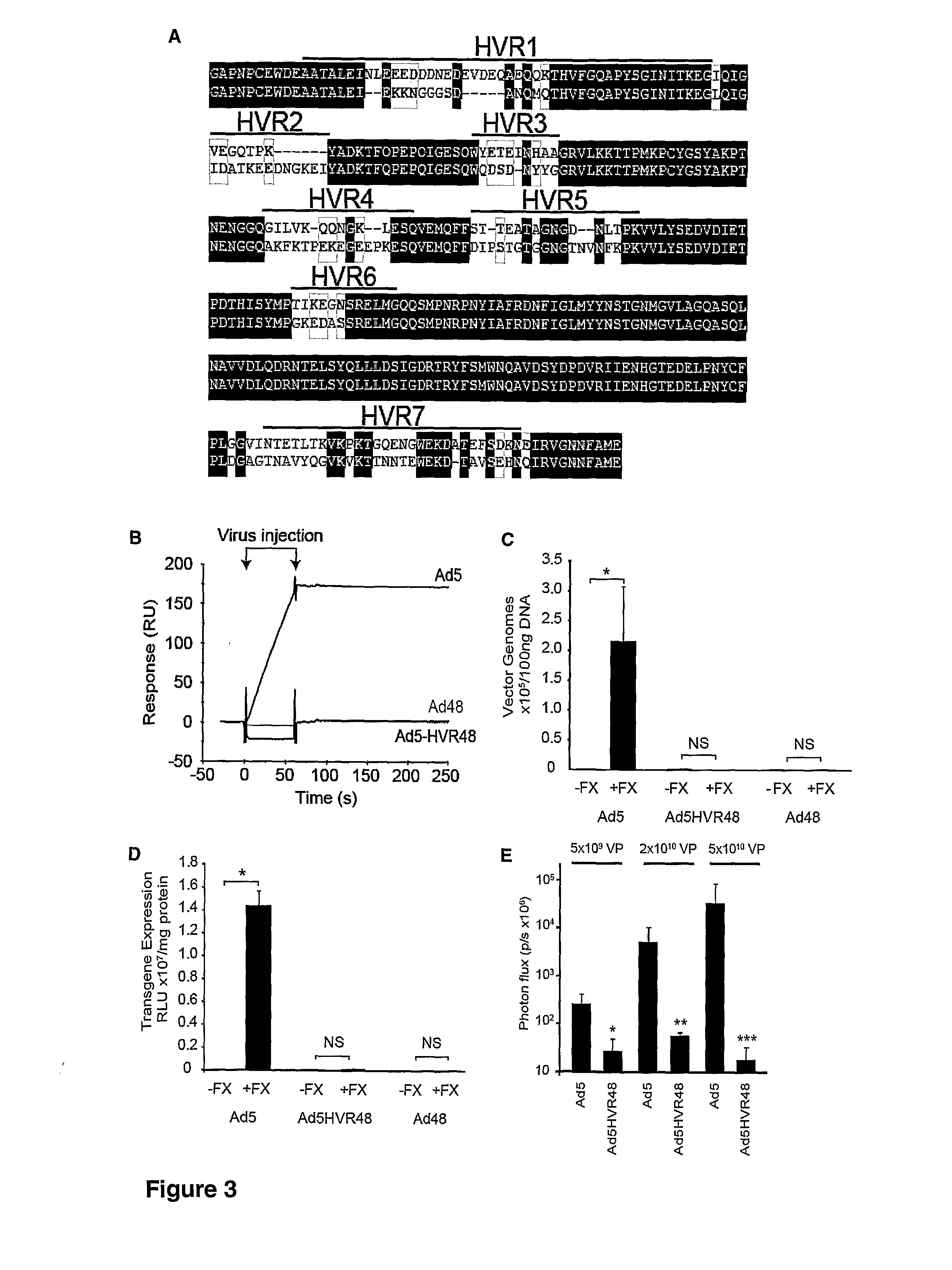
Modification of internal sites of the adenovirus fiber protein and hexon protein permit effective targeting of adenovirus vectors. Accessible sites to redirect adenovirus targeting were identified. The HVR5 loop of the hexon protein and the HI loop of the fiber protein (knob) were highly permissive for the insertion of foreign protein sequences, which apparently did not impact on the viability and productivity of corresponding viruses. Accessibility and functionality of the epitope strongly depend on the size of the neighboring spacers. Other results suggest that short targeting peptides can be effectively fused to the C-terminus of the fiber protein. In a specific embodiment, a series of adenovirus vectors modified at the HVR5 site, the fiber protein HI loop, or the fiber protein C-terminus to target urokinase-type plasminogen activator receptor bearing cells were prepared. Such vectors are particularly useful for targeting the vasculature, e.g., for gene therapy of cancers or cardiovascular conditions.
Owner:CENTELION SAS
Adenoviral vectors and uses thereof
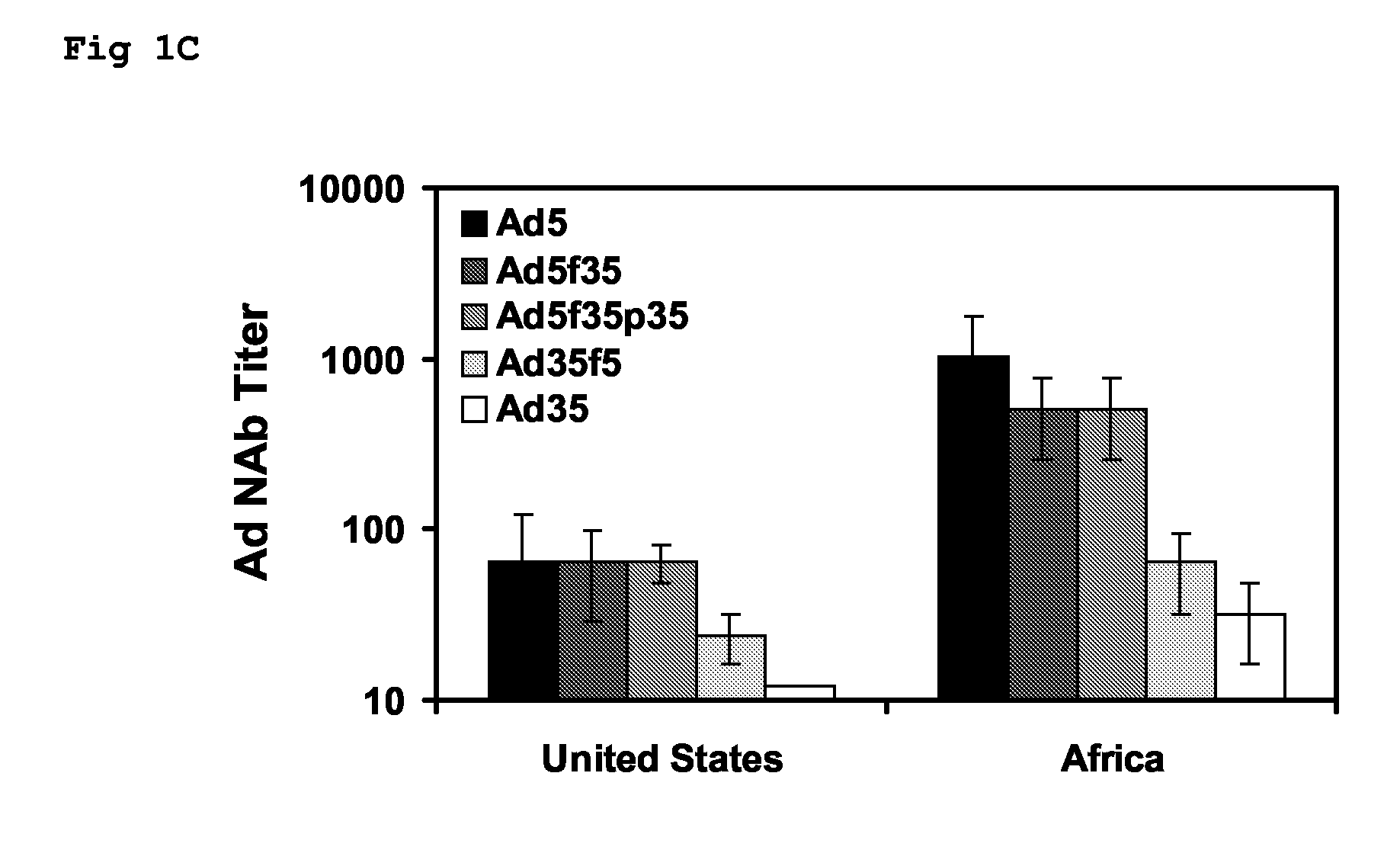
The present invention relates to recombinant adenoviral vectors based on adenoviruses that encounter pre-existing immunity in a minority of the human population and which harbor a chimeric capsid. The chimeric capsid comprises fiber proteins that have at least the knob domain of a human adenovirus that binds to the Coxsackievirus and Adenovirus Receptor (CAR) and a hexon protein from an adenovirus serotype that encounters pre-existing immunity in a low percentage of the human population.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
Improved adenoviral vectors and uses thereof
The present invention relates to recombinant adenoviral vectors based on adenoviruses that encounter pre-existing immunity in a minority of the human population and which harbour a chimeric capsid. The chimeric capsid comprises fiber proteins that have at least the knob domain of a human adenovirus that binds to the Coxsackievirus and Adenovirus Receptor (CAR) and a hexon protein from an adenovirus serotype that encounters pre-existing immunity in a low percentage of the human population.
Owner:JANSSEN VACCINES & PREVENTION BV +1
Affenadenovirus (gorilla) or adenoviral vectors and methods of use
The invention provides an adenovirus or adenoviral vector characterized by comprising one or more particular nucleic acid sequences or one or more particular amino acid sequences, or portions thereof, pertaining to, for example, an adenoviral pIX protein, DNA polymerase protein, penton protein, hexon protein, and / or fiber protein.
Owner:GEN VEC INC
Simian (gorilla) adenovirus or adenoviral vectors and methods of use
The invention provides an adenovirus or adenoviral vector characterized by comprising one or more particular nucleic acid sequences or one or more particular amino acid sequences, or portions thereof, pertaining to, for example, an adenoviral pIX protein, DNA polymerase protein, penton protein, hexon protein, and / or fiber protein.
Owner:GEN VEC INC
Modulation of Adenoviral Tropism
InactiveUS20110104788A1Increase opportunitiesHelp studyCompound screeningApoptosis detectionProviding materialTissues types
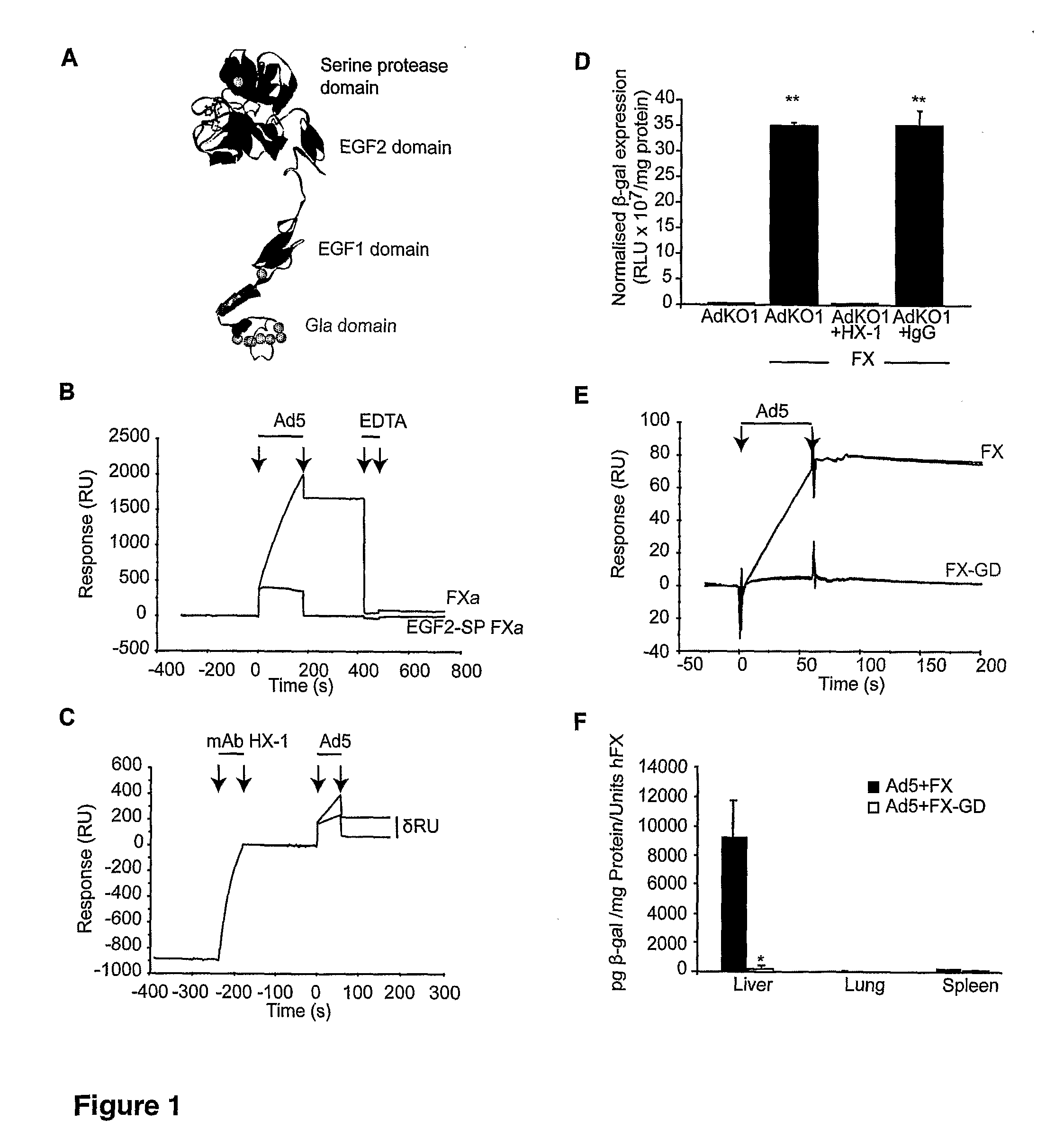
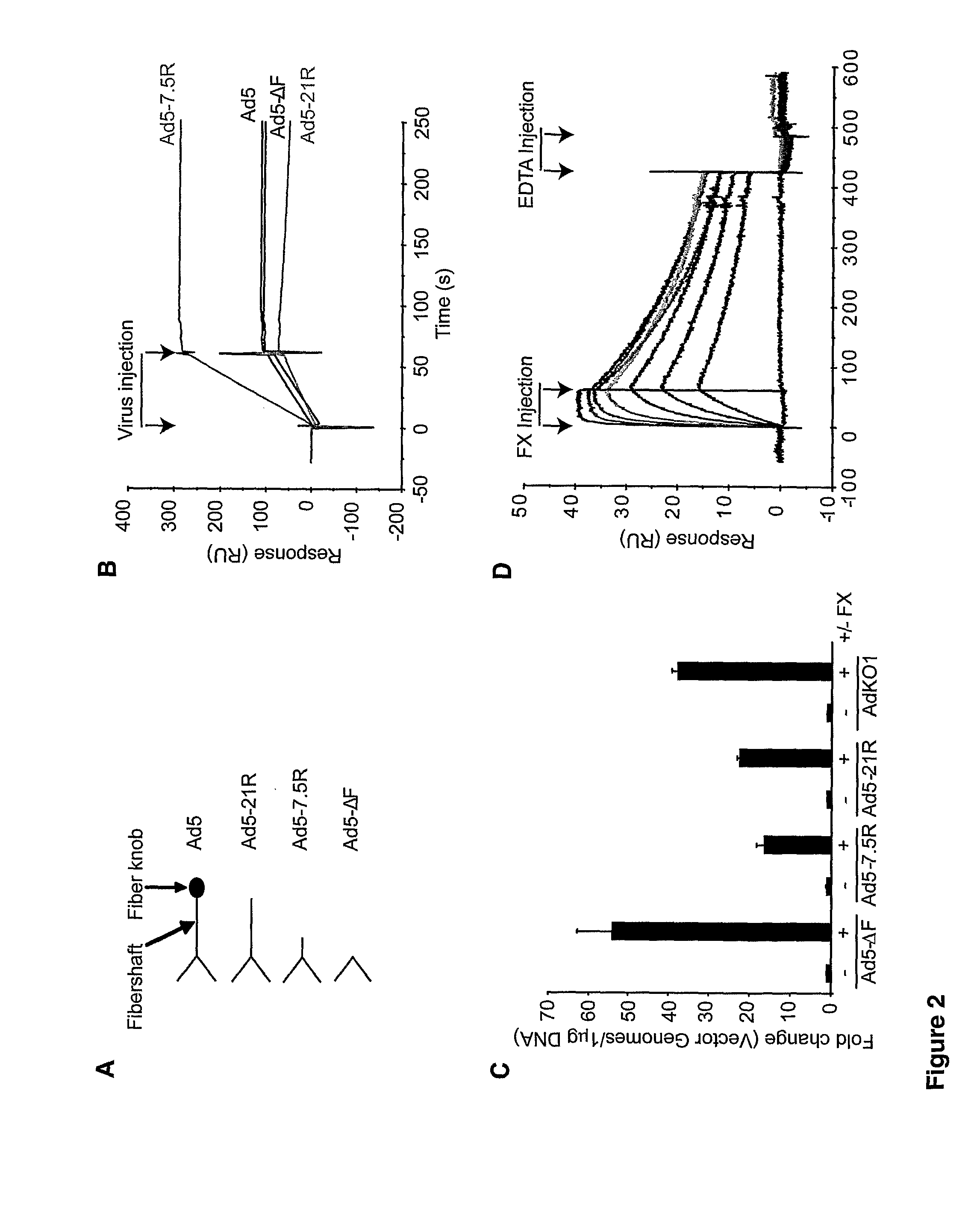
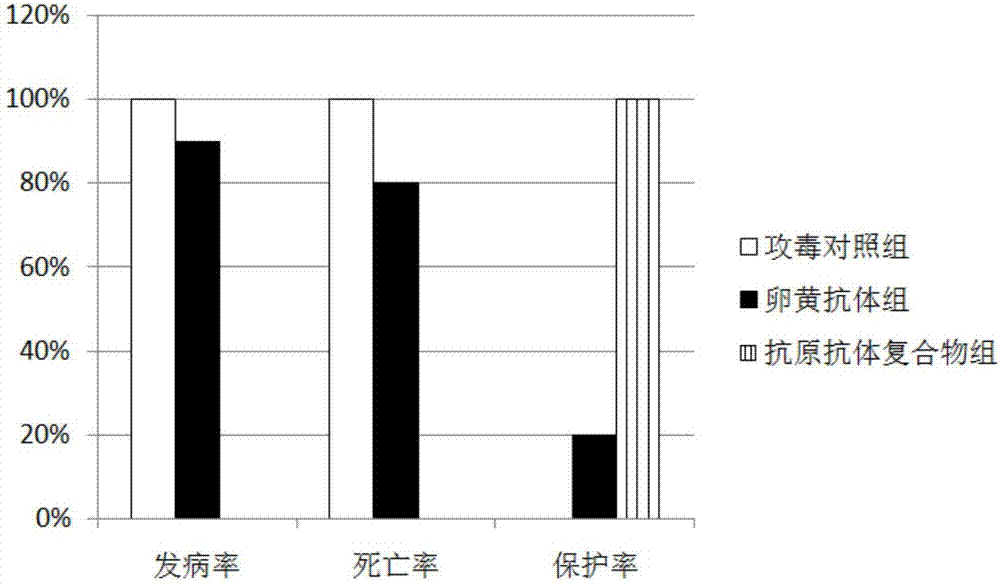
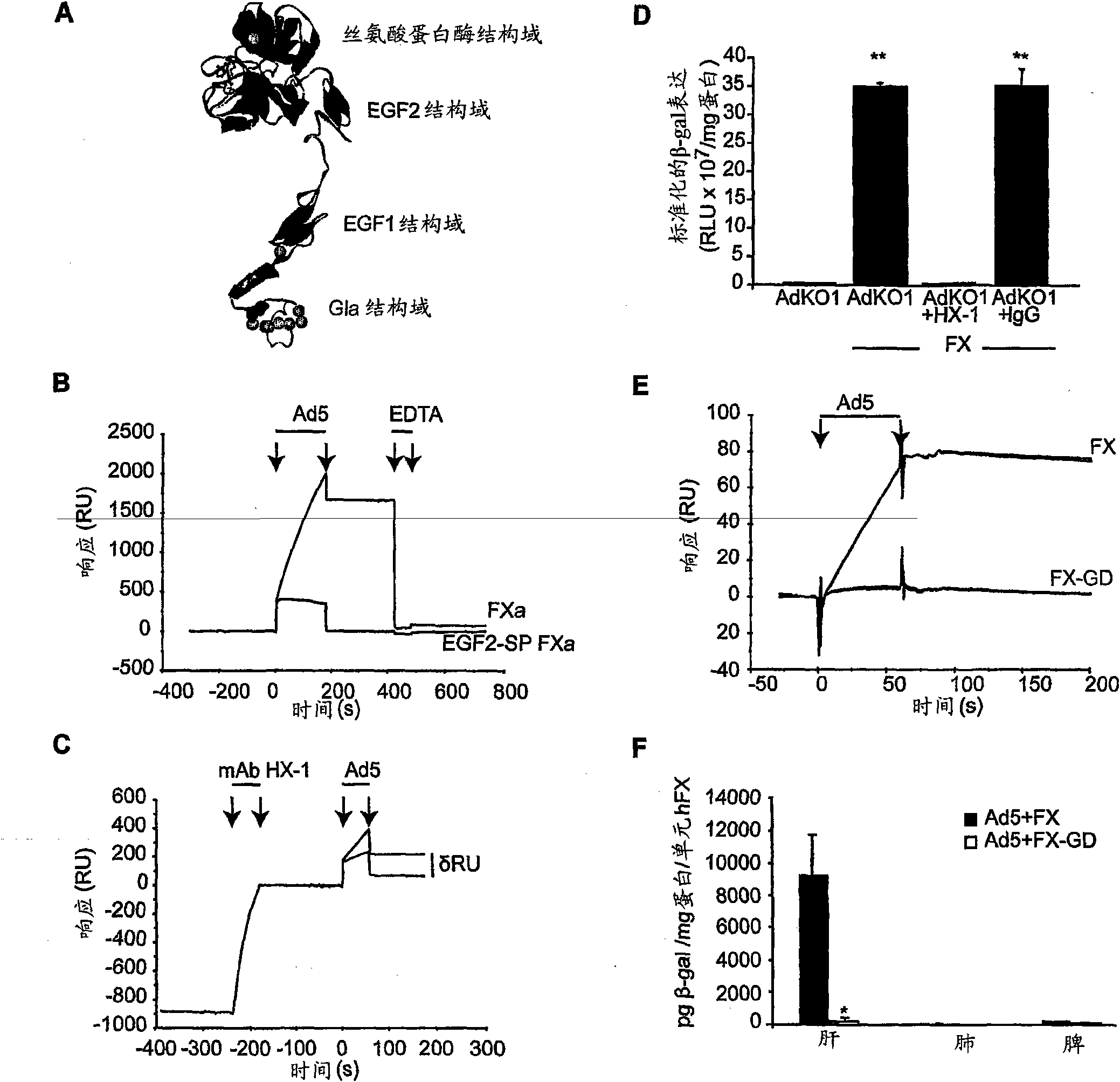
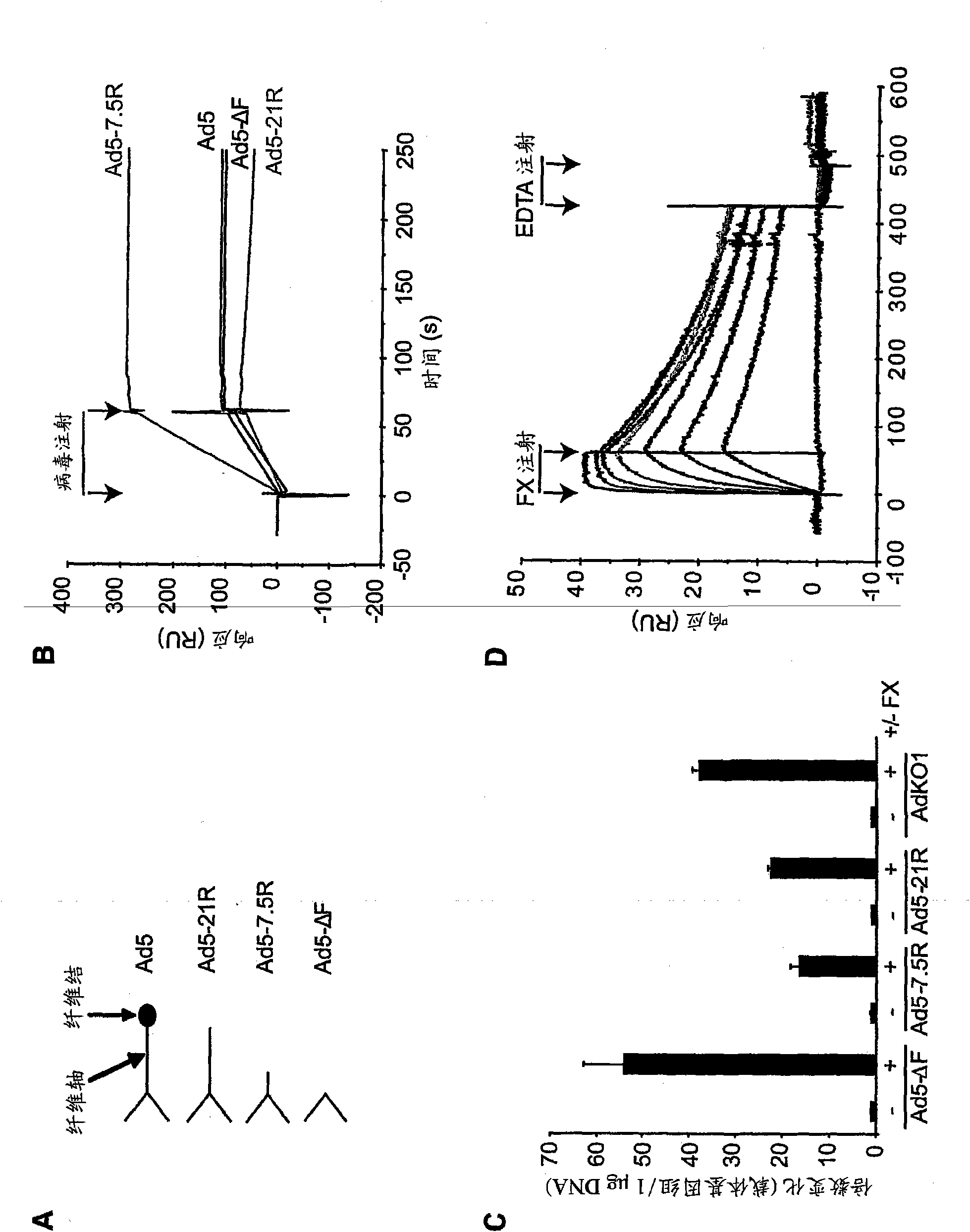
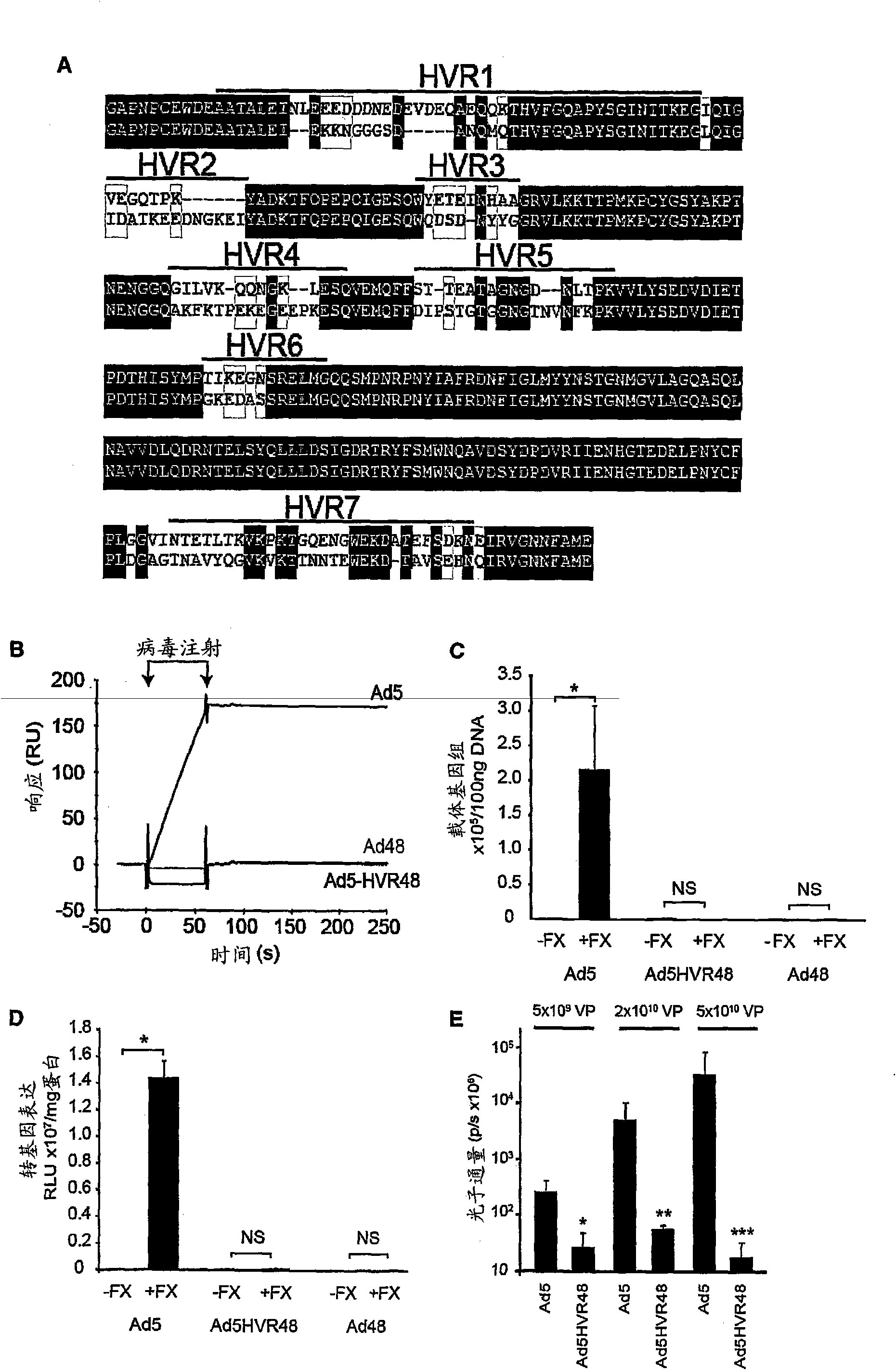
The invention provides materials and methods for modulating adenoviral tropism for hepatocytes and other cell types such as splenocytes. It relates to the findings that hypervariable regions (HVRs) of the viral hexon protein interact with the Gla domain of the blood clotting factor FX as part of the infective process in vivo. The invention provides means to disrupt the interaction between hexon and FX, thus reducing infection of hepatocytes and splenocytes, as well as use of targeting agents comprising the Gla domain or a fragment thereof to direct adenoviral vectors to desired target cell or tissue types.
Owner:BAKER ANDREW +2
Targeted adenovirus vectors for delivery of heterologous genes
Modification of internal sites of the adenovirus fiber protein and hexon protein permit effective targeting of adenovirus vectors. Accessible sites to redirect adenovirus targeting were identified. The HVR5 loop of the hexon protein and the HI loop of the fiber protein (knob) were highly permissive for the insertion of foreign protein sequences, which apparently did not impact on the viability and productivity of corresponding viruses. Accessibility and functionality of the epitope strongly depend on the size of the neighboring spacers. Other results suggest that short targeting peptides can be effectively fused to the C-terminus of the fiber protein. In a specific embodiment, a series of adenovirus vectors modified at the HVR5 site, the fiber protein HI loop, or the fiber protein C-terminus to target urokinase-type plasminogen activator receptor bearing cells were prepared. Such vectors are particularly useful for targeting the vasculature, e.g., for gene therapy of cancers or cardiovascular conditions.
Owner:AVENTIS PHARMA SA (US)
Human adenovirus antigen epitope chimeric protein as well as preparation and application thereof
ActiveCN105820257ALow costEasy to purifyAntibody mimetics/scaffoldsVirus peptidesChemical synthesisNucleotide
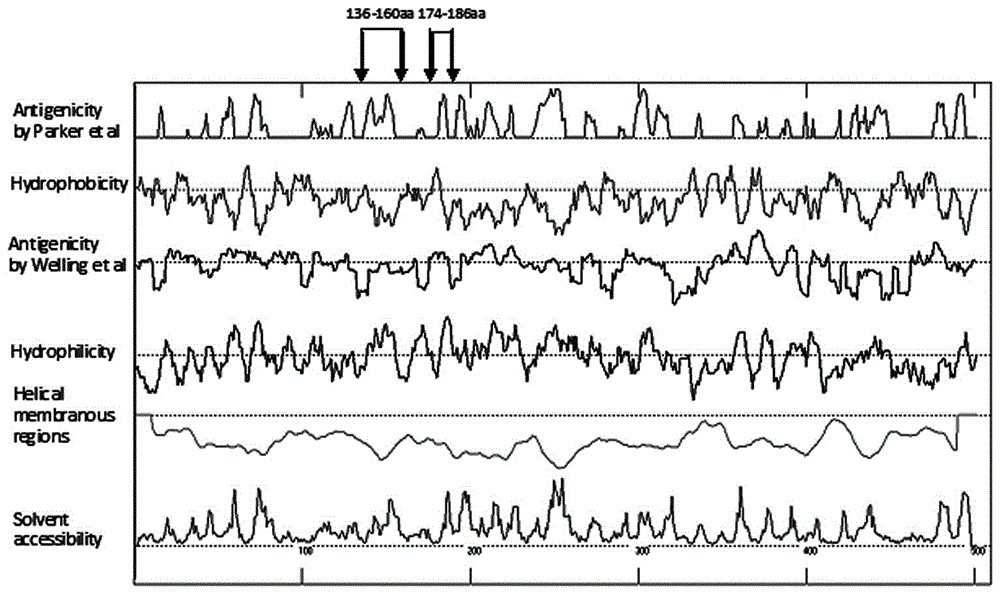
The invention discloses human adenovirus antigen epitope chimeric protein as well as preparation and application thereof, and relates to fields of gene engineering techniques, vaccines and diagnostic reagents. The amino acid sequences of hexon protein of type 3, type 7, type 11, type 14 and type 55 of adenovirus are analyzed through computer analysis, protein fragments with good antigenicity can be screened respectively, the fragments are connected by using two glycine and one serine, then chimeric protein with multiple antigen fragments in serial connection can be formed, pronucleus preferred codons are selected and interpreted into corresponding nucleotide sequences, and full-length genes can be synthesized in a chemical manner. The chimeric protein can be obtained through expression purification by using a gene engineering technique, and the chimeric protein comprises 363 amino acids in whole length. The expressed chimeric protein can be applied to vaccine research, HAdV antibody or antigen detection and the like, and is related to fields such as gene engineering techniques, vaccines and diagnostic reagents.
Owner:中国人民解放军东部战区疾病预防控制中心
Evaluating biological material for unassociated virus-size particles having an adenovirus hexon protein epitope
ActiveUS9816912B2Easy to useComplicate to differentiateSsRNA viruses negative-senseSsRNA viruses positive-senseEpitopeAdeno associate virus
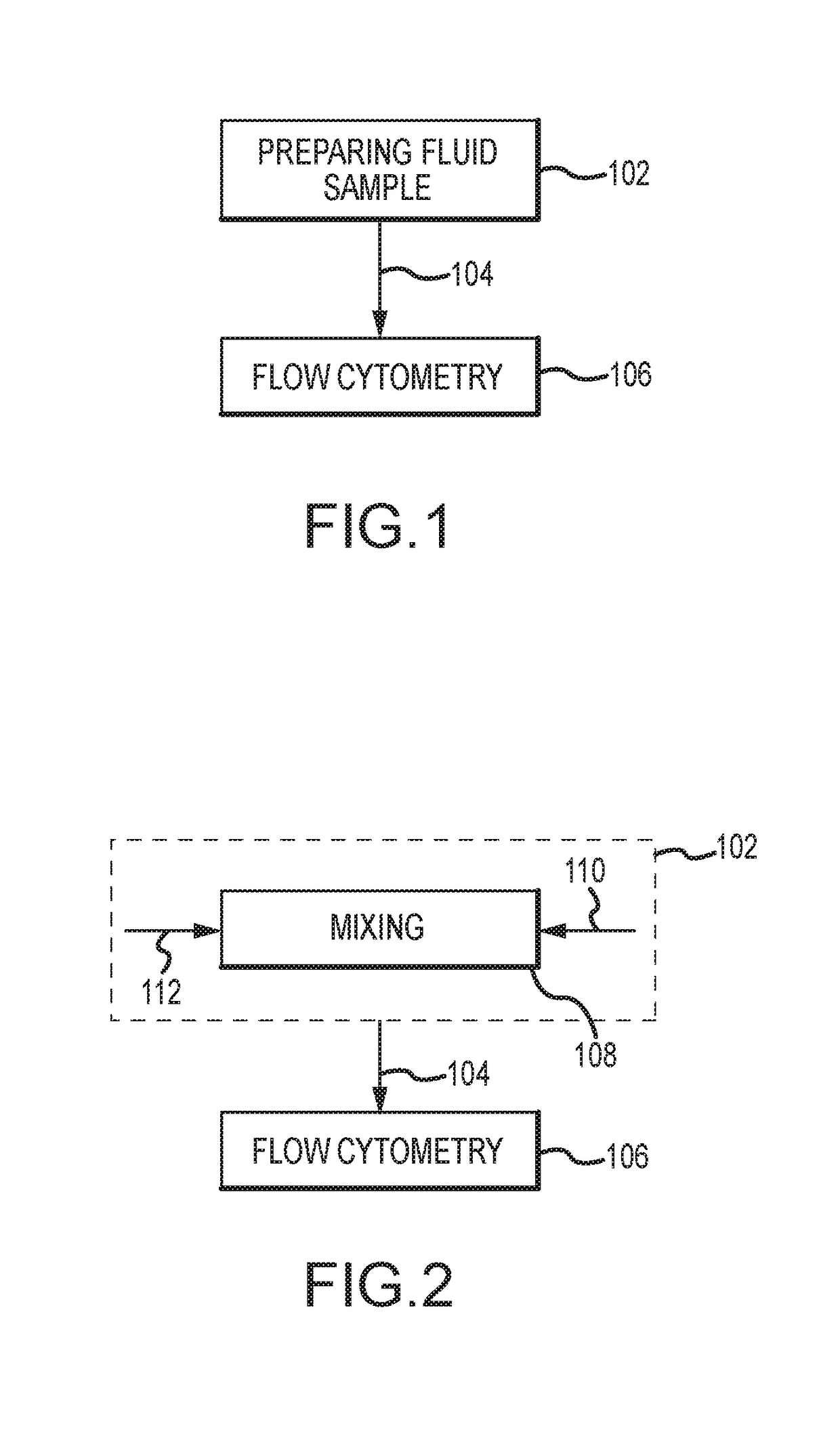
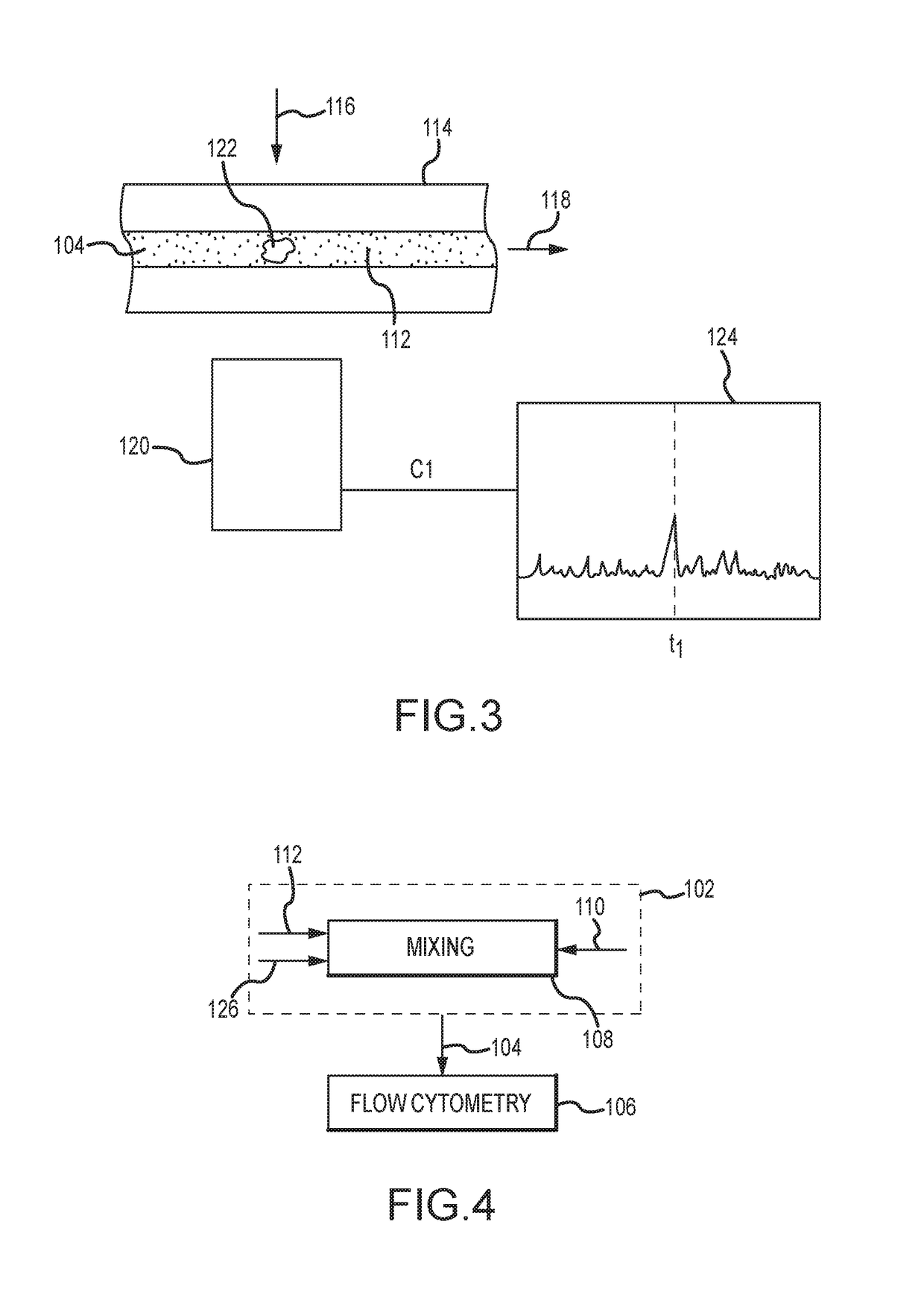
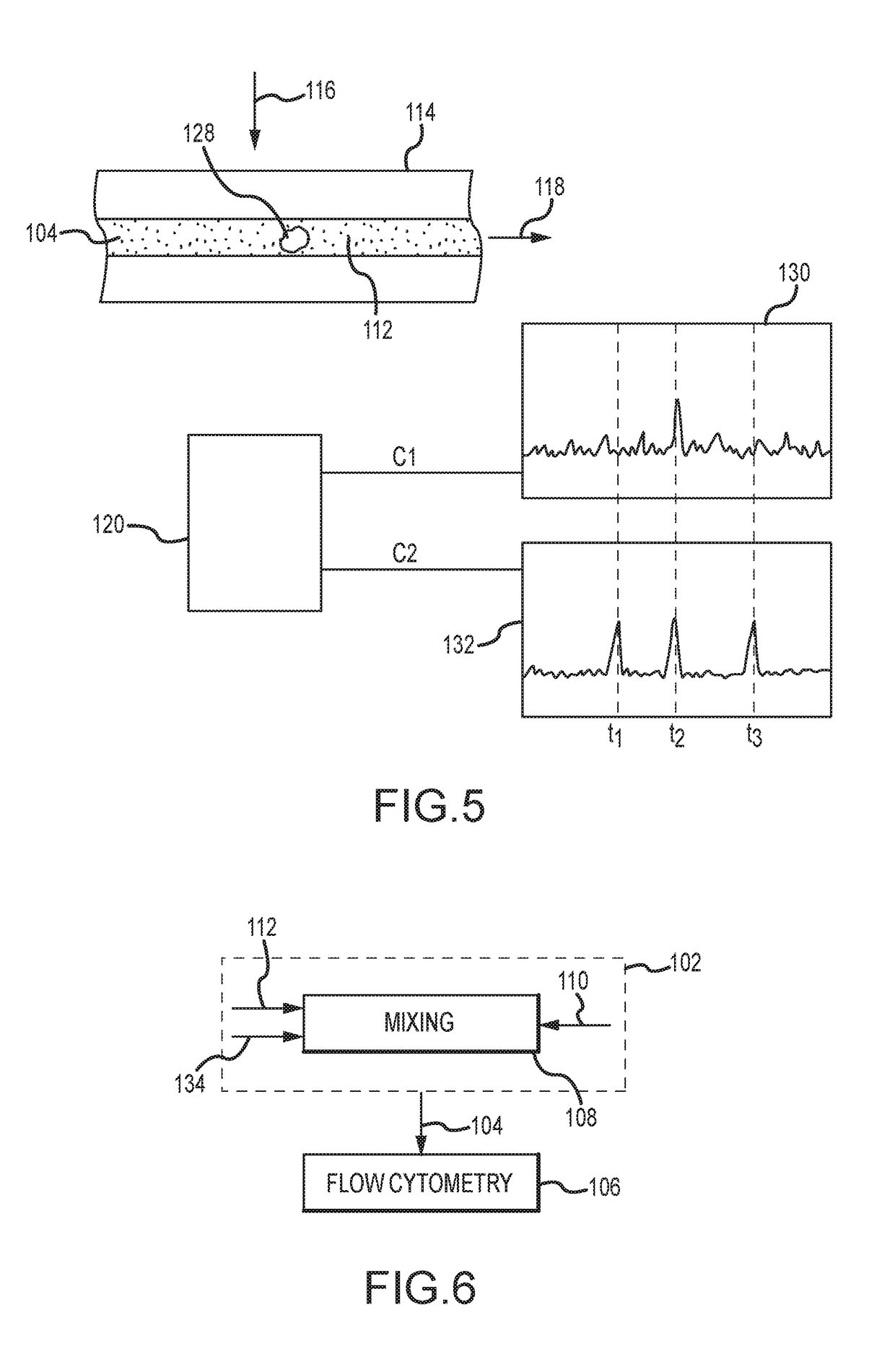
A method for evaluating a biological material for unassociated virus-size particles having a particular epitope indicative of an adeno-associated virus viral type or an adenovirus viral type uses a fluorescent antibody stain specific for binding with the epitope and a fluid sample with the virus-size particles and fluorescent antibody stain is subjected to flow cytometry with identification of fluorescent emission detection events indicative of passage through a flow cell of a flow cytometer of unassociated labeled particles of virus size including such a virus-size particle and fluorescent antibody stain.
Owner:SARTORIUS BIOANALYTICAL INSTR INC
Indirect ELISA kit for detecting aviadenovirus group I antibody, and detection method and application thereof
InactiveCN107102138ALow production costLower control costsMaterial analysis by observing effect on chemical indicatorBiological material analysisSerum igeI antibody
The invention discloses an indirect ELISA kit for detecting an aviadenovirus group I antibody, and a detection method and an application thereof and relates to the technical field of biological detection. The ELISA kit disclosed by the invention comprises a coated elisa plate, a washing liquid, a diluting liquid, a primer developing liquid, a rabbit-anti-chicken elisa second antibody, a terminating liquid, FAVI standard positive serum and FAVI standard negative serum, wherein the coated elisa plate takes a recombinant hexon protein of aviadenovirus group I FAVI as a coating antigen. The ELISA kit disclosed by the invention is used for detecting the aviadenovirus group I antibody, has the advantages of high efficiency, sensitive specificity and repeatability, and is simple and fast to operate, low in cost and suitable for clinical application and popularization.
Owner:YANGLING VOCATIONAL & TECHN COLLEGE +1
Recombinant adenovirus and tetravalent adenovirus vaccine and preparation method thereof
ActiveCN106318916ANo recombinationHigh neutralization potencyViral antigen ingredientsVirus peptidesHuman typeSerotype
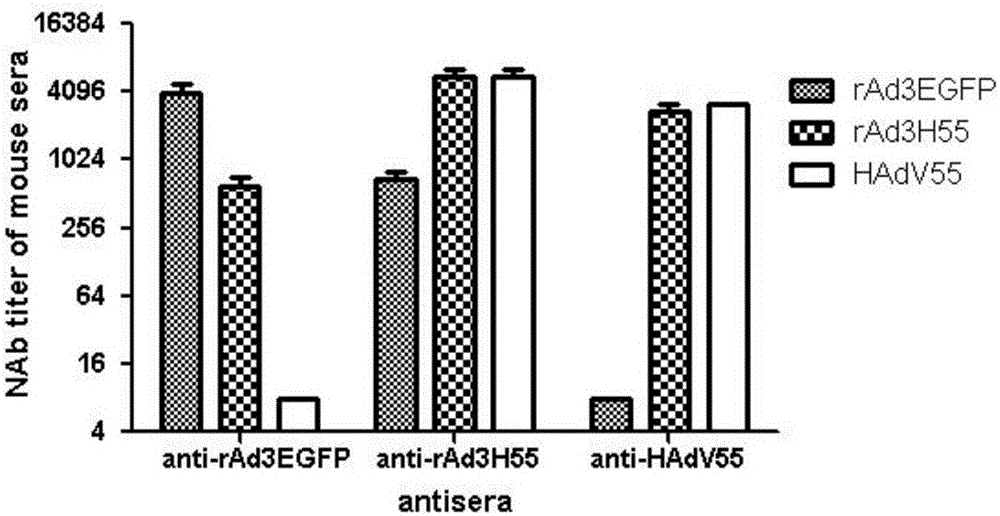
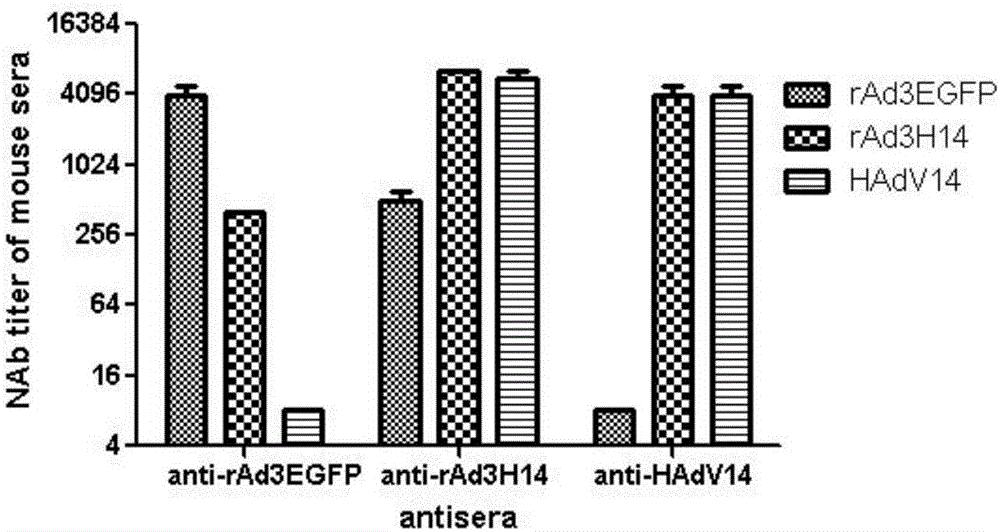
The invention discloses a recombinant adenovirus and tetravalent adenovirus vaccine and a preparation method thereof. The tetravalent recombinant adenovirus vaccine contains a recombinant type 3 adenovirus strain, a recombinant type 7 adenovirus strain, a recombinant type 14 adenovirus strain and a recombinant type 55 adenovirus strain. The preparation method disclosed by the invention comprises the following steps: preparing recombinant shuttle plasmids containing hexon gene segments, and performing in-bacteria homologous recombination with a recombinant human type 3 adenovirus strain, thereby obtaining a recombinant adenoviral genome in which the hexon gene segments are replaced by type 7, type 14 and type 55; transfecting cells, rescuing to obtain recombinant human type 3, 7, 14 and 55 recombinant adenoviruses with different main capsid protein-hexon proteins; purifying, mixing according to the same protein content, and inactivating by using beta-propiolactone, thereby obtaining the tetravalent adenovirus vaccine. The tetravalent adenovirus vaccine is capable of inducing neutralizing antibody responses to four types of serotype adenoviruses, and the neutralizing titer is 500-1000.
Owner:GUANGZHOU GIR MEDICINE CO LTD +1
Recombinant aviadenovirus type 4 fiber2 protein as well as preparation method and application thereof
ActiveCN112142830AImprove solubilityImproving immunogenicityBacteriaViral antigen ingredientsAntigen epitopeHighly pathogenic
The invention discloses a recombinant fowl adenovirus type 4 (FAdV-4) fiber2 protein as well as a preparation method and application thereof, and belongs to the technical field of gene engineering. The recombinant protein is obtained by removing 274 amino acid residues at the N end of the fowl adenovirus type 4 fiber2 protein and then recombining with the 21-55th amino acid sequence of the fowl adenovirus type 4 hexon protein; and can be used for preparing the avian adenovirus 4-type genetic engineering subunit vaccine. Aiming at the defects of poor solubility and immunogenicity of prokaryoticexpression natural fiber2 protein, the fiber2 protein coding gene is subjected to series modification, and compared with the unmodified fiber2 protein, the recombinant protein has the advantages thatthe gene coding sequence of 274 amino acid residues at the N end of the fiber2 protein is removed, and the gene coding sequences of two important antigen epitopes of the hexon protein are fused; so that the solubility and the immunogenicity are obviously improved, so that the subunit vaccine which is controllable in quality, safe and effective and can prevent highly pathogenic FAdV-4 virus infection can be prepared, and complete protection can be obtained by once immunization.
Owner:YANGTZE UNIVERSITY +1
Adenoviral vector-based dengue fever vaccine
The invention relates to a replication-deficient adenoviral vector comprising two or more nucleic acid sequences encoding Dengue virus antigens and a chimeric hexon protein. The chimeric hexon protein comprises a first portion and a second portion. The first portion comprises at least 10 contiguous amino acid residues from a first adenovirus serotype (e.g., serotype 5 adenovirus hexon protein), optionally with one amino acid substitution. The second portion comprises (a) at least one hypervariable region (HVR) of a hexon protein of an adenovirus of a second adenovirus serotype, or (b) at least one synthetic hypervariable region (HVR) that is not present in the hexon protein of the wild-type adenovirus of the first adenovirus serotype.
Owner:GEN VEC INC
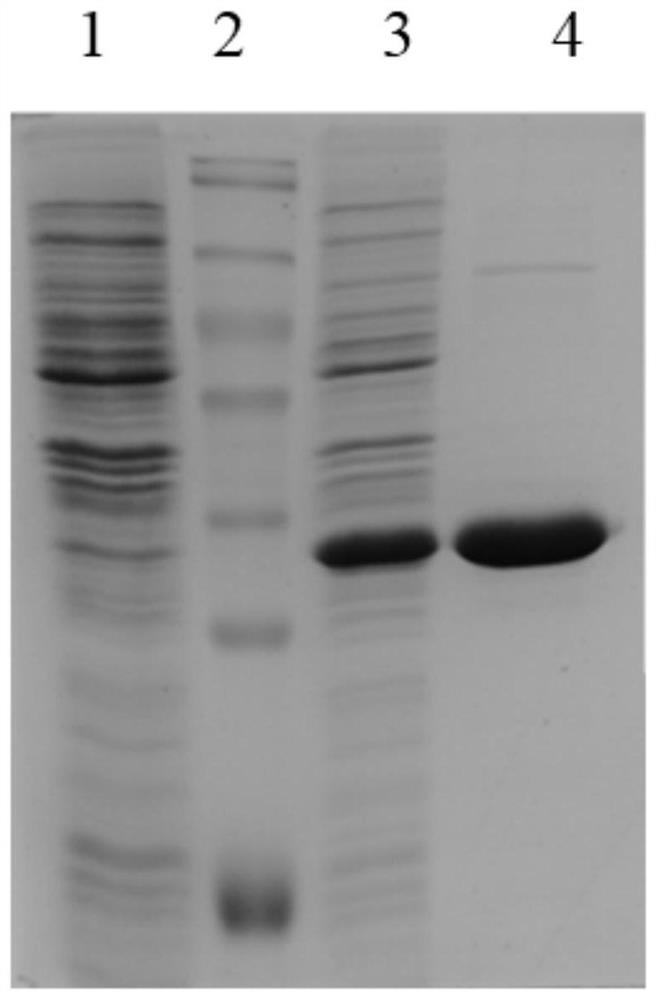
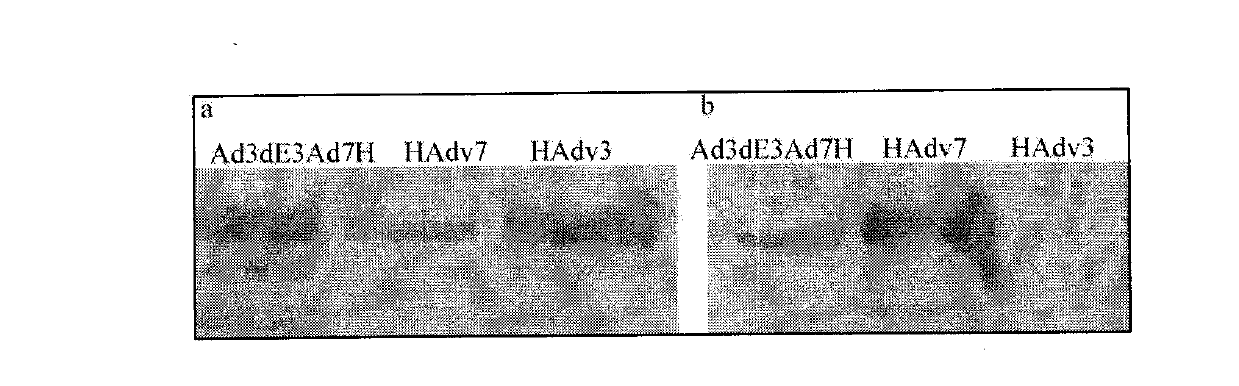
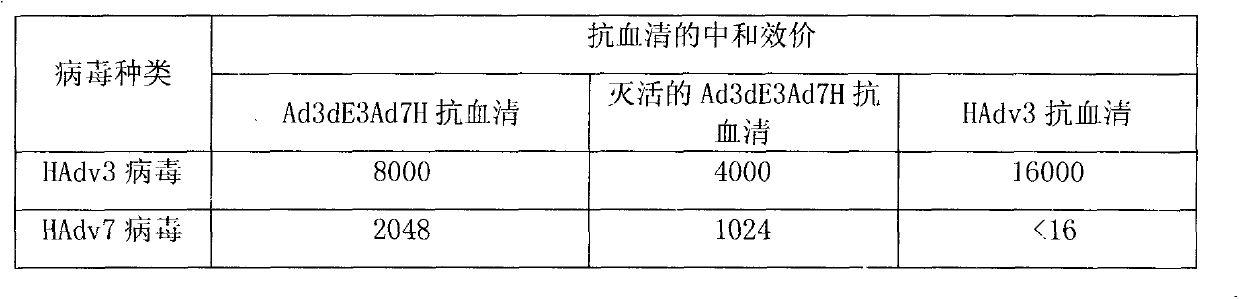
Recombinant human adenovirus 3, and preparation method and application thereof
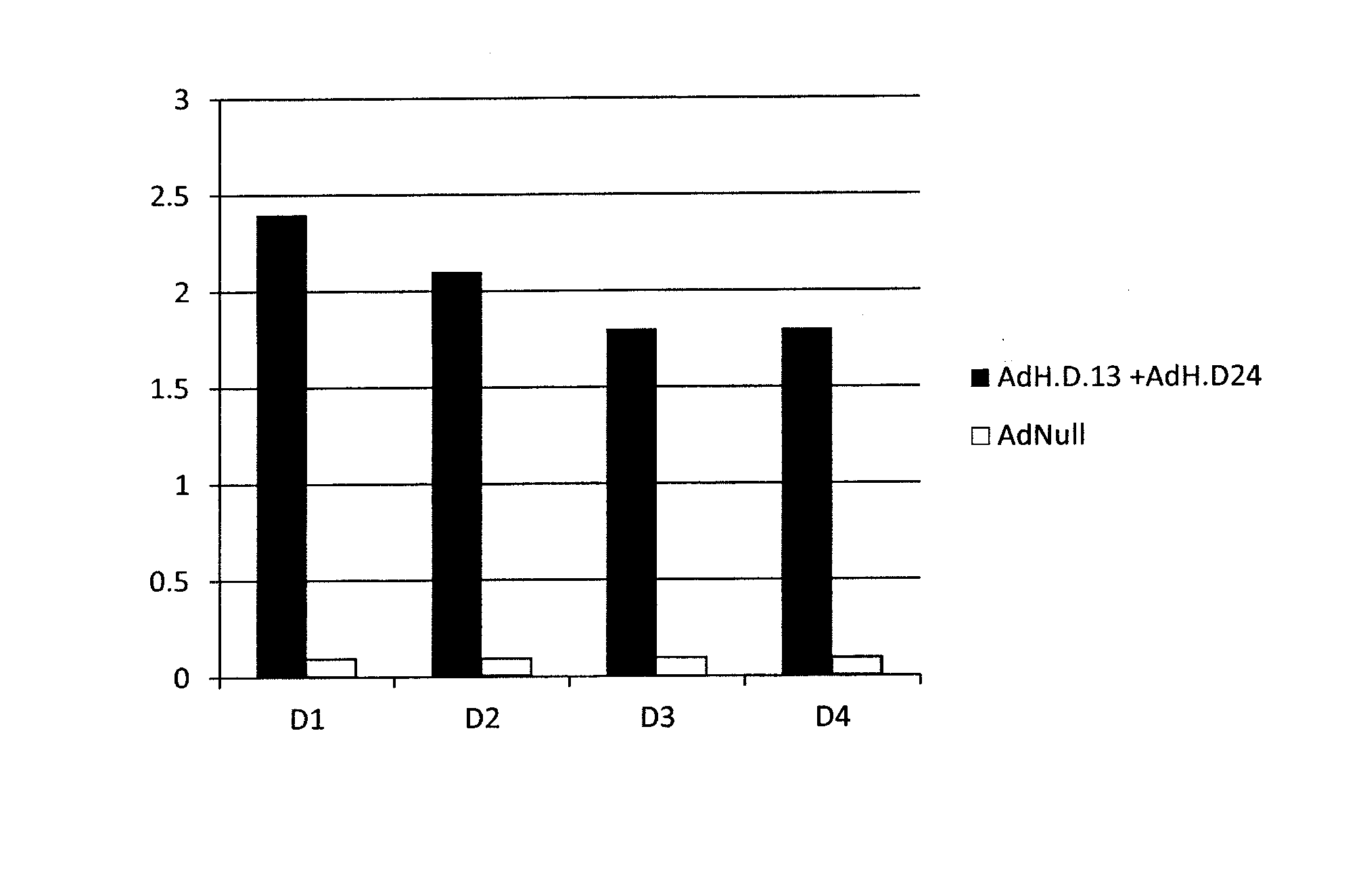
The invention discloses a novel human adenovirus 3 and 7 bivalent vaccine candidate strain with human adenovirus 3 (HAdv3) as a carrier, and a preparation method thereof. The capsid of the HAdv3 includes an HAdv3 hexon and an HAdv7 hexon. An HAdv7 hexon protein expression cassette is inserted to human adenovirus 3 genome E3 region, rescued packaging is carried out in in-vitro cells to obtain a hexon chimeric recombinant adenovirus, and the capsid of the hexon chimeric recombinant adenovirus includes an HAdv3 hexon and an HAdv7 hexon. The inactivated or non-inactivated vaccine candidate strain can induce an anti-HAdv3 and anti-HAdv 7 neutralization effect antibody, and can be used for making bivalent vaccines for preventing the EV71 infection and the HAdv3 infection.
Owner:GUANGZHOU GIR MEDICINE CO LTD +1
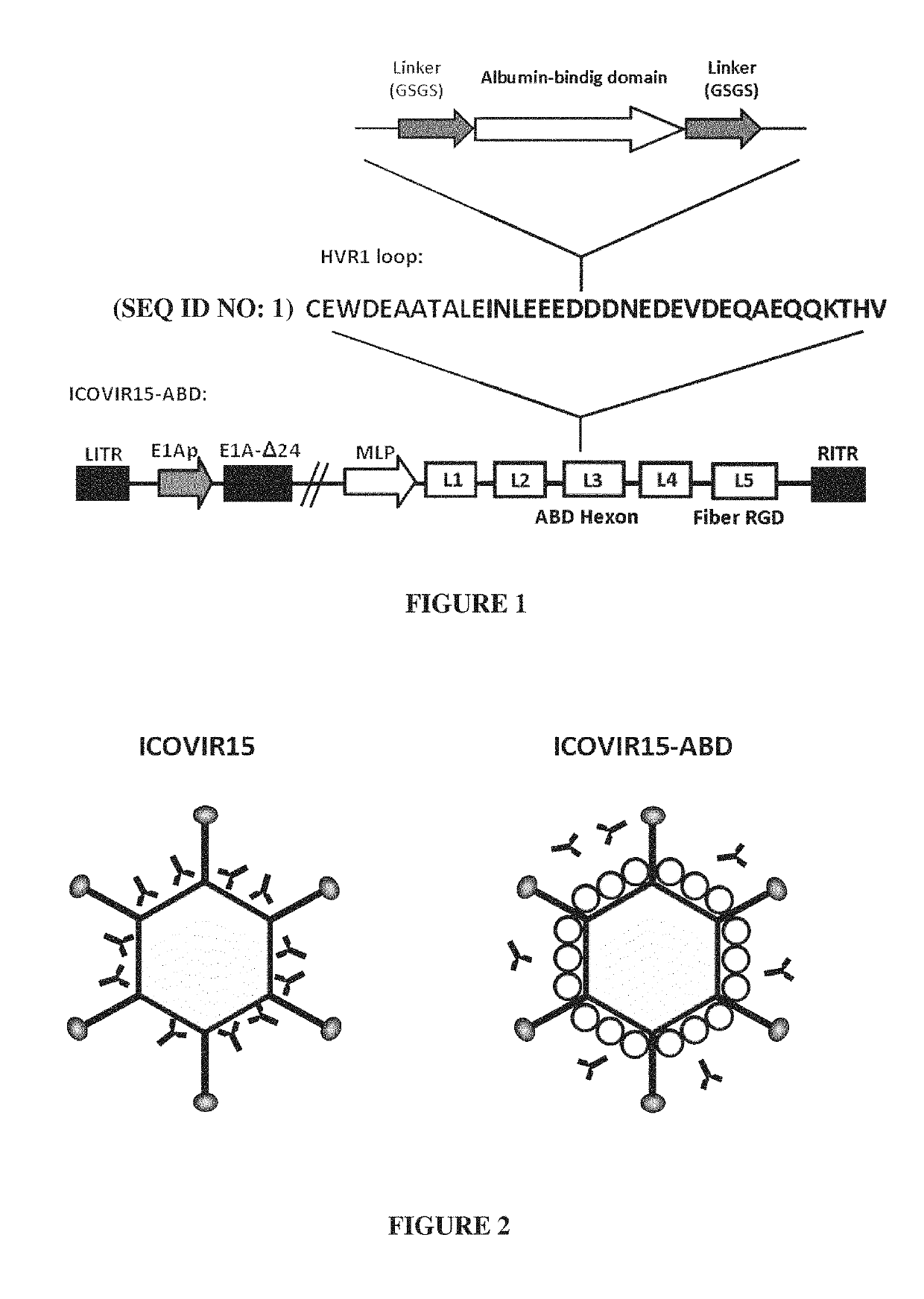
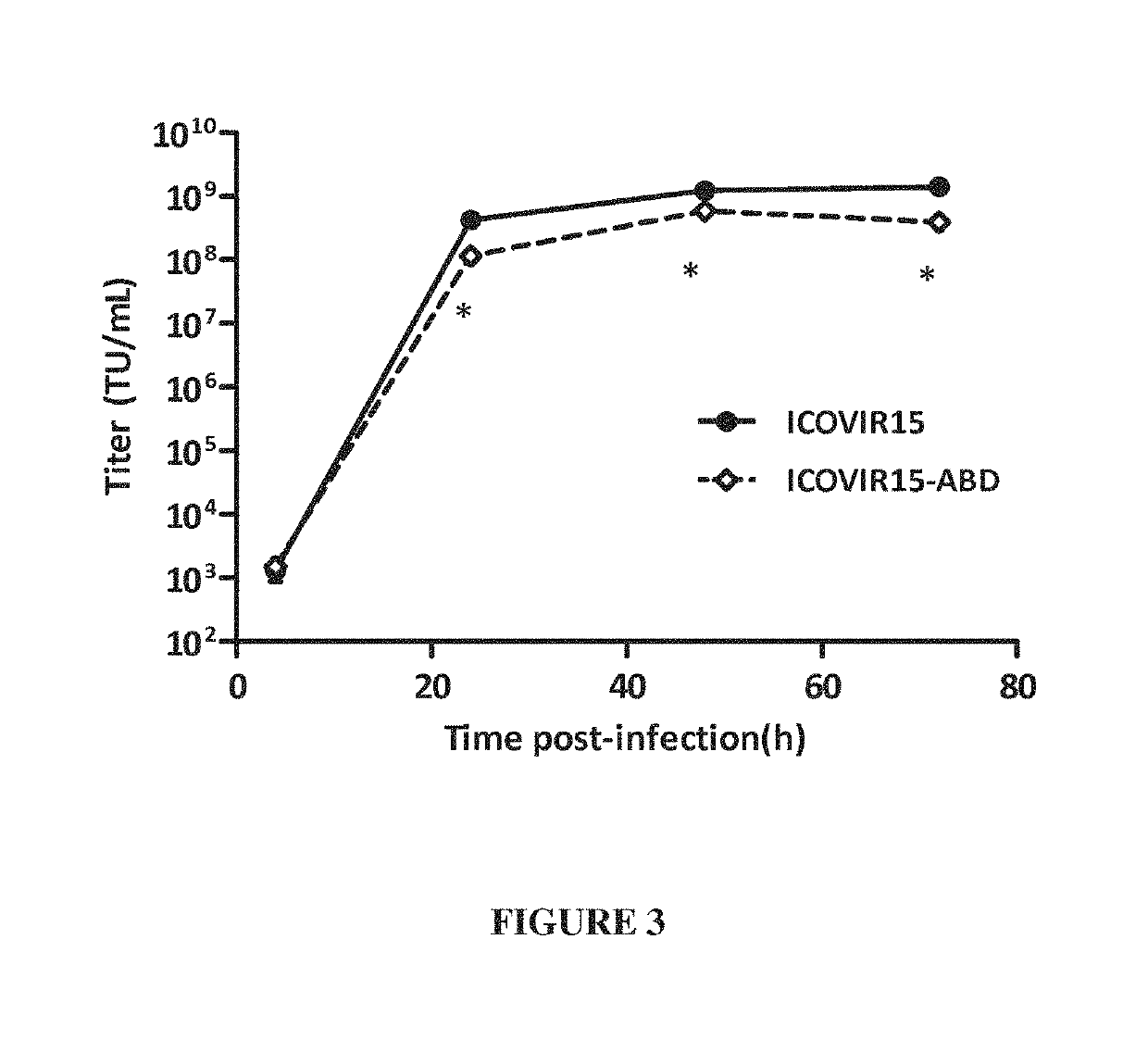
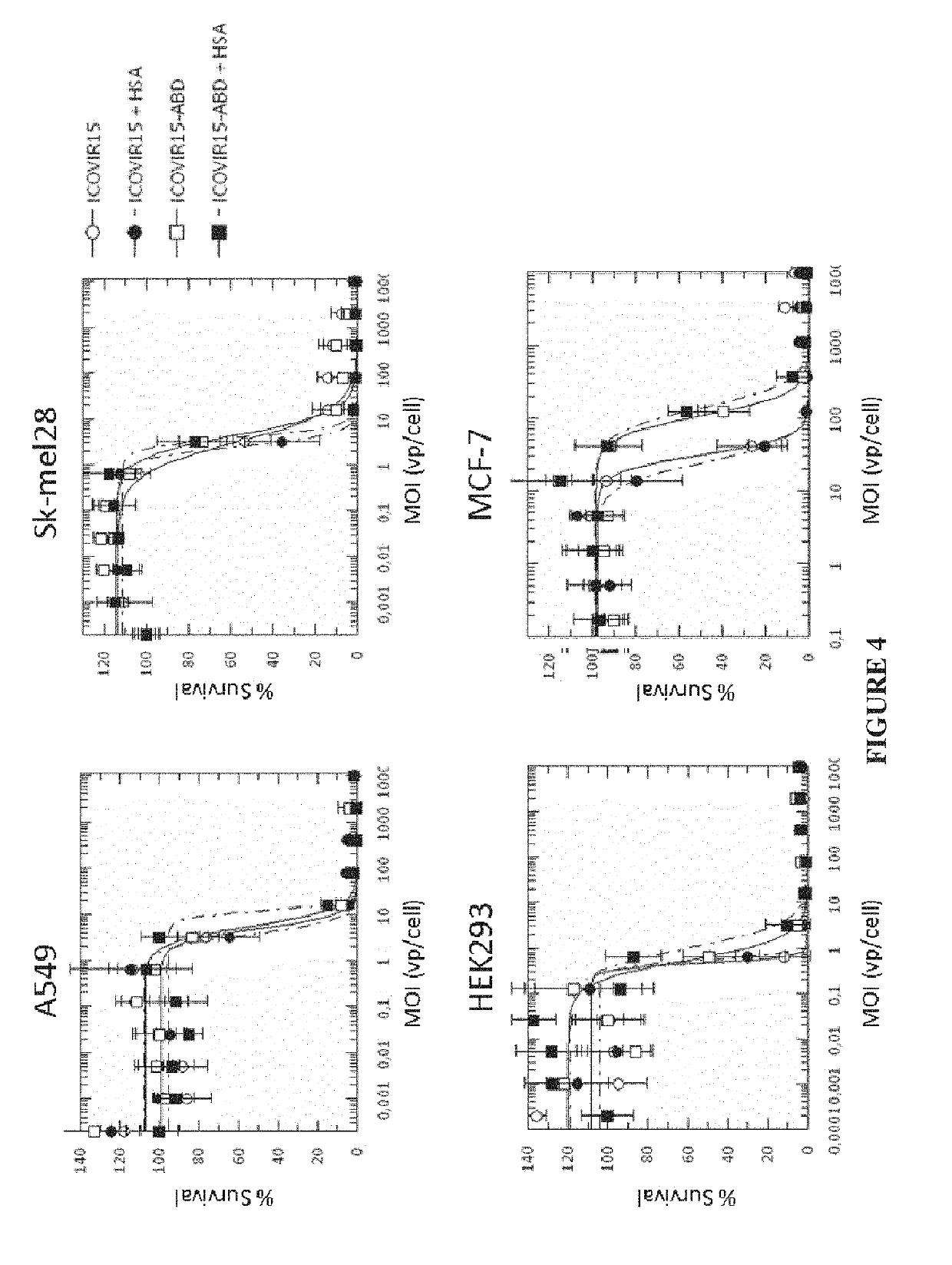
Adenovirus comprising an albumin-binding moiety
ActiveUS10604549B2Peptide/protein ingredientsAntibody mimetics/scaffoldsOncolytic adenovirusPharmaceutical drug
Owner:FUNDACIO INST DINVESTIGACIO BIOMEDICA DE BELLVITGE IDIBELL +1
ELISA (Enzyme Linked Immunosorbent Assay) kit for detecting fowl adenovirus antibody based on hexon protein N-terminal conservative area
InactiveCN106443015AStrong specificityBiological material analysisBiological testingHorseradish peroxidaseAdenovirus Antibody
The invention belongs to the field of biotechnical detection, and particularly relates to an indirect ELISA (Enzyme Linked Immunosorbent Assay) kit for detecting a fowl adenovirus antibody based on a hexon protein N-terminal conservative area. The kit comprises an ELISA enzyme standard plate coated with the expression product of the hexon protein N-terminal conservative area, an HRP (Horseradish Peroxidase) marked rabbit anti-chicken antibody, a sample diluting solution and a washing solution; the sequence of the hexon protein N-terminal conservative area is as shown by SEQ ID NO. 5. By using the indirect ELISA kit for the fowl adenovirus antibody, which is established by the invention, an antibody resisting a fowl adenovirus can be detected specifically, but an antibody resisting other pathogens cannot be detected. Therefore, the kit has favorable specificity on the fowl adenovirus, and can be used for the epidemiological investigation of the infection status of the fowl adenovirus.
Owner:YANGZHOU UNIV
Genetically-engineered vaccine against fowl adenovirus type 4, and preparation method and application thereof
PendingCN111040024AStrong antigen immunityHigh antigen immunityViral antigen ingredientsVirus peptidesProtective antigenAntigen
The invention provides a genetically-engineered vaccine against fowl adenovirus type 4, and a preparation method and application thereof. According to the invention, protective antigens, namely pentonand hexon proteins of fowl adenovirus serotype 4 are connected by a linker, and are expressed by an insect cell-baculovirus expression system to form virus-like particles of fowl adenovirus type 4 onspatial conformation; an adjuvant is added; and emulsification is carried out so as to prepare the genetically-engineered vaccine against fowl adenovirus type 4. The preparation method for the vaccine provided by the invention is simple in process, capable of preparing a large amount of fowl adenovirus type 4 antigen protein, short in time consumption, high in expression quantity and beneficial to large-scale production; and the obtained genetically-engineered vaccine is good in immune effect and capable of effectively preventing infection of the fowl adenovirus type 4.
Owner:乾元浩生物股份有限公司
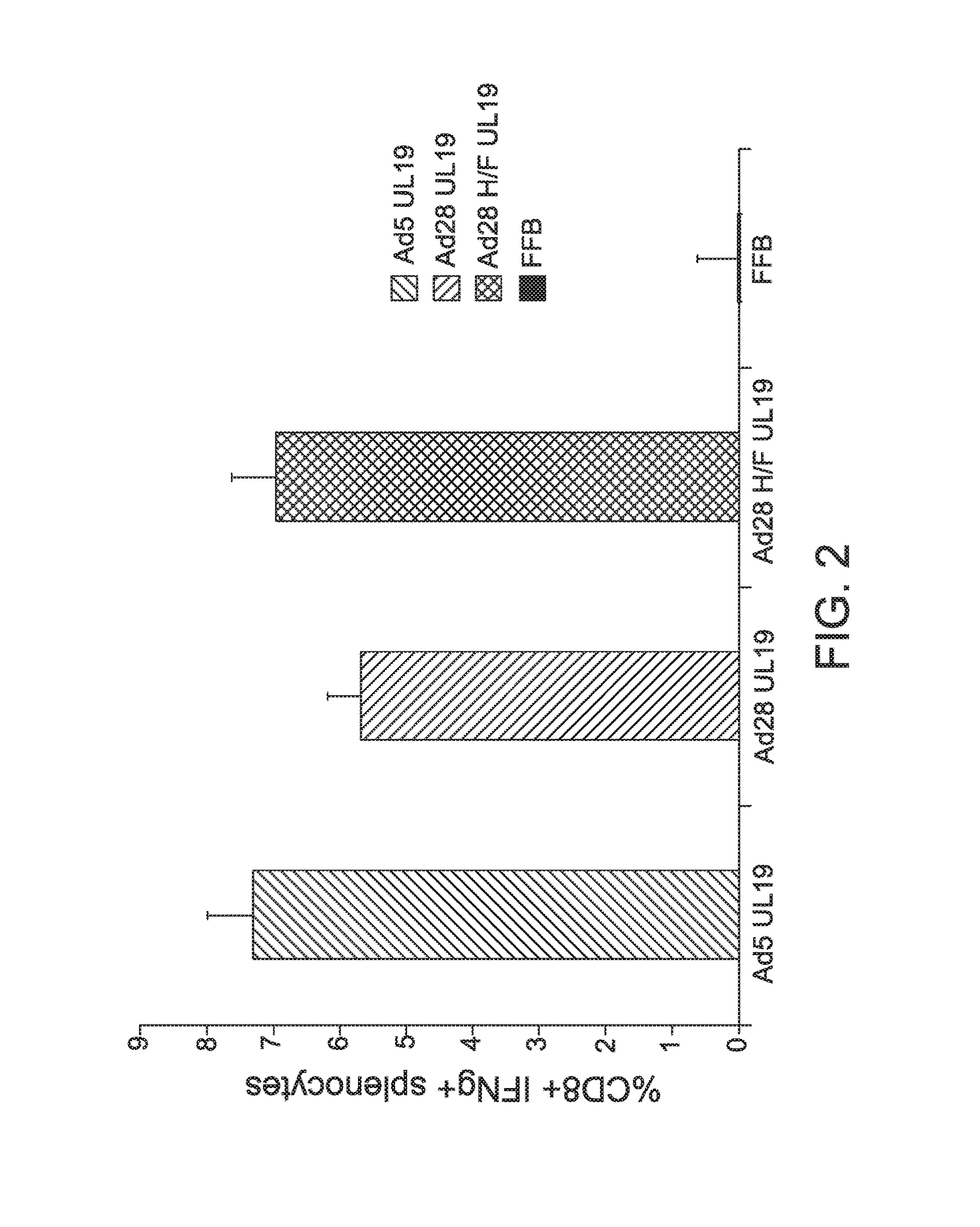
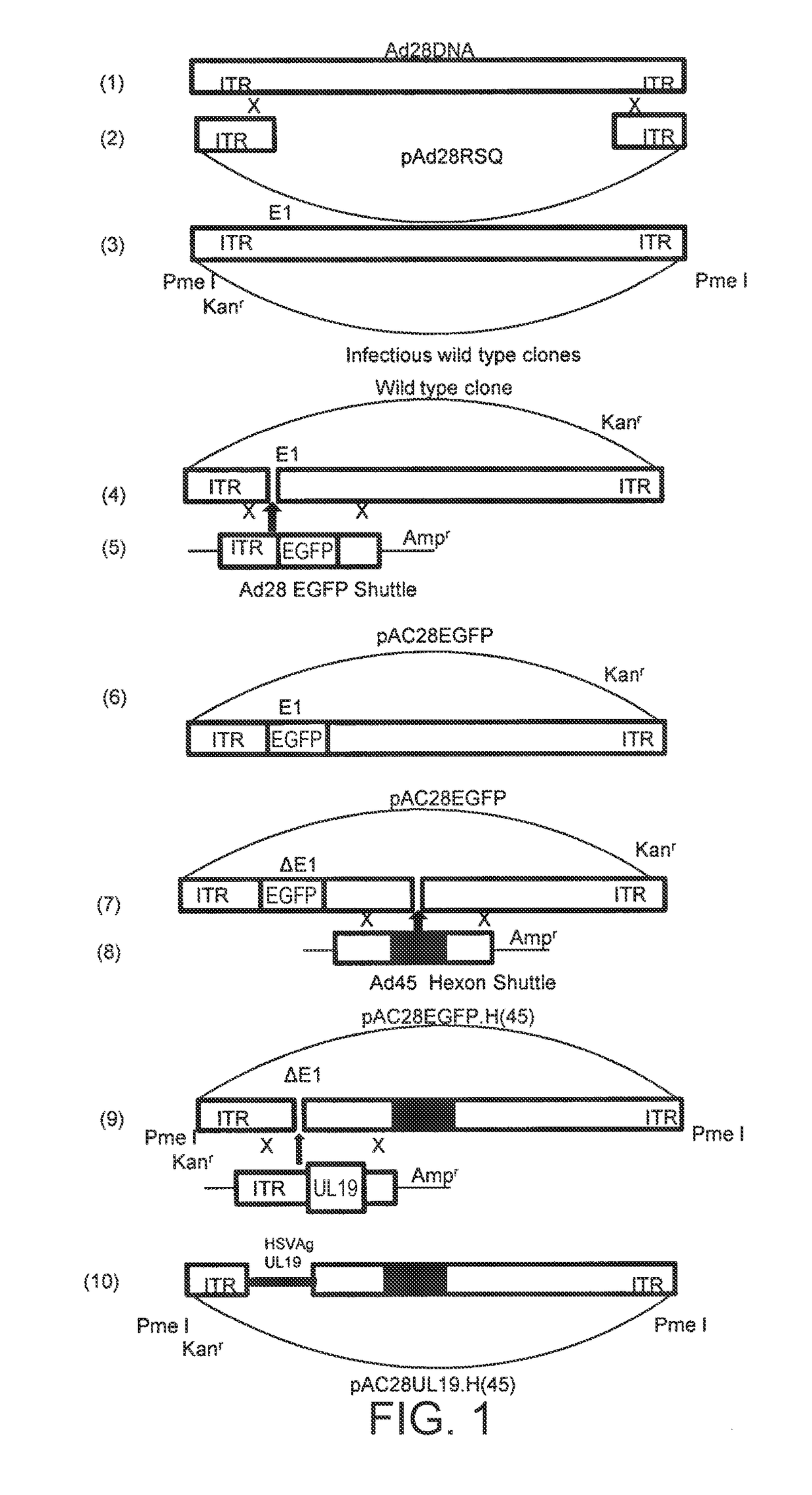
Modified serotype 28 adenoviral vectors
The invention provides a replication-deficient serotype 28 adenoviral vector characterized by comprising a portion of a serotype 45 adenoviral hexon protein and / or a portion of a serotype 45 fiber protein in place of the endogenous serotype 28 hexon and / or fiber protein.
Owner:GENVEC INC
Fowl adenovirus group I immunity related fusion protein as well as encoding gene and application thereof
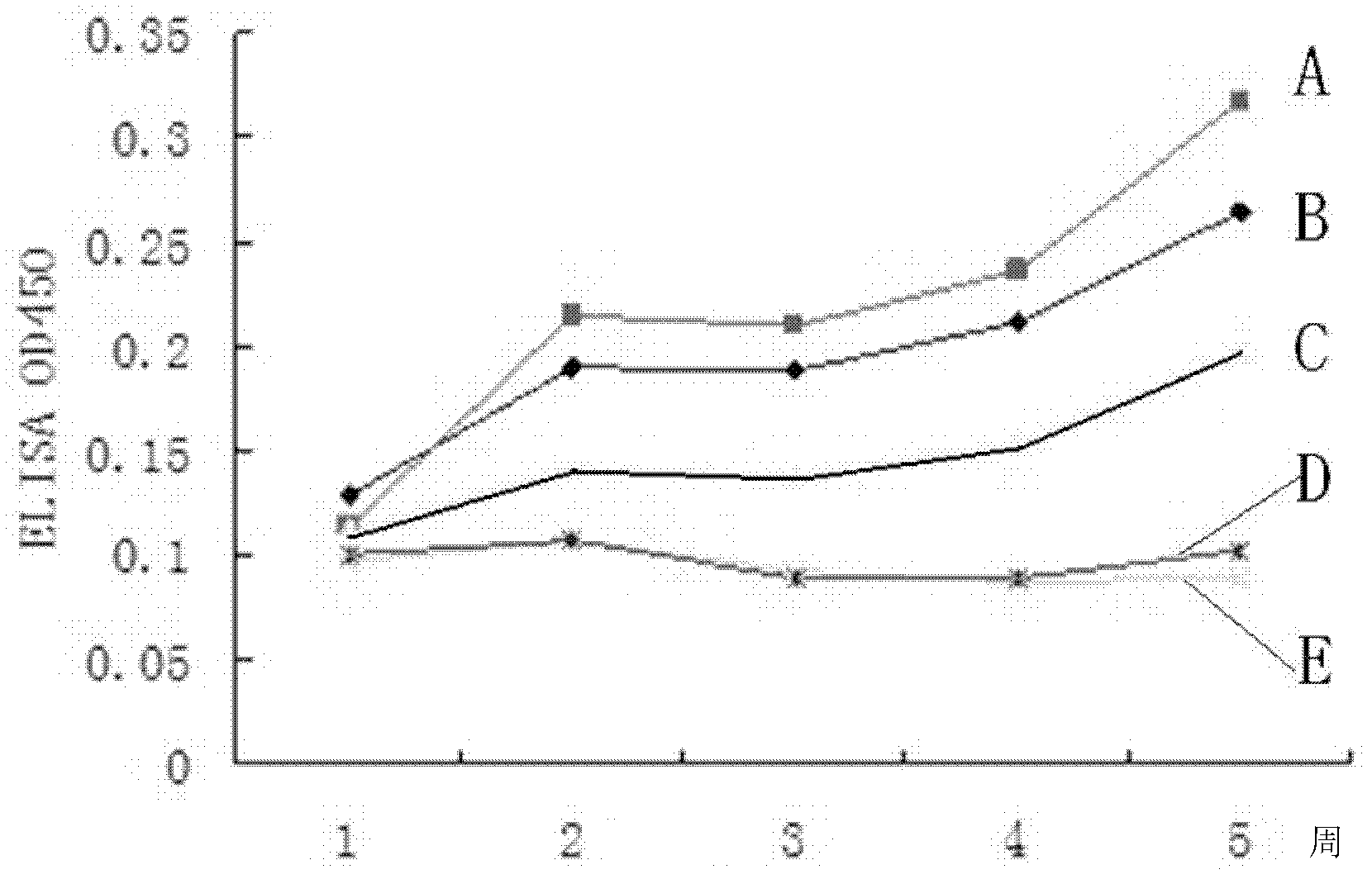
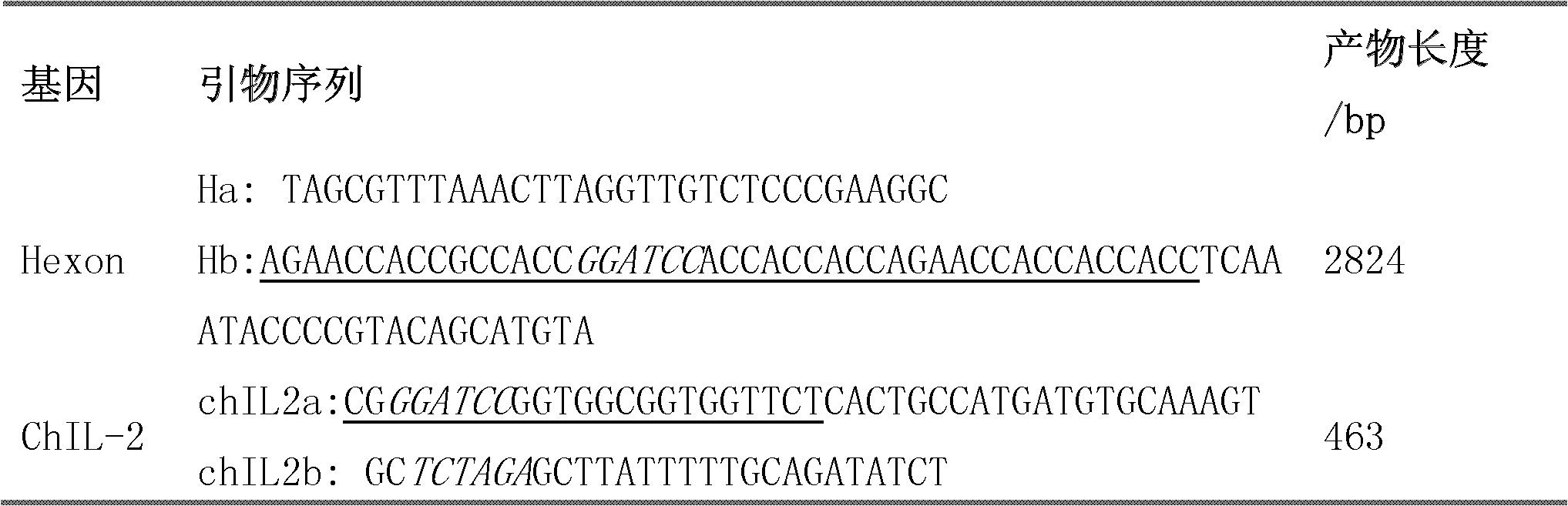
The invention discloses a fowl adenovirus group I immunity related fusion protein as well as an encoding gene and application of the fowl adenovirus group I immunity related fusion protein. The fusion protein provided by the invention comprises Hexon protein and chicken interleukin-2. The Hexon protein is the protein composed of an amino acid sequence from the 1st position to the 914th position of N' tail end of the sequence 6 in a sequence table, and the chicken interleukin-2 is the protein composed of the amino acid sequence from the 933rd position to the 1075th position of the N' tail end of the sequence 6 in the sequence table. Experiments show that a DNA (deoxyribonucleic acid) molecule composed of a Hexon gene and a chicken interleukin-2 (chIL-2) gene, which contain fowl adenovirus group I and are connected in series, is provided, a Hexon specific antibody can be generated when the DNA molecule is injected to chicken muscle by an expression vector, and the DNA molecule has good immunity protection effect and can be used for effectively preventing the fowl adenovirus group I after fowl adenovirus group I challenge experiments.
Owner:GUANGXI VETERINARY RES INST
Adenoviral vector-based dengue fever vaccine
The invention relates to a replication-deficient adenoviral vector comprising two or more nucleic acid sequences encoding Dengue virus antigens and a chimeric hexon protein. The chimeric hexon protein comprises a first portion and a second portion. The first portion comprises at least 10 contiguous amino acid residues from a first adenovirus serotype (e.g., serotype 5 adenovirus hexon protein), optionally with one amino acid substitution. The second portion comprises (a) at least one hypervariable region (HVR) of a hexon protein of an adenovirus of a second adenovirus serotype, or (b) at least one synthetic hypervariable region (HVR) that is not present in the hexon protein of the wild-type adenovirus of the first adenovirus serotype.
Owner:GENVEC INC
Modified serotype 28 adenoviral vectors
The invention provides a replication-deficient serotype 28 adenoviral vector characterized by comprising a portion of a serotype 45 adenoviral hexon protein and / or a portion of a serotype 45 fiber protein in place of the endogenous serotype 28 hexon and / or fiber protein.
Owner:GENVEC INC
Cytotoxic t-cell epitope peptide and use thereof
Owner:MEDICAL & BIOLOGICAL LAB CO LTD
Simian (gorilla) adenovirus or adenoviral vectors and methods of use
The invention provides an adenovirus or adenoviral vector characterized by comprising one or more particular nucleic acid sequences or one or more particular amino acid sequences, or portions thereof, pertaining to, for example, an adenoviral pIX protein, DNA polymerase protein, penton protein, hexon protein, and / or fiber protein.
Owner:GEN VEC INC
Preparation method for immunoglobulinlg of adenovirus anti-Fi,anti-Pb and anti-Hx
The invention discloses a preparation method for the immunoglobulin of an adenoviruses anti-Fi, anti-Pb and anti-Hx, which firstly extracts a fibrin, a pentamer protein and a hexon from the adenoviruses as antigens, then purifies the antigens with discontinuous density centrifugation with the virus antigens prepared through the tissue culture, and then uses the prepared purified antigens for establishing and screening the method for the immunoglobulin of high titer adenoviruses anti-Fi, anti-Pb and anti-Hx and the virus neutralization experiment in vitro, etc. while at last the immunoglobulin from the immunoglobulin blood source of high titer adenoviruses anti-Fi, anti-Pb and anti-Hx by means of low temperature ethanol technique, ion exchange chromatography or affinity chromatography is extracted.
Owner:TONROL BIOLOGICAL PHARM CO LTD
Blocking ELISA (enzyme-linked immunosorbent assay) kit for detection of fowl adenovirus I type-4 antibody and application thereof
The invention discloses a hybridoma strain prepared with fowl adenovirus I type-4 Hexon protein, a monoclonal antibody secreted by the hybridoma strain, and a blocking ELISA (enzyme-linked immunosorbent assay) kit for detection of fowl adenovirus I type-4 antibody. The kit herein includes a coated plate, a horse radish peroxidase-marked fowl adenovirus type-4 monoclonal antibody, a cleaning solution, serum diluent, FAdV-4 standard positive serum, FAdV-4 standard negative serum, a substrate rendering liquid, and a stop solution, wherein the hybridoma strain is collected under CGMCC No. 16203; the fowl adenovirus type-4 monoclonal antibody is secreted by the hybridoma strain collected under CGMCC No. 16203; the coated plate employs Escherichia coli system expressed recombinant fowl adenovirus type-4 Hexon protein as a coating antigen. The kit herein employs the blocking ELISA as a principle to detect fowl adenovirus I type-4 antibody in a fowl serum sample, has the advantages of high sensitivity and specificity, good repeatability, high detection speed, good detection convenience, good convenience of standardization and the like, and is very convenient to popularize and apply in basic-level farming units.
Owner:北京市动物疫病预防控制中心
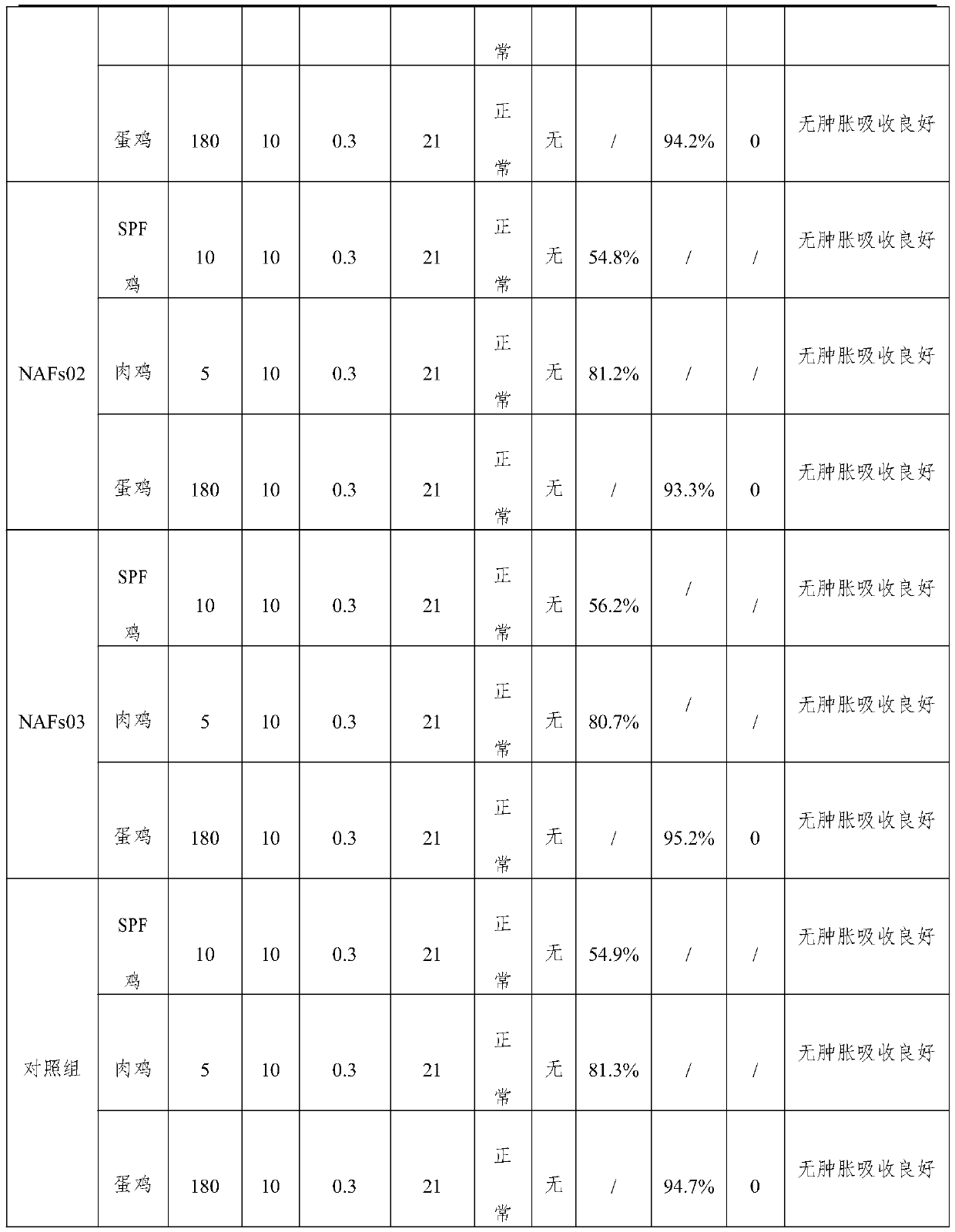
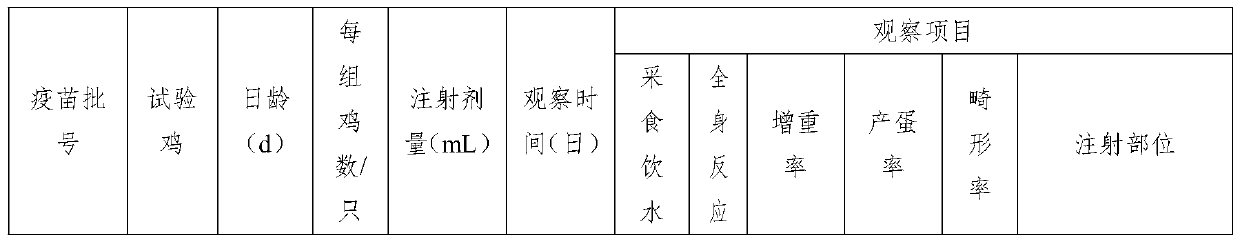
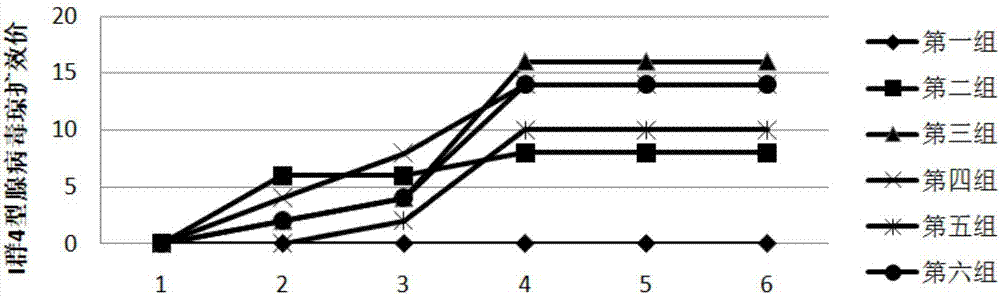
Triple inactivated vaccine and preparation method thereof
InactiveCN110680914AHexon is high in proteinImproving immunogenicitySsRNA viruses negative-senseViral antigen ingredientsImmunogenicityTGE VACCINE
The invention provides a triple vaccine. Antigens of the triple inactivated vaccine include an inactivated chicken newcastle disease La Sota virus strain, an inactivated H9 subtype avian influenza QF01 strain and an inactivated I-group 8b type fowl adenovirus Hexon protein. The invention further provides a preparation method of the triple inactivated vaccine. The preparation method comprises the following steps of enabling the I-group 8b type fowl adenovirus Hexon protein to be inactivated through pyrrole, and performing mixing and emulsifying on the inactivated I-group 8b type fowl adenovirusHexon protein, an inactivated newcastle disease virus concentrated solution and an inactivated avian influenza virus concentrated solution to obtain the triple inactivated vaccine. The I-group 8b type fowl adenovirus Hexon protein inactivated by the triple inactivated vaccine is high in content, high in immunogenicity and low in formaldehyde content and endotoxin content, has better protection effects on current epidemic I-group 8b type fowl adenovirus, is good in safety properties and true in immune effects, can resist infection of clinical chicken groups on newcastle disease virusese, avianinfluenza and I-group 8b type fowl adenovirus, and is long in persistent period, the immunity persistent period can reach 5 months under the condition that 0.3mL of the vaccine is only used once, andat least 70% of the protection ratio can be provided for the chicken groups.
Owner:洛阳职业技术学院 +2
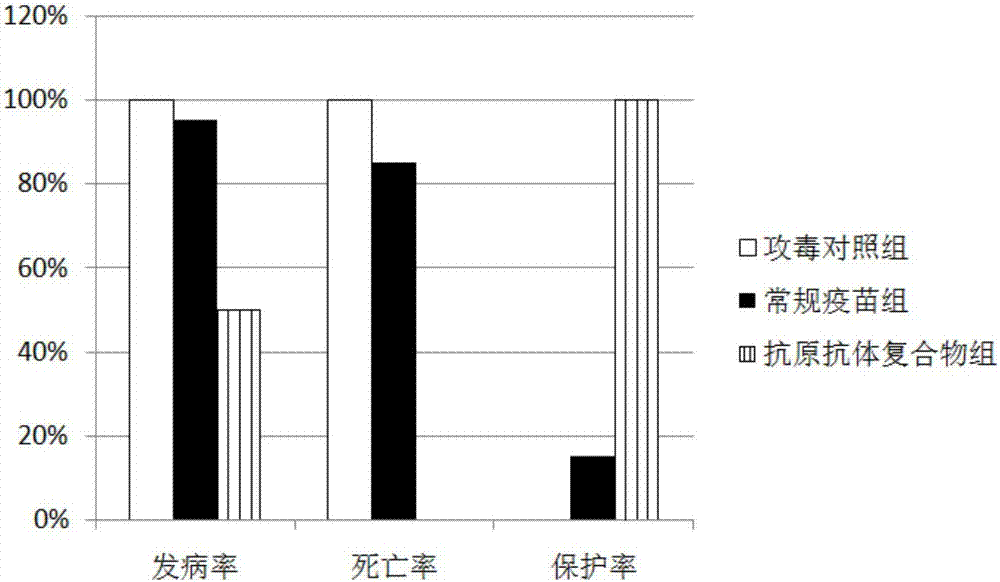
Group-I type-4 adenovirus long-acting egg yolk antibody preparation method
InactiveCN107880119ASolve the technical problem of poor effect of artificial immunization methodAvoid overdoseEgg immunoglobulinsVirus peptidesAntibiotic YInactivated vaccine
The invention provides a group-I type-4 adenovirus long-acting egg yolk antibody preparation method. According to the technical scheme, on one hand, a group-I type-4 adenovirus inactivated vaccine isutilized for preparing an egg yolk antibody, on the other hand, a group-I type-4 adenovirus hexon protein antigen expression vector is built, and a prokaryotic expression system is utilized for preparing an antigen protein; on the basis, the influence of antigen-antibody valence matching on an antigen-antibody mixed liquor immunoprotection effect is investigated, an optimal compound method is determined on the basis, and a freeze-drying dosage form is designed. According to the method, on the basis of an original adenovirus egg yolk antibody, an adenovirus hexon protein antigen is added, and acompound is prepared. The adenovirus antigen-antibody compound can resist persistent infection caused by a pathogenic microorganism, the overdose of antibiotics is avoided, and the used antigen is not a complete viral antigen but an antigen prepared from a hexon protein of an adenovirus, so that the danger of outward dispersing toxicity can be reduced, and more safety and effectiveness are realized.
Owner:TIANJIN RINGPU BIO TECH
Modulation of adenoviral tropism
The invention provides materials and methods for modulating adenoviral tropism for hepatocytes and other cell types such as splenocytes. It relates to the findings that hypervariable regions (HVRs) of the viral hexon protein interact with the Gla domain of the blood clotting factor FX as part of the infective process in vivo. The invention provides means to disrupt the interaction between hexon and FX, thus reducing infection of hepatocytes and splenocytes, as well as use of targeting agents comprising the Gla domain or a fragment thereof to direct adenoviral vectors to desired target cell or tissue types.
Owner:安德鲁·贝克 +2
Affenadenovirus (gorilla) or adenoviral vectors and methods of use
The invention provides an adenovirus or adenoviral vector characterized by comprising one or more particular nucleic acid sequences or one or more particular amino acid sequences, or portions thereof, pertaining to, for example, an adenoviral pIX protein, DNA polymerase protein, penton protein, hexon protein, and / or fiber protein.
Owner:GENVEC INC
Affenadenovirus (gorilla) or adenoviral vectors and methods of use
The invention provides an adenovirus or adenoviral vector characterized by comprising one or more particular nucleic acid sequences or one or more particular amino acid sequences, or portions thereof, pertaining to, for example, an adenoviral pIX protein, DNA polymerase protein, penton protein, hexon protein, and / or fiber protein.
Owner:GEN VEC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com