Improved adenoviral vectors and uses thereof
An adenovirus, recombinant adenovirus technology, applied in the direction of virus/phage, application, vector, etc., can solve the problem of low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
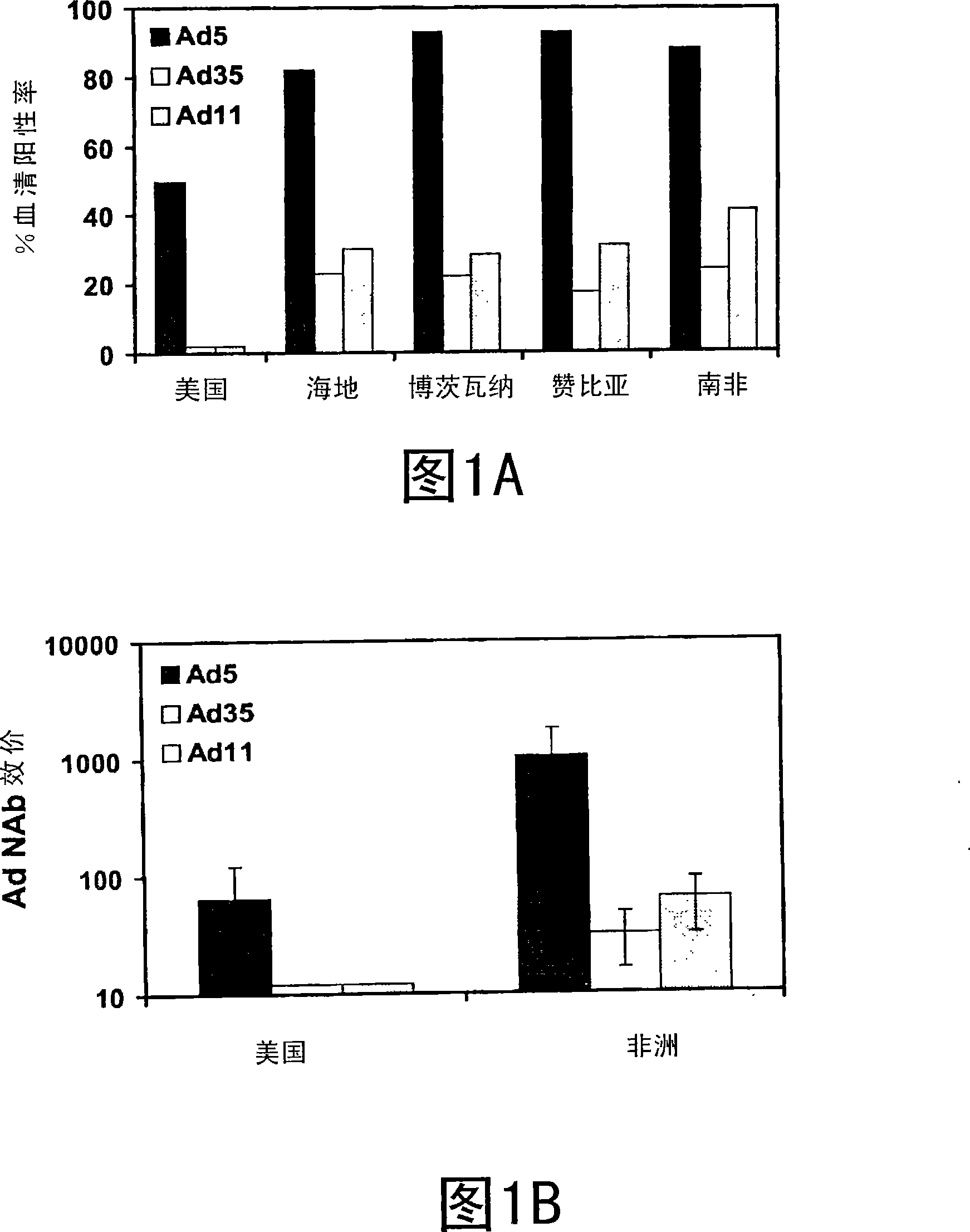
[0087] Example 1: International seropositivity and NAb titers for Ad5, Ad35 and AdI1
[0088] Ad-specific neutralizing antibody (NAb) responses were assessed by a luciferase-based virus neutralization assay as described by Sprangers et al. (2003). Briefly, A549 human lung cancer cells were treated with 1×10 4 Cells / well density were plated in 96-well plates and infected at a multiplicity of infection (moi) of 500 with El-deleted non-replicative Ad-luciferase reporter constructs in 200 μl reaction volumes of 2-fold serially diluted serum. After 24 hours of incubation, luciferase activity in cells was determined using the Steady-Glo Luciferase Reagent system (Promega). The 90% neutralization titer refers to the maximum serum dilution that neutralizes 90% of the luciferase activity.
[0089] In addition to the studies disclosed in WO 00 / 70071, experiments were performed to assess seropositivity and NAb titers for Ad5 and additional Ad serotypes in the developing world. Using t...
Embodiment 2
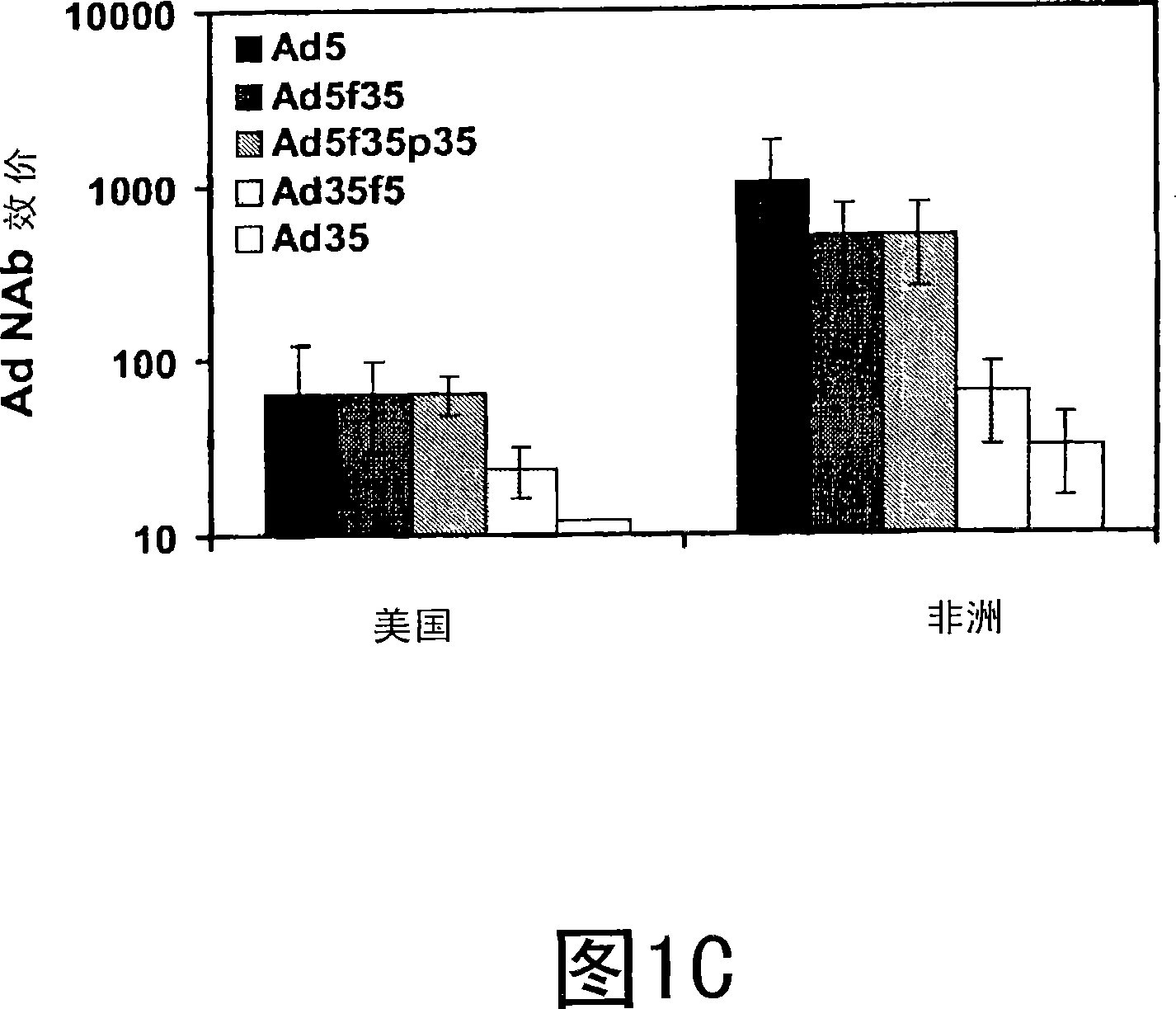
[0090] Example 2: Immunodominant Targets of Ad5-Specific Neutralizing Antibodies
[0091] The samples from the previous examples were used to further determine the predominant antigenic targets of Ad5-specific NAbs in the United States and sub-Saharan Africa. Assuming no detectable NAb cross-reactivity between Ad5 and Ad35, a capsid chimeric Ad5 / Ad35 virus expressing the luciferase targeted in the virus neutralization assay was used. In the case of intact virions with wild-type growth kinetics, these vectors consist of various combinations of Ad5 and Ad35 hexons, pentons, and fibrin (Havenga et al. 2002; Rea et al. 2001). Chimeric vectors used in this study included Ad5f35 (Ad5 with Ad35 fiber knob, shaft, and Ad35-Ad5 chimeric tail), Ad5f35p35 (Ad5 with Ad35 fiber and shaft, Ad35-Ad5 chimeric tail, and penton) and Ad35f5 (Ad35 containing the Ad5 fiber knob, shaft and Ad5-Ad35 chimeric tail).
[0092] As shown in Figure 1C, similar NAb titers were observed for Ad5, Ad5f35 an...
Embodiment 3
[0093] Example 3: Production of Ad35f5 and Ad35k5
[0094] Recombinant Ad35-based vaccines are immunogenic in the presence of anti-Ad5 immunity. However, recombinant Ad35 vaccines are inherently less immunogenic than recombinant Ad5 vaccines in the absence of anti-Ad5 immunity (Barouch et al. 2004). This problem is now overcome by constructing a capsid chimeric recombinant Ad35 vector in which at least the Ad35 fiber knob is replaced by an Ad5 fiber knob (Ad35k5 refers to knob replacement, Ad35f5 refers to most fiber replacement).
[0095] Recombinant Ad35k5 vectors were generated by replacing the Ad35 fiber protein-encoding gene with a chimeric fiber protein-encoding gene consisting of the Ad35 fiber tail and shaft linked to the Ad5 fiber knob (amino acids 133 to 314) (amino acids 1 to 132). Ad5 fiber-specific antibodies did not attenuate the immunogenicity of recombinant Ad5 vaccines. The Ad35 fiber knob does not interact with CAR because it is a subgroup B adenovirus (Ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com