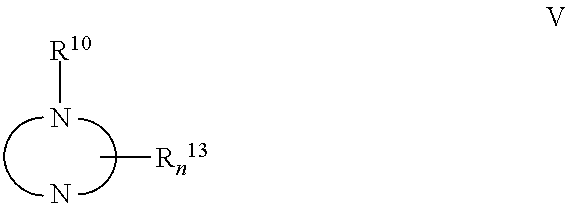Patents
Literature
69 results about "Coxsackie meningitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
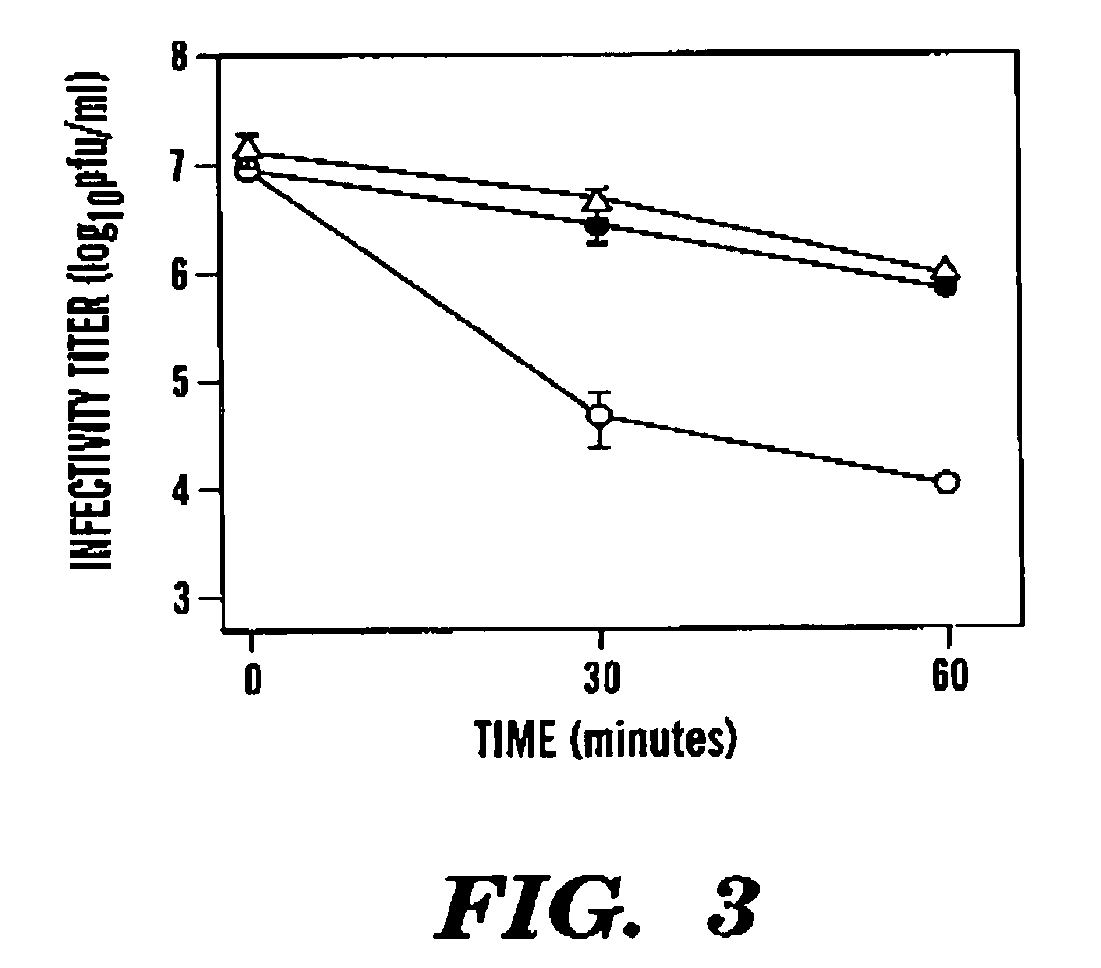
Coxsackieviruses are among the leading causes of aseptic meningitis (the other usual suspects being echovirus and mumps virus). The entry of coxsackievirus into cells, especially endothelial cells, is mediated by Coxsackie virus and adenovirus receptor.
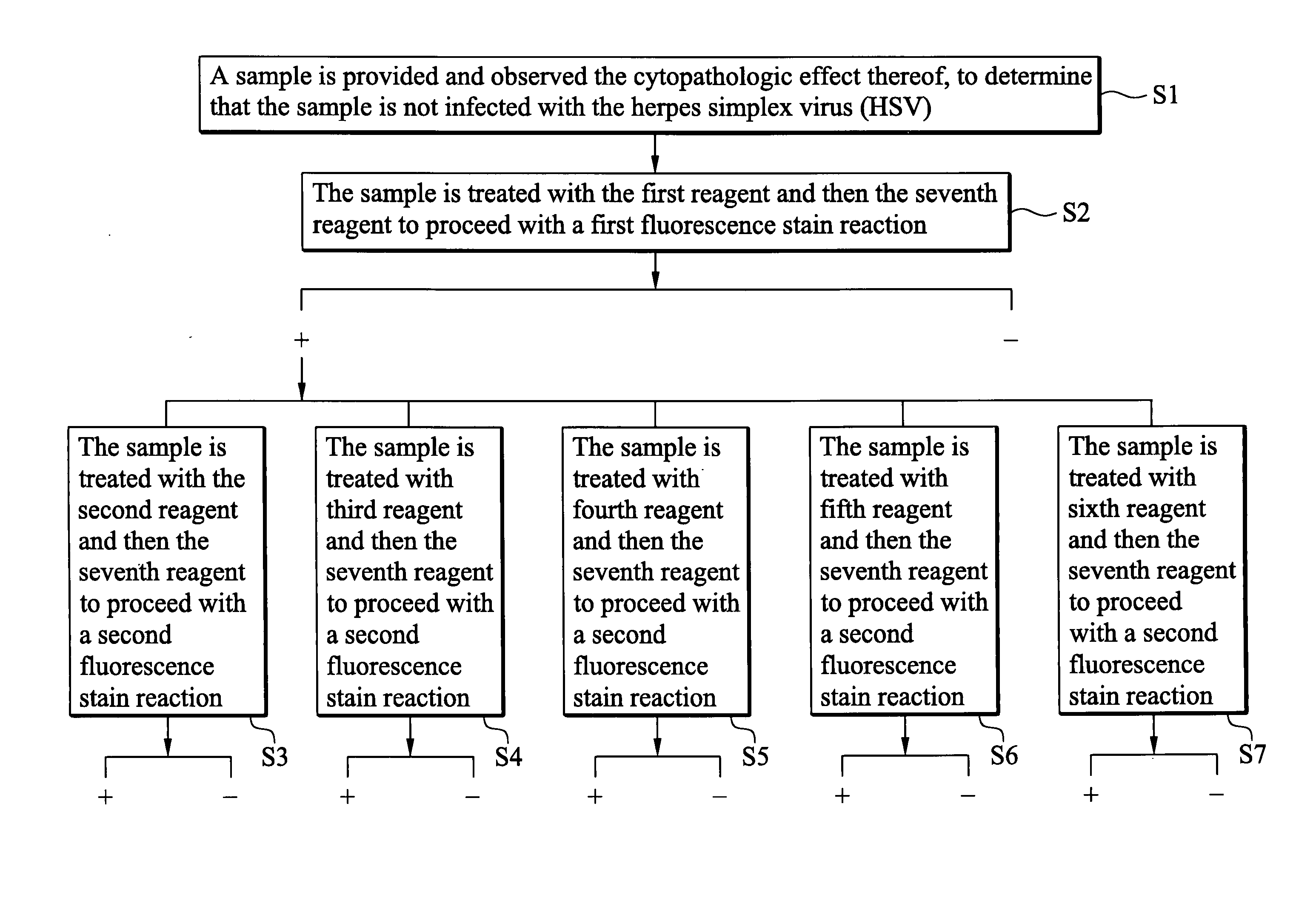
Indirect immunofluorescence assay typing kit for coxsackievirus A group and method for typing coxsackievirus A group
InactiveUS20090317796A1Microbiological testing/measurementBiological material analysisImmunofluorometric AssaysTyping
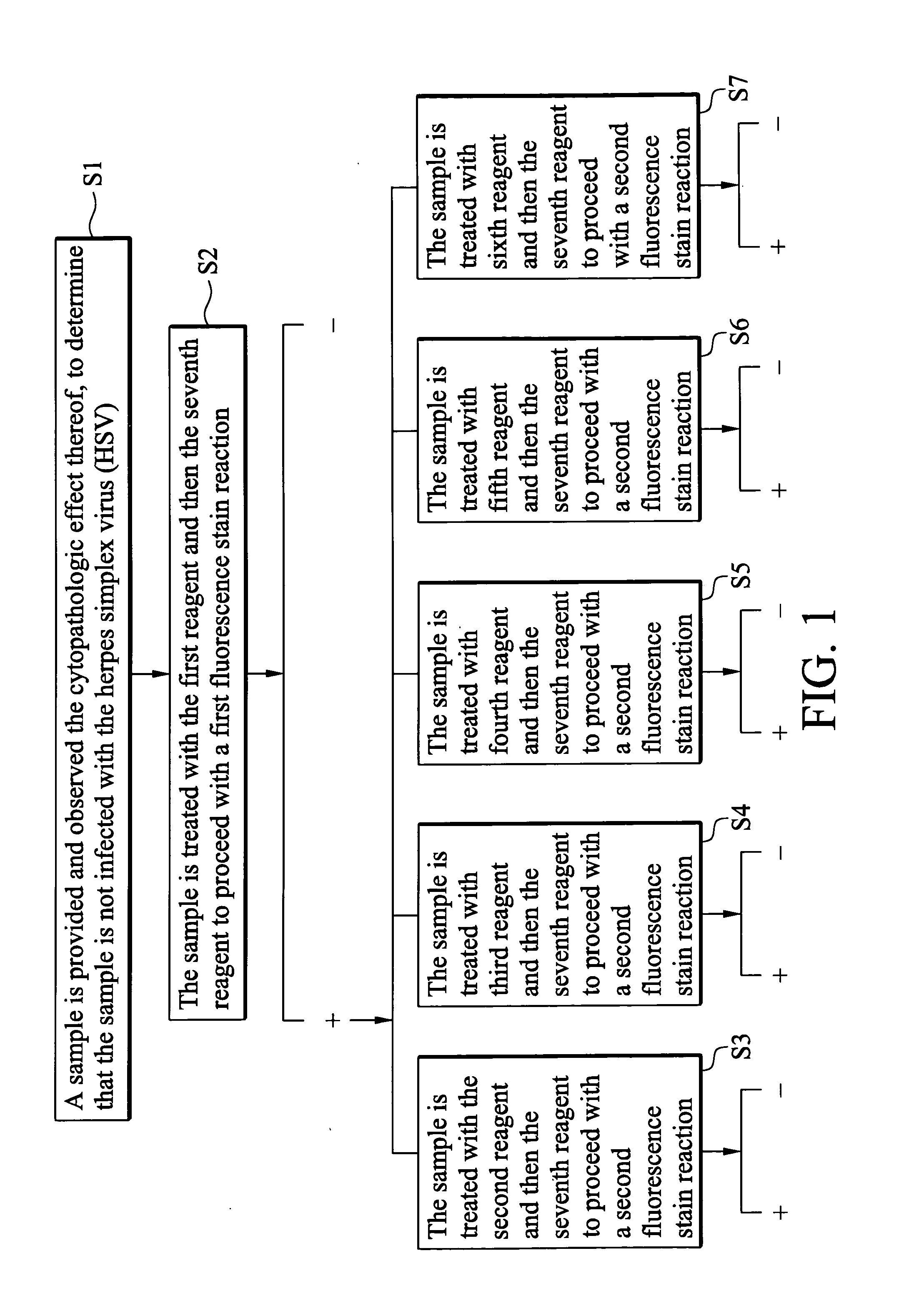
An indirect immunofluorescence assay typing kit for coxsackievirus, comprising: a first reagent comprising a mixture of an anti-coxsackievirus A2 polyclonal antibody, an anti-coxsackievirus A4 polyclonal antibody, an anti-coxsackievirus A5 polyclonal antibody, an anti-coxsackievirus A6 polyclonal antibody, and an anti-coxsackievirus A10 polyclonal antibody; a second reagent comprising the anti-coxsackievirus A2 polyclonal antibody; a third reagent comprising the anti-coxsackievirus A4 polyclonal antibody; a fourth reagent comprising the anti-coxsackievirus A5 polyclonal antibody; a fifth reagent comprising the anti-coxsackievirus A6 polyclonal antibody; a sixth reagent comprising the anti-coxsackievirus A10 polyclonal antibody; and a seventh reagent comprising a secondary antibody labeled with a fluorescence compound, wherein the secondary antibody is used for detecting the antibody anti-coxsackieviruses A2, A4, A5, A6 and A10 polyclonal antibodies and a titer of the anti-coxsackieviruses A2, A4, A5, A6 or A10 polyclonal antibody is about 1:5000-151:70000.
Owner:CENTS FOR DISEASE CONTROL DEPT OF HEALTH
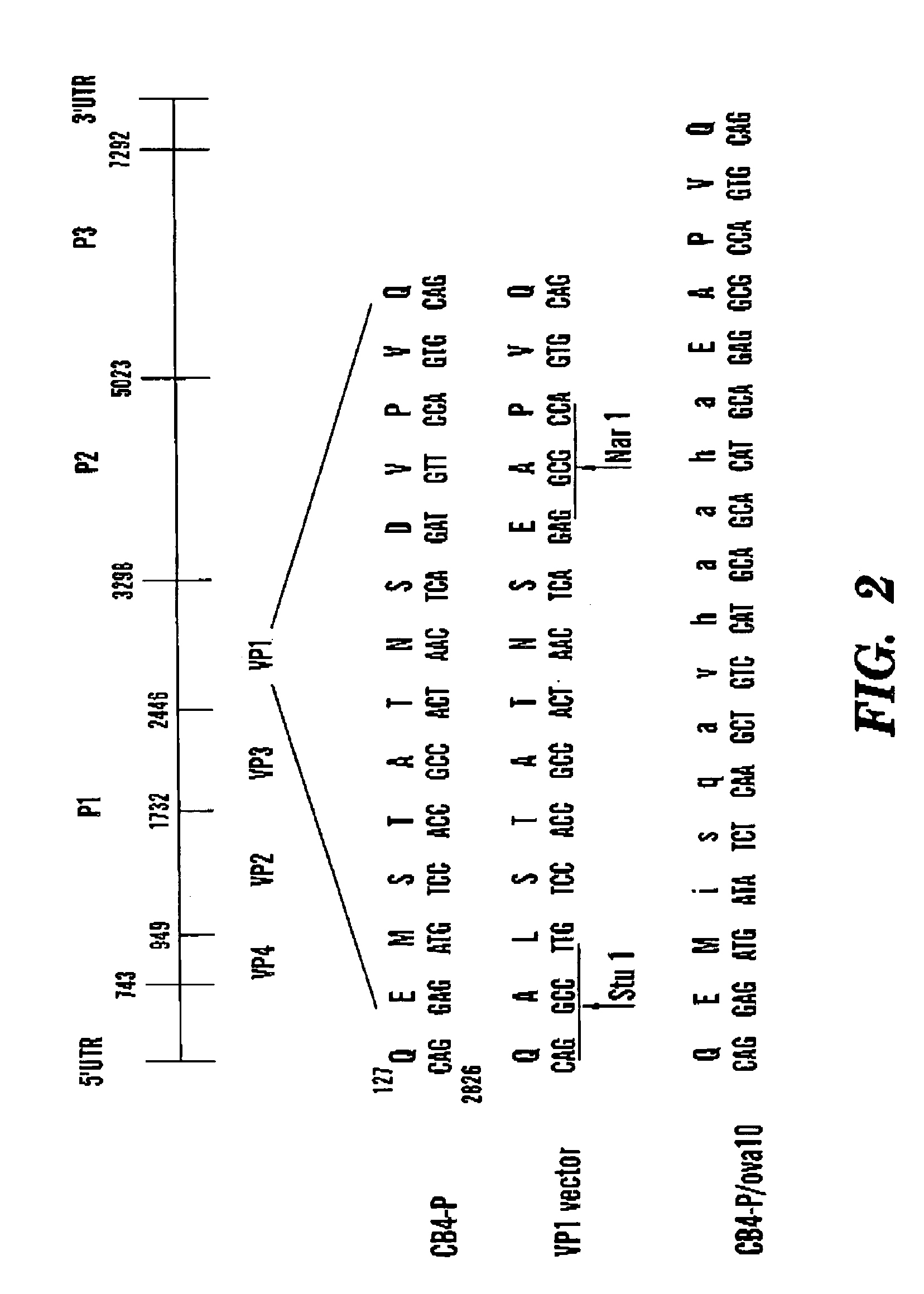
Coxsackievirus B4 expression vectors and uses thereof
InactiveUS7270997B2Prevents and inhibits disease progressionSsRNA viruses positive-senseVirus peptidesHeterologousCoxsackieviruses B
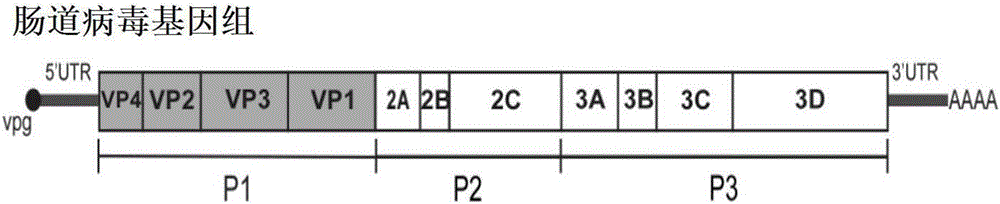
Disclosed is a recombinant attenuated coxsackievirus B4 virion which is engineered to contain a heterologous nucleic acid within the open reading frame of its genome, wherein the heterologous nucleic acid encodes a heterologous polypeptide which is expressed by the virion. Specific examples of attenuated coxsackievirus B4 virions suitable for use in the present invention are CB4-P and JVB. In one embodiment the heterologous nucleic acid is inserted into the P1 region of the genome such that the heterologous polypeptide is expressed as a fusion of a viral capsid protein. Methods of use of the recombinant attenuated coxsackievirus B4 virion include inducing an immune response in an individual to the heterologous polypeptide contained therein.
Owner:RAMSINGH ARLENE I
Gene-modified coxsackievirus
ActiveUS20160143969A1Good effectImprove securityBiocideSsRNA viruses positive-senseNucleotideVirotherapy
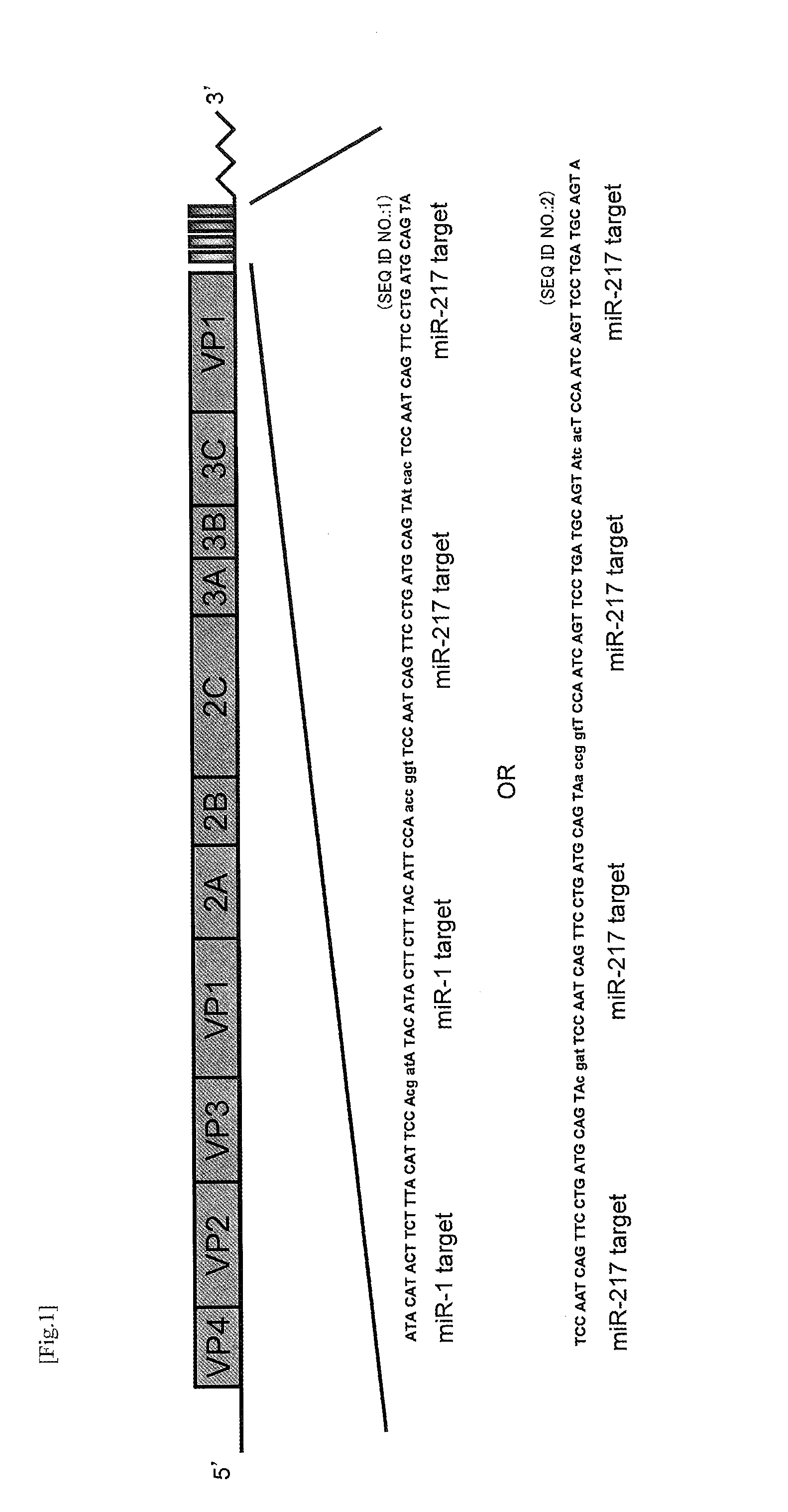
A modified coxsackievirus showing improved safety and / or aggressiveness to be used for oncolytic virotherapy is provided. A modified coxsackievirus showing tissue-specific suppression of proliferation and comprising a imitated genome consisting of the genome of coxsackievirus B3 wild-type (CVB3-WT) inserted with at least one polynucleotide consisting of a target sequence of tissue-specific microR NA (miRNA) is provided. The mutated genome is preferably further inserted with the region encoding GM-CSF in an expressible form.
Owner:NEOPRECISION THERAPEUTICS CO LTD +1
Adenoviral vectors and uses thereof
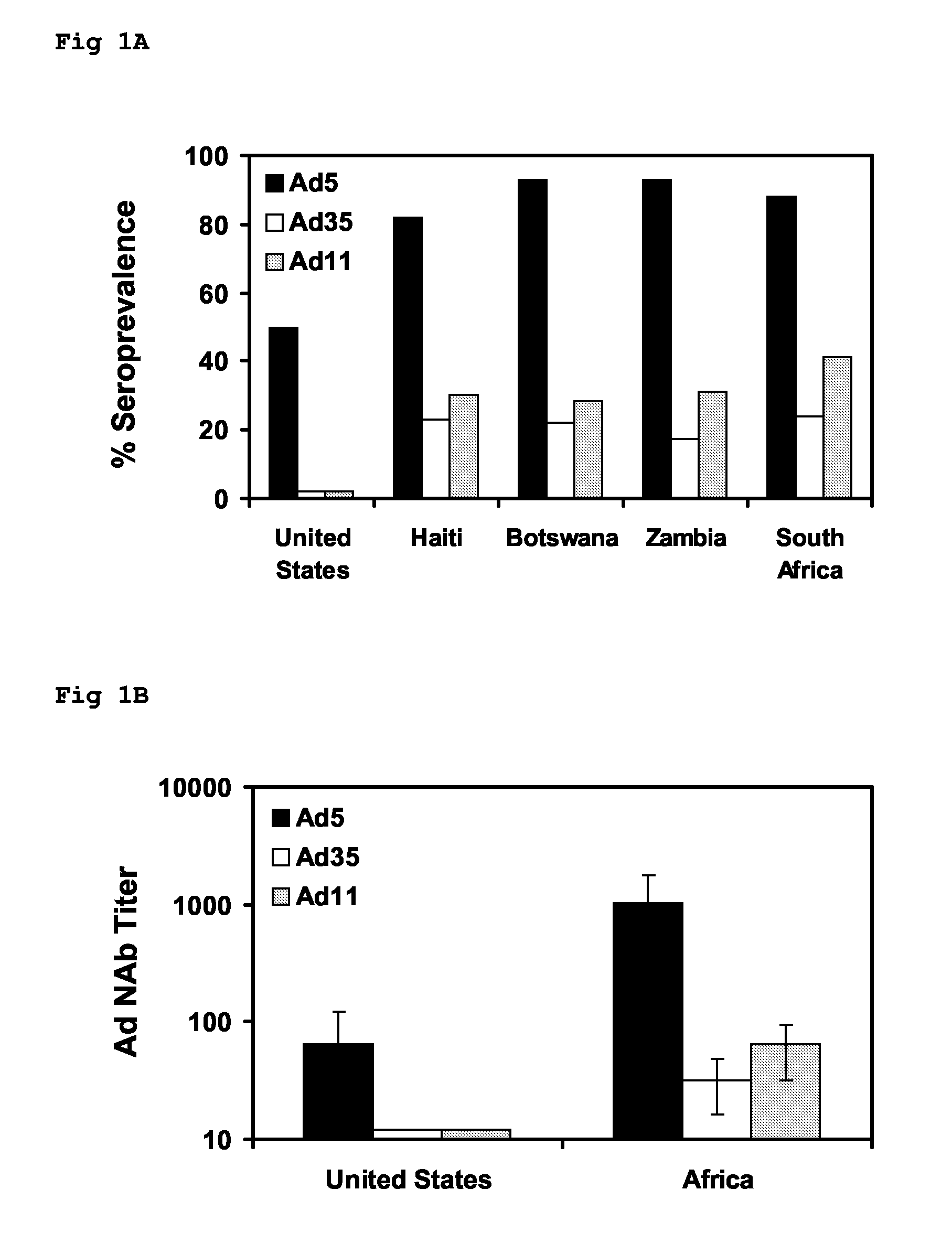
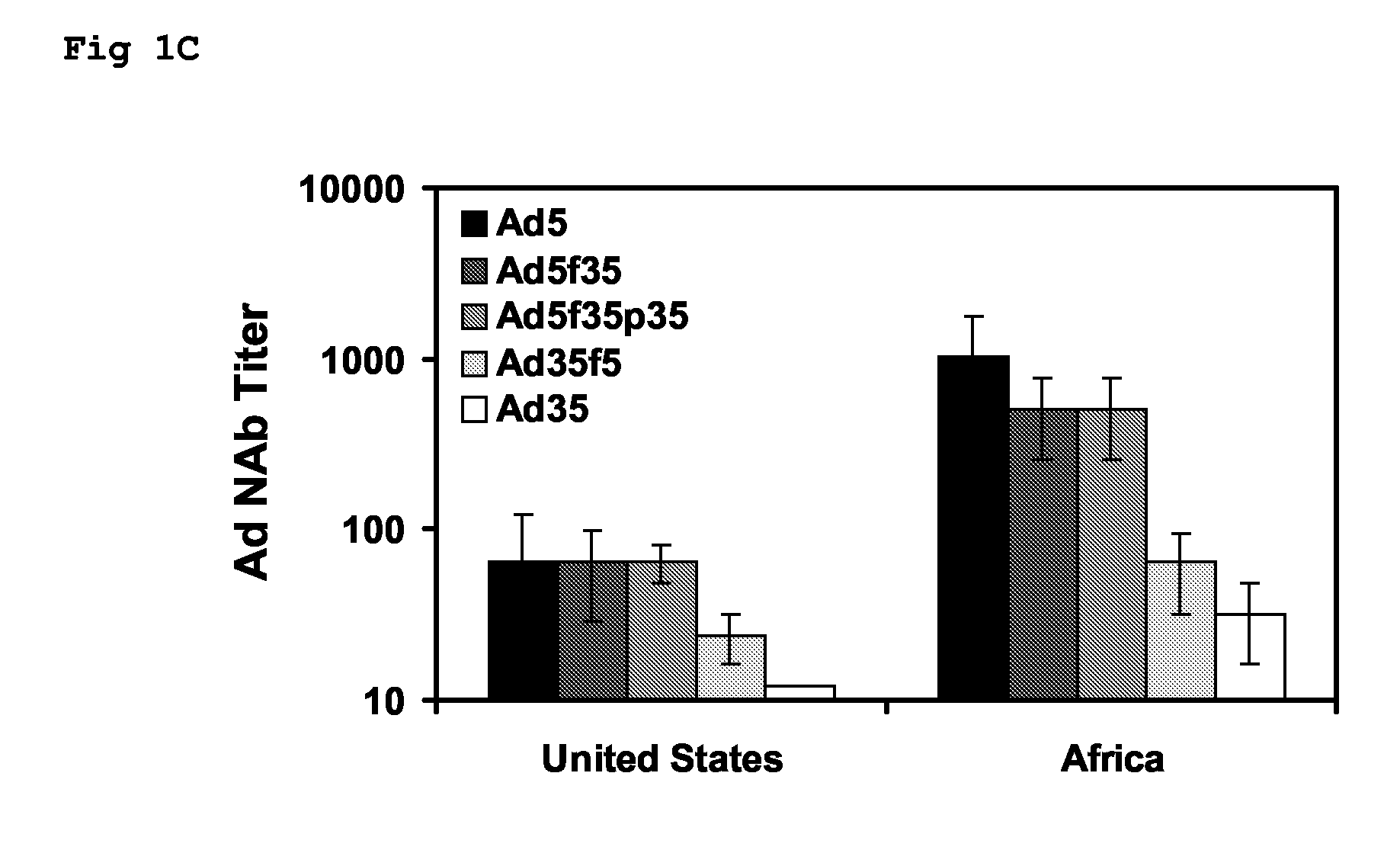
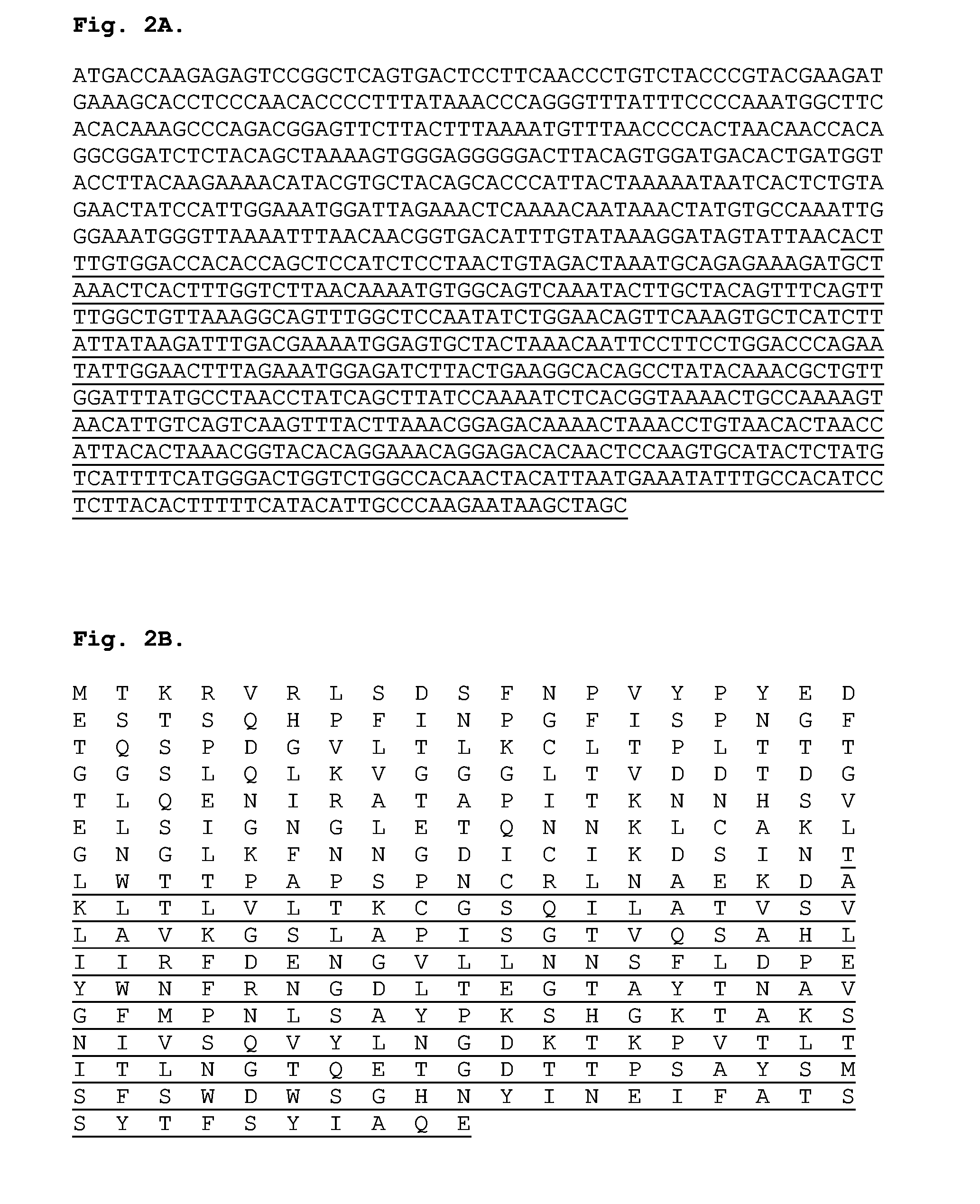
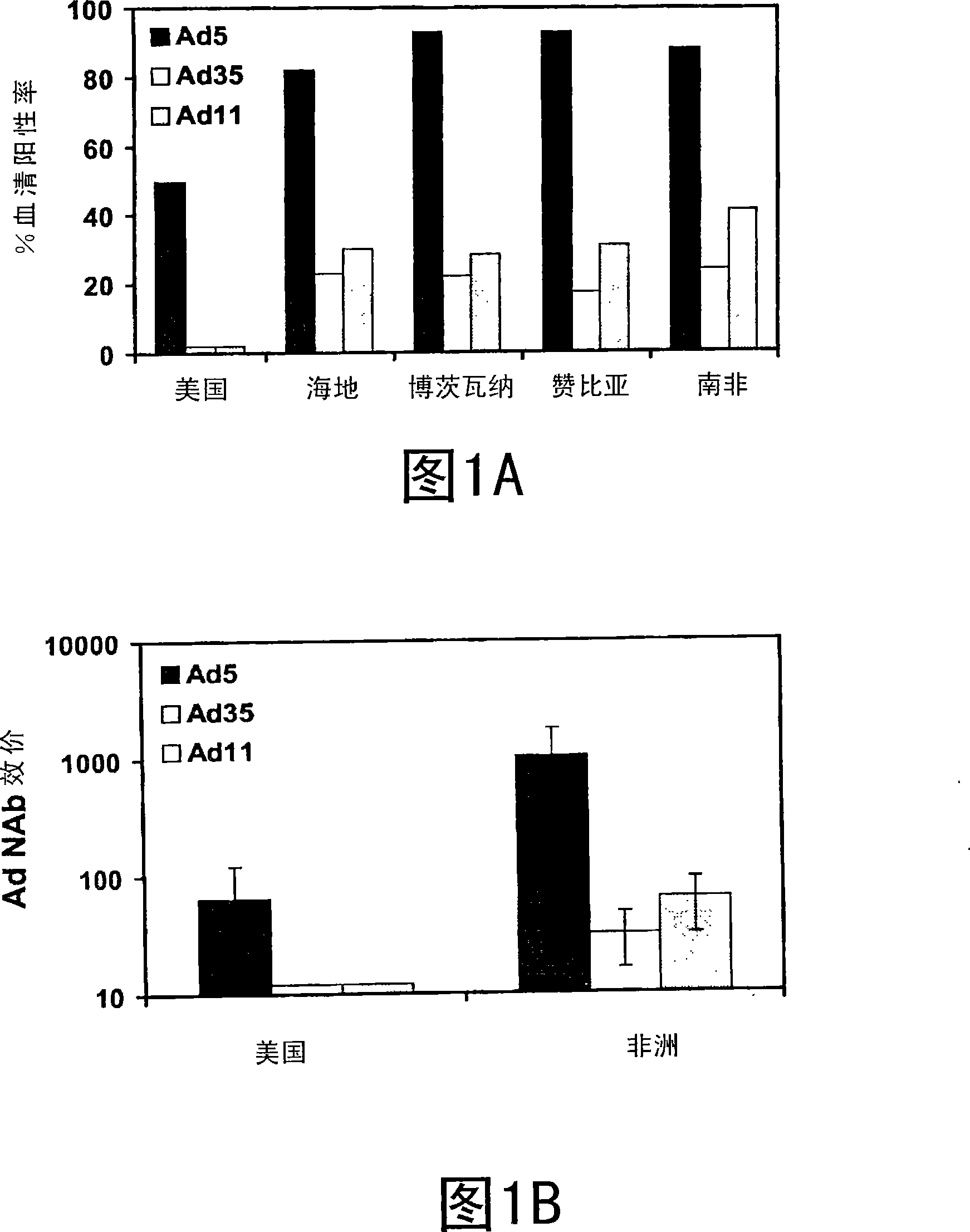
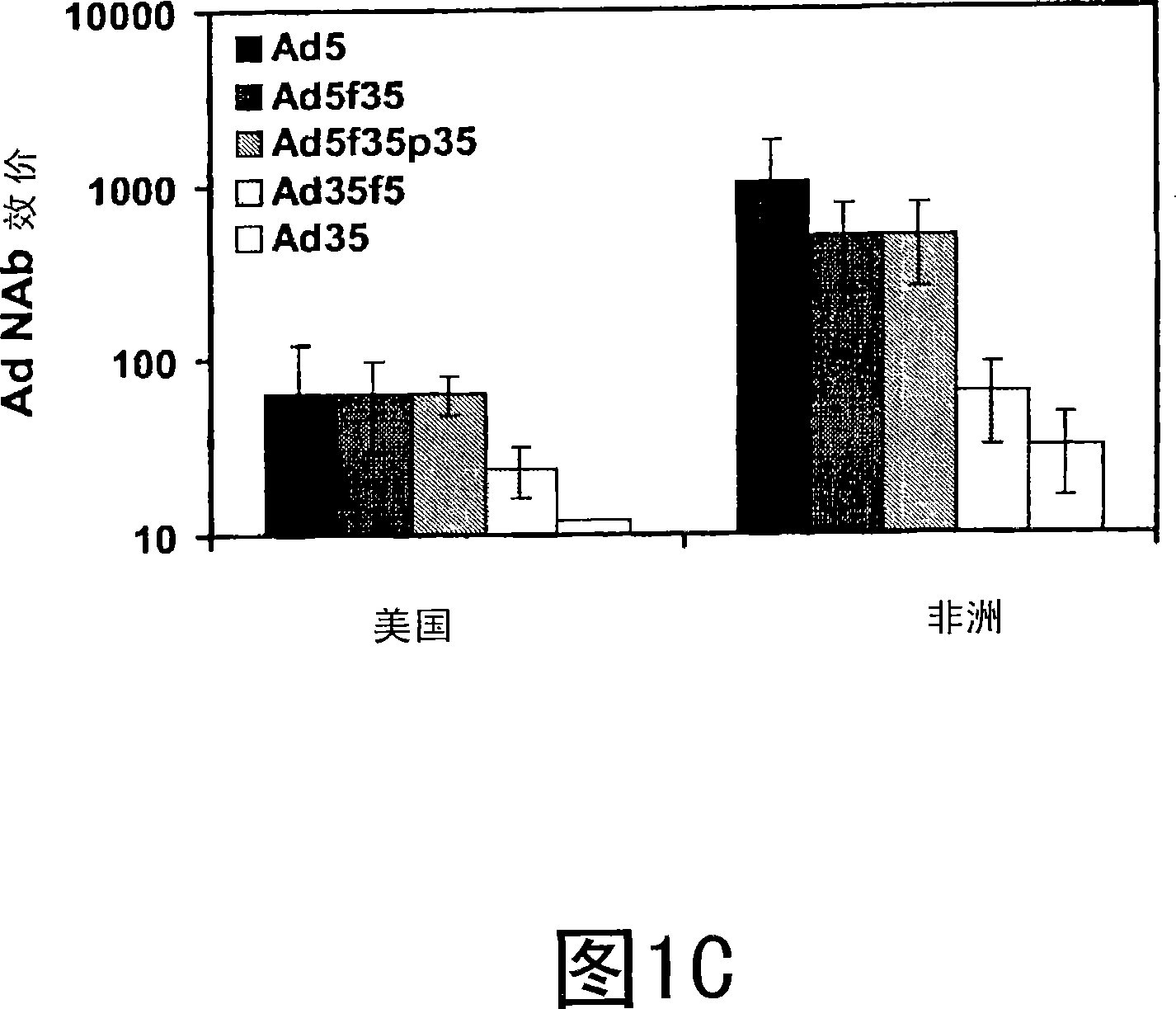
The present invention relates to recombinant adenoviral vectors based on adenoviruses that encounter pre-existing immunity in a minority of the human population and which harbor a chimeric capsid. The chimeric capsid comprises fiber proteins that have at least the knob domain of a human adenovirus that binds to the Coxsackievirus and Adenovirus Receptor (CAR) and a hexon protein from an adenovirus serotype that encounters pre-existing immunity in a low percentage of the human population.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +1
Improved adenoviral vectors and uses thereof
The present invention relates to recombinant adenoviral vectors based on adenoviruses that encounter pre-existing immunity in a minority of the human population and which harbour a chimeric capsid. The chimeric capsid comprises fiber proteins that have at least the knob domain of a human adenovirus that binds to the Coxsackievirus and Adenovirus Receptor (CAR) and a hexon protein from an adenovirus serotype that encounters pre-existing immunity in a low percentage of the human population.
Owner:JANSSEN VACCINES & PREVENTION BV +1
Method of using adenoviral vectors with increased immunogenicity in vivo
The invention provides a method of inducing an immune response in a mammal. The method comprises administering to the mammal an adenoviral vector comprising (a) a subgroup C fiber protein wherein a native coxsackievirus and adenovirus receptor (CAR)-binding site is disrupted, (b) a subgroup C penton base protein wherein a native integrin-binding site is disrupted, and (c) a nucleic acid sequence encoding at least one antigen derived from an infectious agent other than an adenovirus which is expressed in the mammal to induce an immune response.
Owner:UNITED STATES OF AMERICA +1
Anti-viral compound
The present invention relates to compounds of Formula (I), which inhibit the growth of picornaviruses, Hepatitus viruses, enteroviruses, cardioviruses, polioviruses, coxsackieviruses of the A and B groups, echo virus and Mengo virus. In said Formula, A is phenyl, pyridyl, substituted phenyl, substituted pyridyl, or benzyl; R is hydrogen, COR4 or COCF; X is N-OH, O or CHR1 R1 is hydrogen, halo, CN, C -C alkyl -C≡ CH, CO(C -C alkyl), CO (C -C alkyl), or CONR2R3 R2 and R3 are independently hydrogen or C -C alkyl; A' is hydrogen, halo, C -C alkyl, benzyl, naphthyl, thienyl, furyl, pyridyl, pyrollyl, COR4 S(O)nR4 or a group of formula (II); R4 is C -C alkyl, phenyl, or substituted phenyl; n is 0,1, or 2; R5 is independently at each occurance hydrogen or halo; m is 1,2,3, or 4; and R6 is hydrogen, halo, CF, OH, CO H, NH, NO, CONHOCH, C -C alkyl, or CO (C -C alkyl), C -C alkoxy; or pharmaceutically acceptable salts thereof.
Owner:ELI LILLY & CO
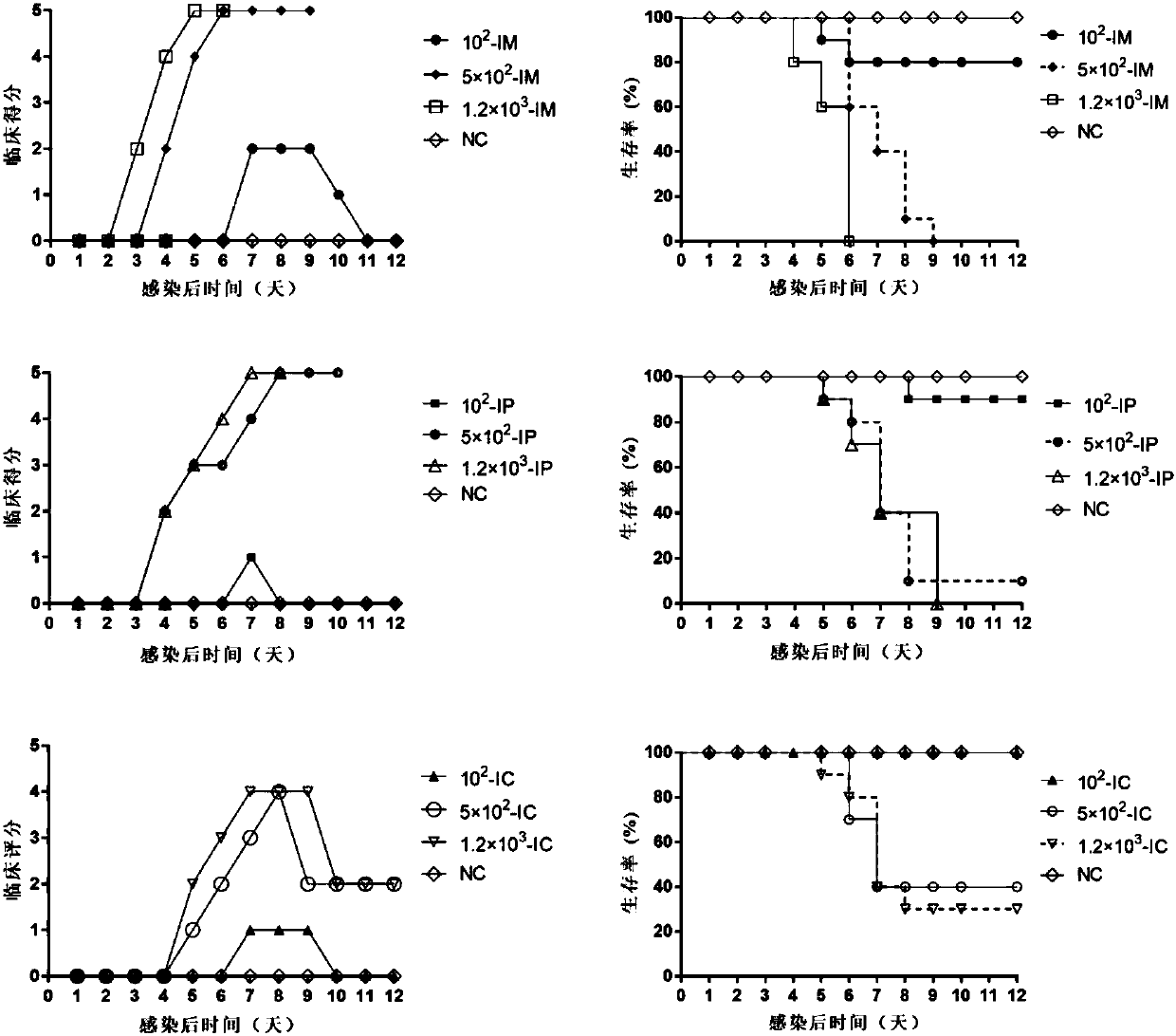
Coxsackievirus A6 type infected animal model as well as preparation method and application thereof
InactiveCN106867974AGood repeatabilitySsRNA viruses positive-senseMicroorganism based processesMicroorganismViral Vaccine
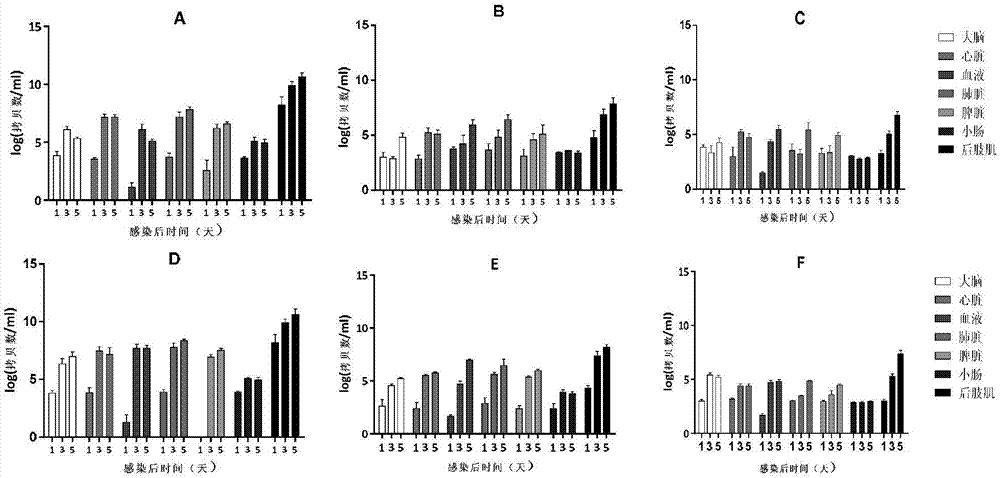
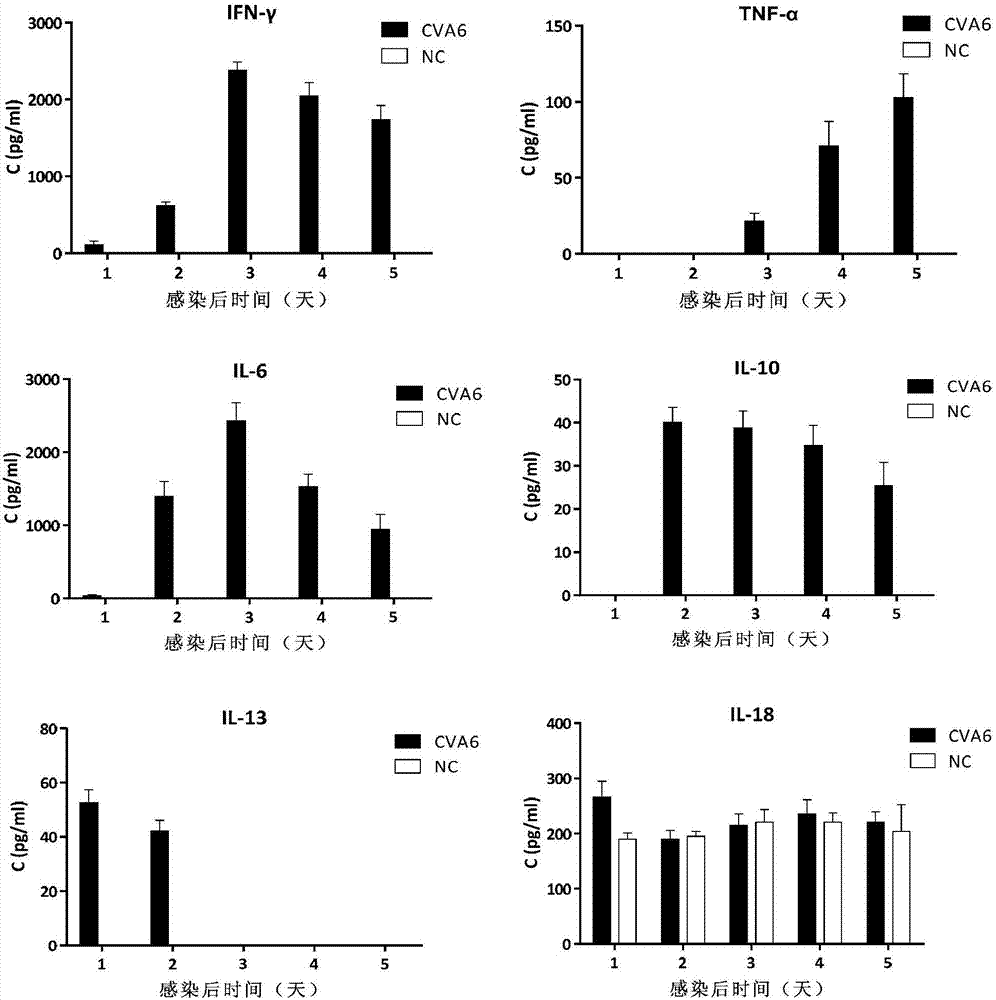
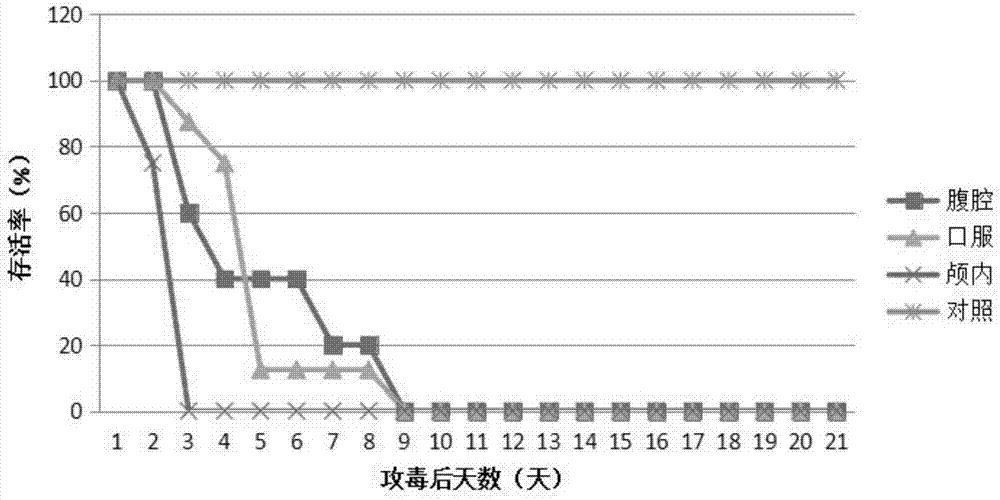
The invention discloses a coxsackievirus A6 type infected animal model as well as a preparation method and an application thereof. The preparation method of the coxsackievirus A6 type infected animal model provided by the invention comprises a step of infecting an animal with an inactivated coxsackievirus A6 type strain, so that the coxsackievirus A6 type infected animal model is obtained, wherein the coxsackievirus A6 type strain is preserved in China General Microbiological Culture Collection Center with preservation number of CGMCC No.13393. The coxsackievirus A6 type infected animal model, which is stable and is good in repeatability, is constructed on the basis of the CVA6 strain WF057R; and with the application of the model, a research tool is provided for next medicine antiviral therapy and assessment of an immune protective effect of an inactivated viral vaccine.
Owner:TAISHAN MEDICAL UNIV
Viral particles as immunogens against enterovirus infection and production thereof
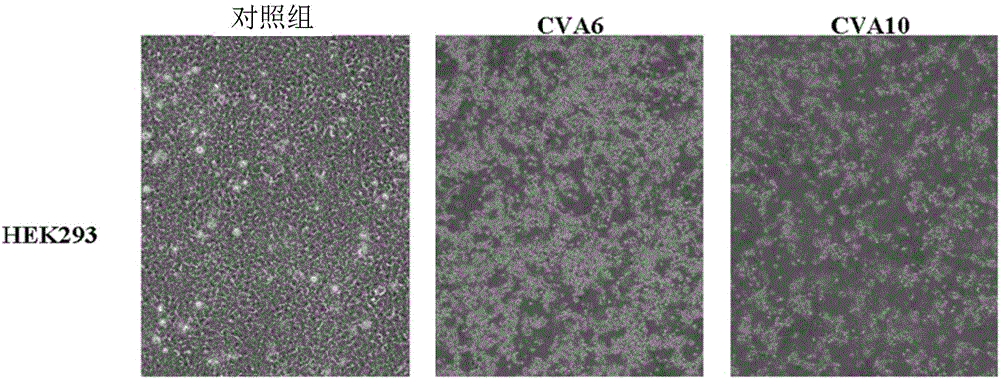
The present invention relates to viral particles as immunogens against enterovirus infection and a method of producing the same. Specifically, the present invention features that human embryo kidney 293 (HEK 293) cells are used to produce viral particles of Enterovirus A, particularly Coxsackievirus A6 (CVA6) particles or Coxsackievirus A10 (CVA10) particles or both and optionally additional viral particles of other Enterovirus A e.g. Coxsackievirus A16 (CVA16) and / or Enterovirus A71 (EV71). The yield of the viral particles in HEK 293 cells is unexpectedly high and effective to induce an immune response against enterovirus infection, especially CVA6 and CVA10. The present invention also relates to an immunogenic composition against enterovirus infection for human use comprising the viral particles as described herein and a method of preventing enterovirus infection or a disease as caused, particularly Hand-Foot-Mouth diseases (HFMD), by administering the immunogenic composition to a subject in need thereof.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH +1
Swine vesicular disease virus and mutant strains and preparation process and use thereof
InactiveUS6200576B1SsRNA viruses positive-senseSugar derivativesSwine vesicular diseaseCoxsackievirus
The present invention relates to a gene of swine vesicular disease virus (SVDV) and the mutant strains of the gene, and the expression plasmids, the preparation process thereof. The invention also relates to a vaccine for use in the prophylaxis of swine vesicular disease composition containing the mutant strains. Furthermore, the invention provides a process for differentiating mutant strains of SVDV from the wild type strain of SVDV, coxsackievirus and foot-and-mouth disease virus by polymerase chain reaction.
Owner:DEV CENT FOR BIOTECHNOLOGY
Human embryo lung fibroblast strain and method for using human embryo lung fibroblast strain for producing hand-foot-mouth viral vaccine
InactiveCN102911910AAvoid Residual EffectsReduced purification stepsAntiviralsEmbryonic cellsEmbryoViral Vaccine
The invention provides a novel human embryo lung diploid fibroblast strain Walvax-2, CCTCCC201055. The cell strain is sensitive to main epidemic disease viral strains-coxsackievirus 16-type COX.A16 in a group A and enterovirus 71-type EV71 second-strain virus of a hand-foot-mouth disease, and virus output is high. The invention further provides a method for preparing a divalent hand-foot-mouth virus inactivated vaccine and application of the human embryo lung diploid fibroblast strain in preparation of the hand-foot-mouth virus inactivated vaccine. The divalent inactivated vaccine produced by the human embryo lung diploid fibroblast strain can effectively prevent hand-foot-mouth disease.
Owner:云南沃森生物技术股份有限公司
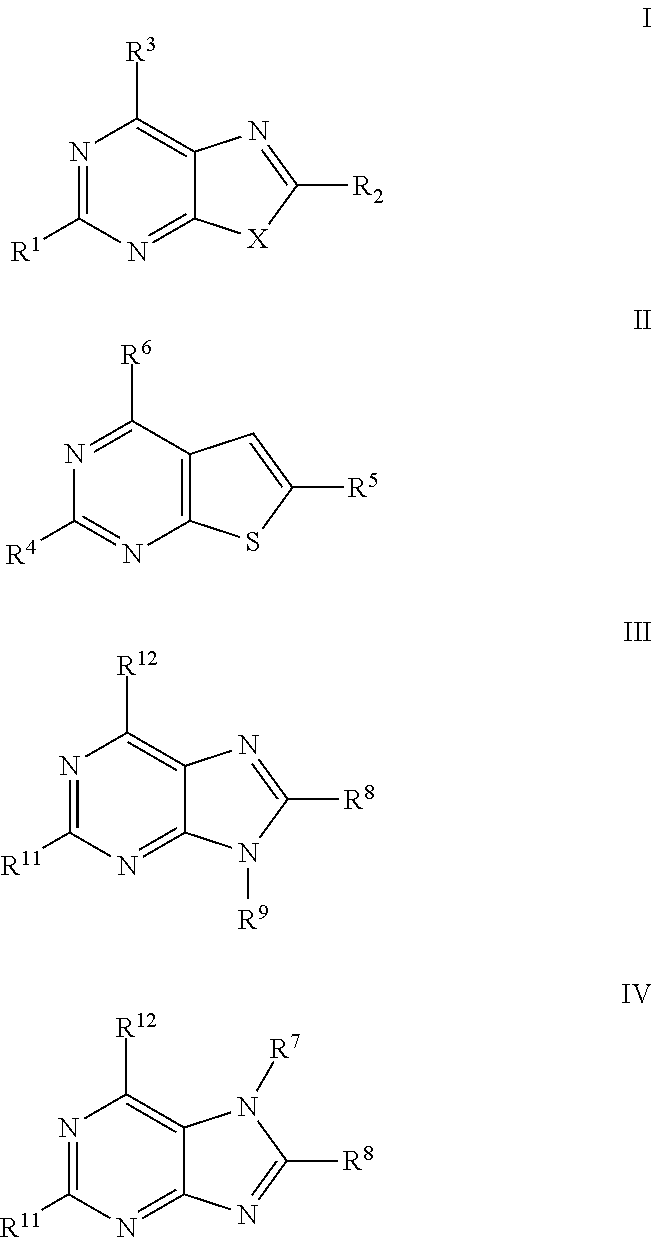
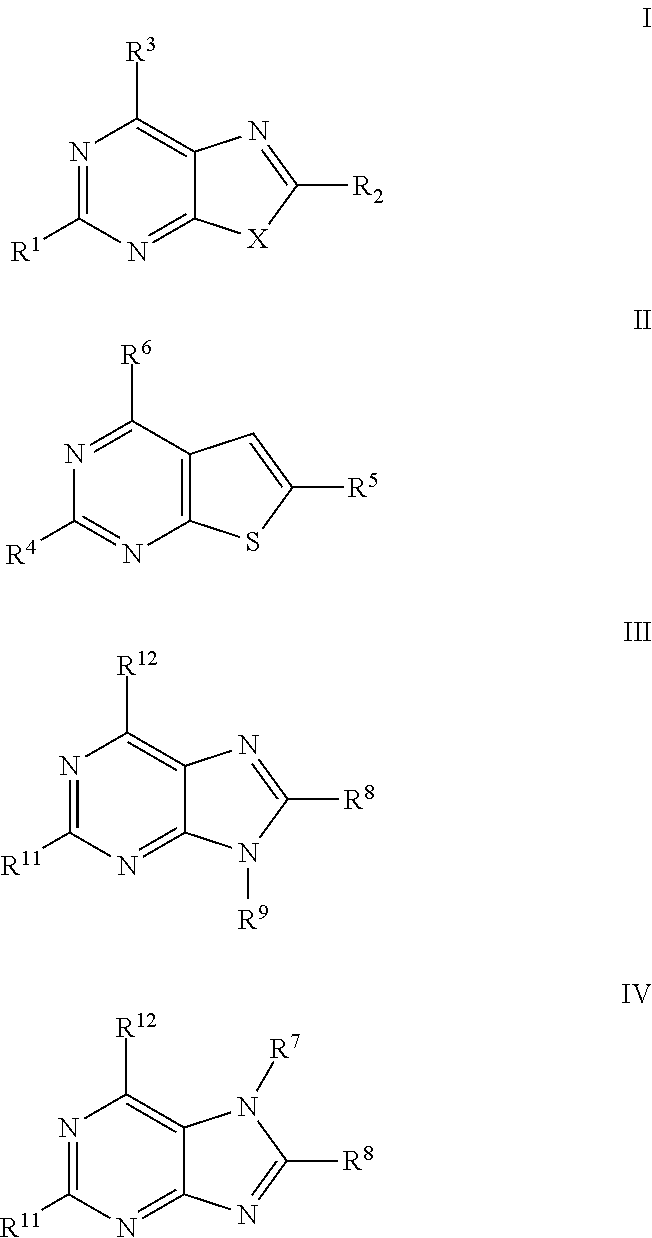
Antiviral Activity of Novel Bicyclic Heterocycles
The present invention relates to compound of Formula I, II, III, or IV, and / or a pharmaceutical acceptable addition salt thereof and / or a stereoisomer thereof and / or a solvate thereof, wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, R11, and R12 are as defined in the claim 1 or as described in detail in the description of the invention, and to the use of said compounds to treat or prevent viral infections and their use to manufacture a medicine to treat or prevent viral infections, particularly infections with RNA-viruses belonging to the family of the Retroviridae, the family of the Flaviviridae and the family of the Picornaviridae and more preferably infections with Human Immunodeficiency Virus 1 (HIV1), Human Immunodeficiency Virus 2 (HIV2), Hepatitis C virus (HCV), Dengue virus, and enteroviruses like Coxsackievirus, Rhinovirus and Poliovirus. The present invention also relates to pharmaceutical compositions of said compounds and the use of said pharmaceutical compositions to treat or prevent viral infections. The present invention further relates to the use of said compounds as biologically active ingredients, more specifically as medicaments for the treatment of viral disorders and pathologic conditions such as, but not limited to, viral infections with Human Immunodeficiency Virus 1 (HIV1), Human Immunodeficiency Virus 2 (HIV2), Hepatitis C virus (HCV), Dengue virus, and enteroviruses like Coxsackievirus, Rhinovirus and Poliovirus.
Owner:KATHOLIEKE UNIV LEUVEN
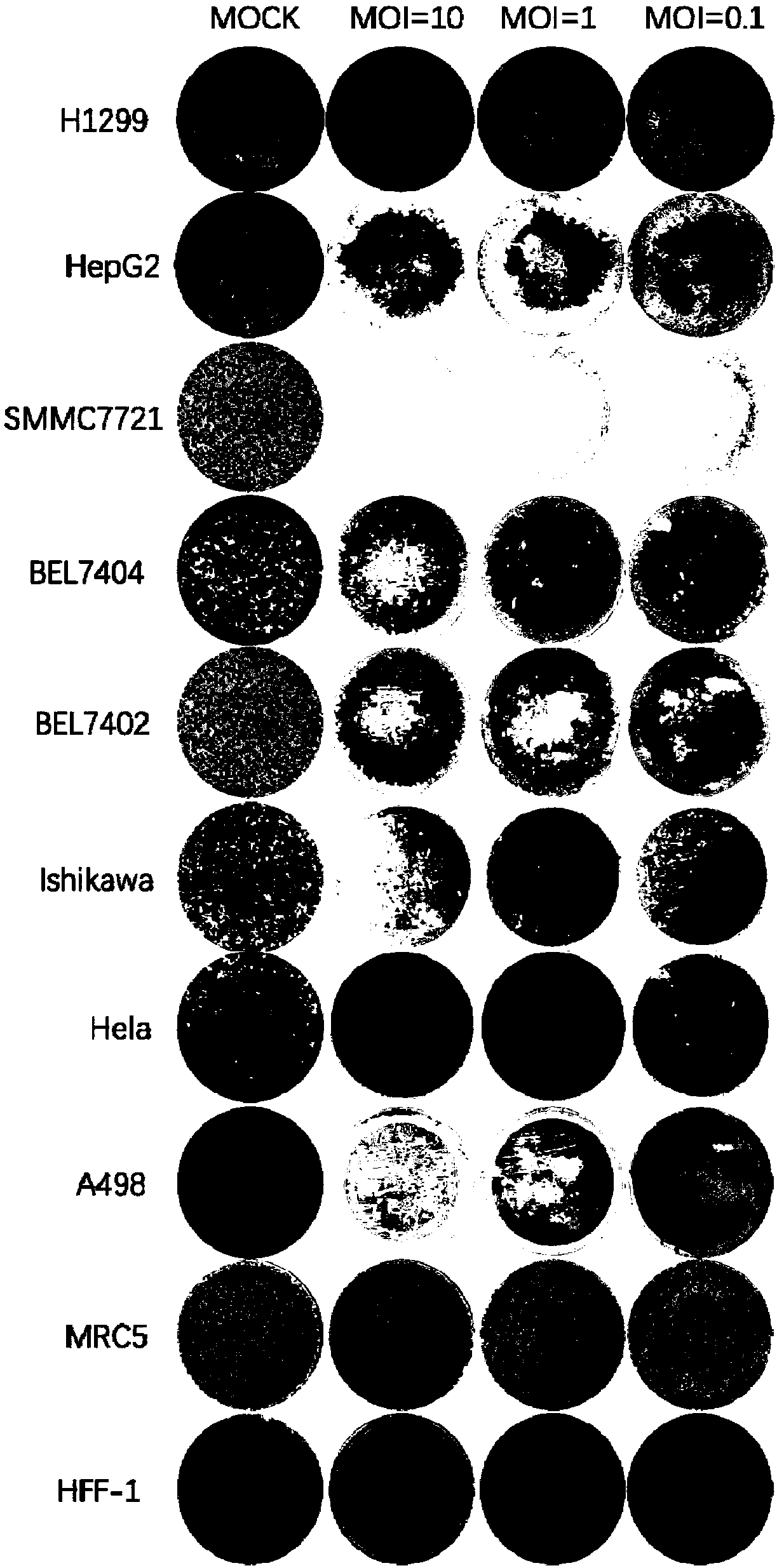
Coxsackievirus and application of coxsackievirus in preparation of anti-tumor drugs
ActiveCN103981152AHigh selection specificityImprove securityMicroorganism based processesViral/bacteriophage medical ingredientsCOXSACKIE A VIRUSMelanoma
The present invention discloses a coxsackievirus, which is a coxsackievirus group B type 3 mutant strain, wherein the genome site 2690 is adenine, and the genome site 3231 is guanine. The coxsackievirus has significant cytolytic capacity and selection specificity, can be used for preparation of anti-tumor drugs, especially anti-lung cancer drugs, anti-liver cancer drugs, anti-prostate cancer drugs, anti-melanoma drugs, anti-breast cancer drugs, anti-colon cancer drugs and anti-rectal cancer drugs, is used for preparation of anti-tumor drugs, and has characteristics of good anti-tumor effect and high safety.
Owner:WUHAN BOWEIDE BIOTECH CO LTD
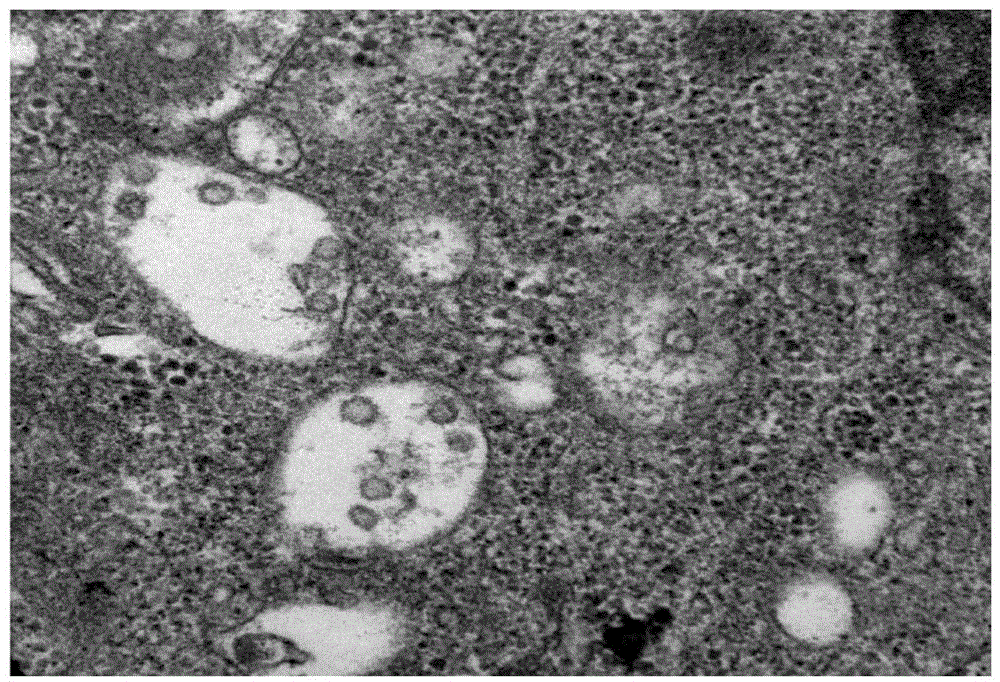
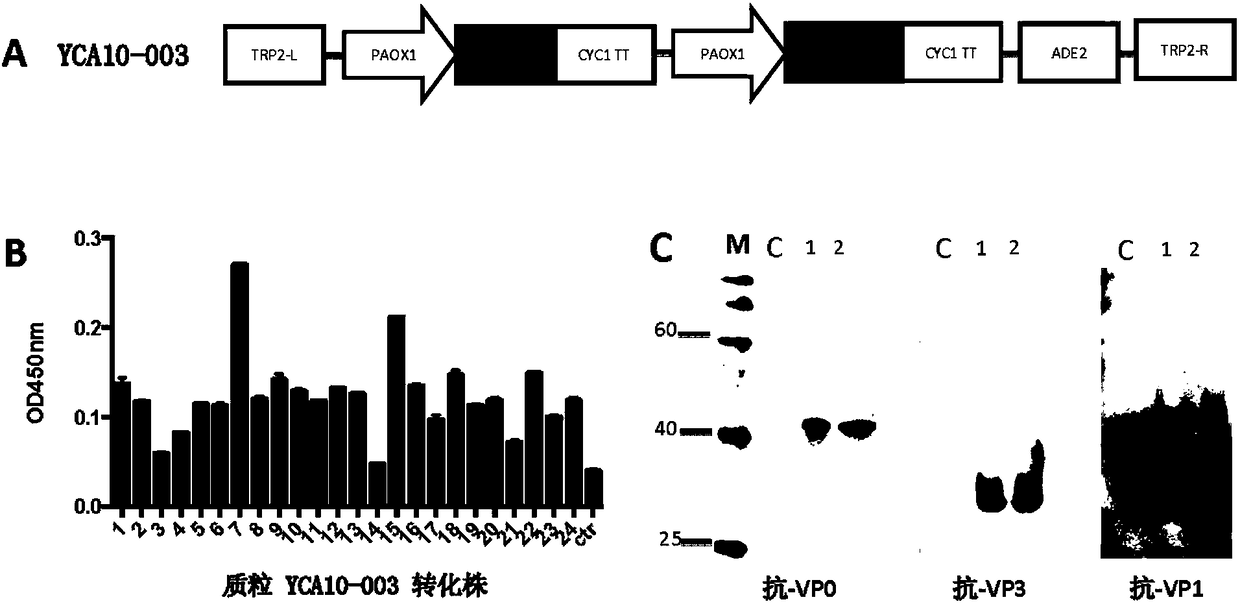
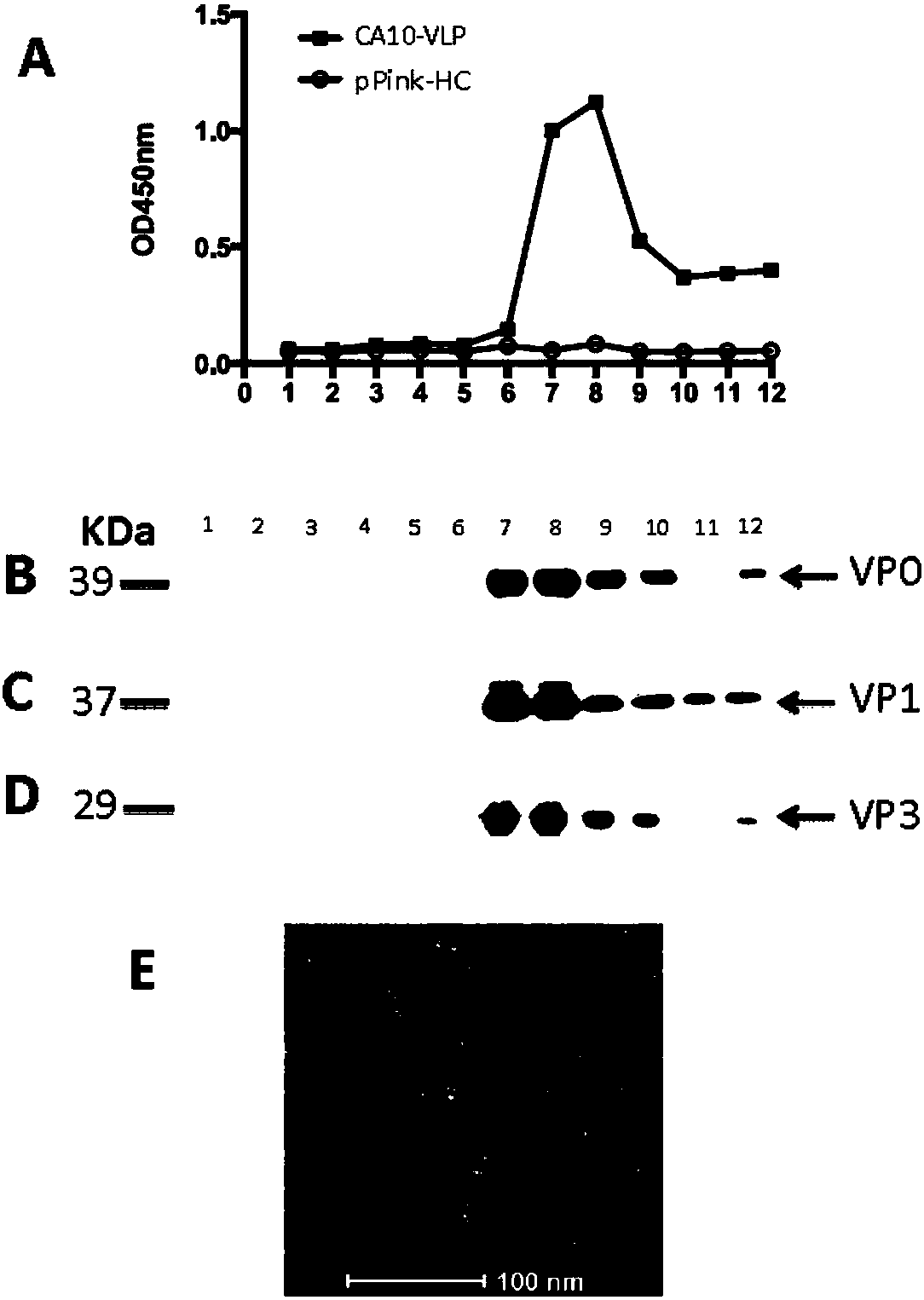
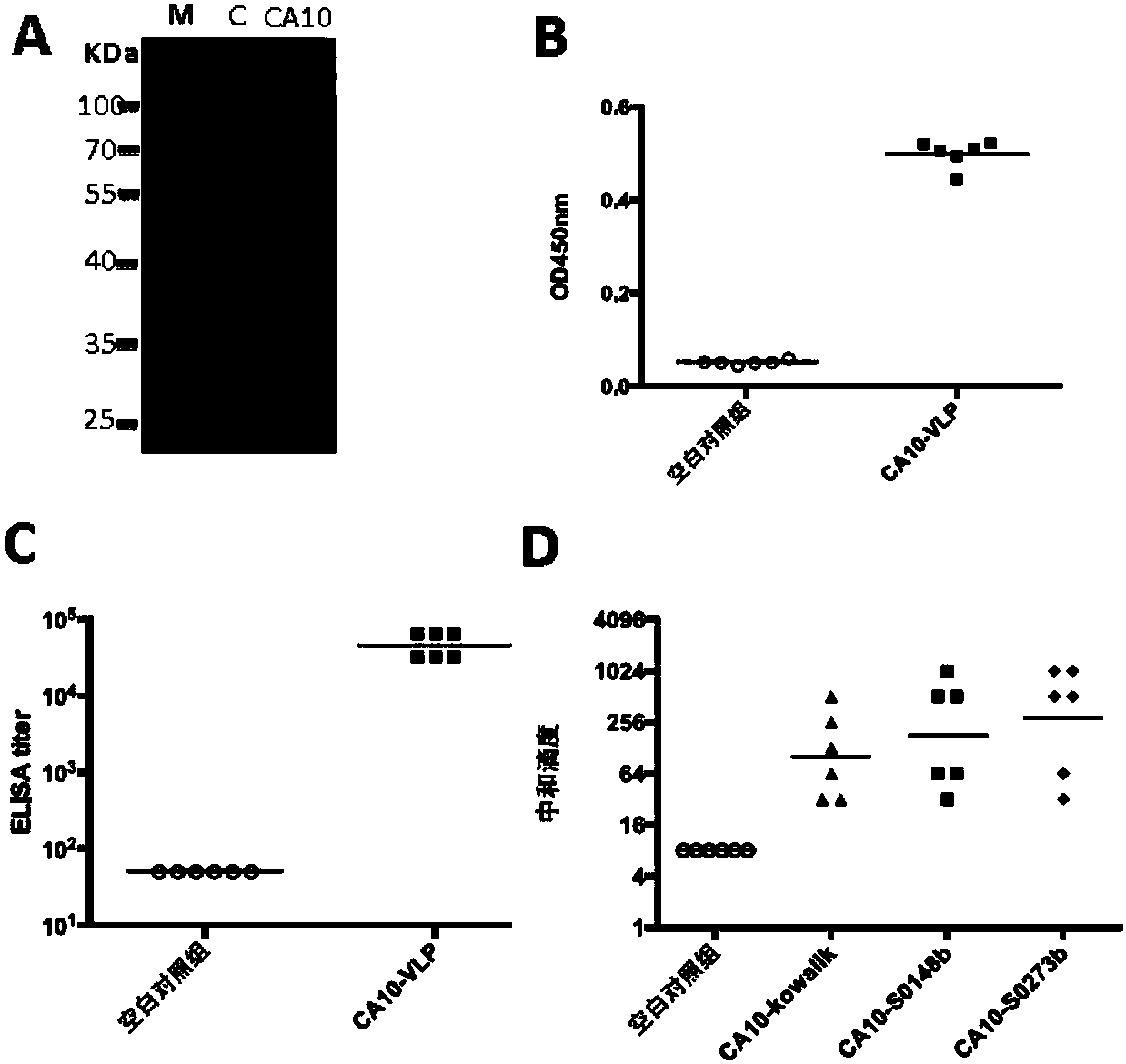
Yeast-expressed Coxsackievirus A10 virus-like particles and applications thereof
The present invention provides yeast-expressed Coxsackievirus A10 virus-like particles and applications thereof. According to the present invention, specifically the gene sequences of the P1 protein and the 3CD protein of Coxsackievirus A10 are transformed into yeast cells, and expression is performed to obtain the novel Coxsackievirus A10 virus-like particles; and the yeast-expressed Coxsackievirus A10 virus-like particles have characteristics of high expression, strong immunogenicity and good specificity.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Mucosa M-cell targeted viral myocarditis gene vaccine and preparation method thereof
ActiveCN103341179AValid responseEfficiently induces a responseGenetic material ingredientsAntiviralsWhole bodyViral Myocarditis
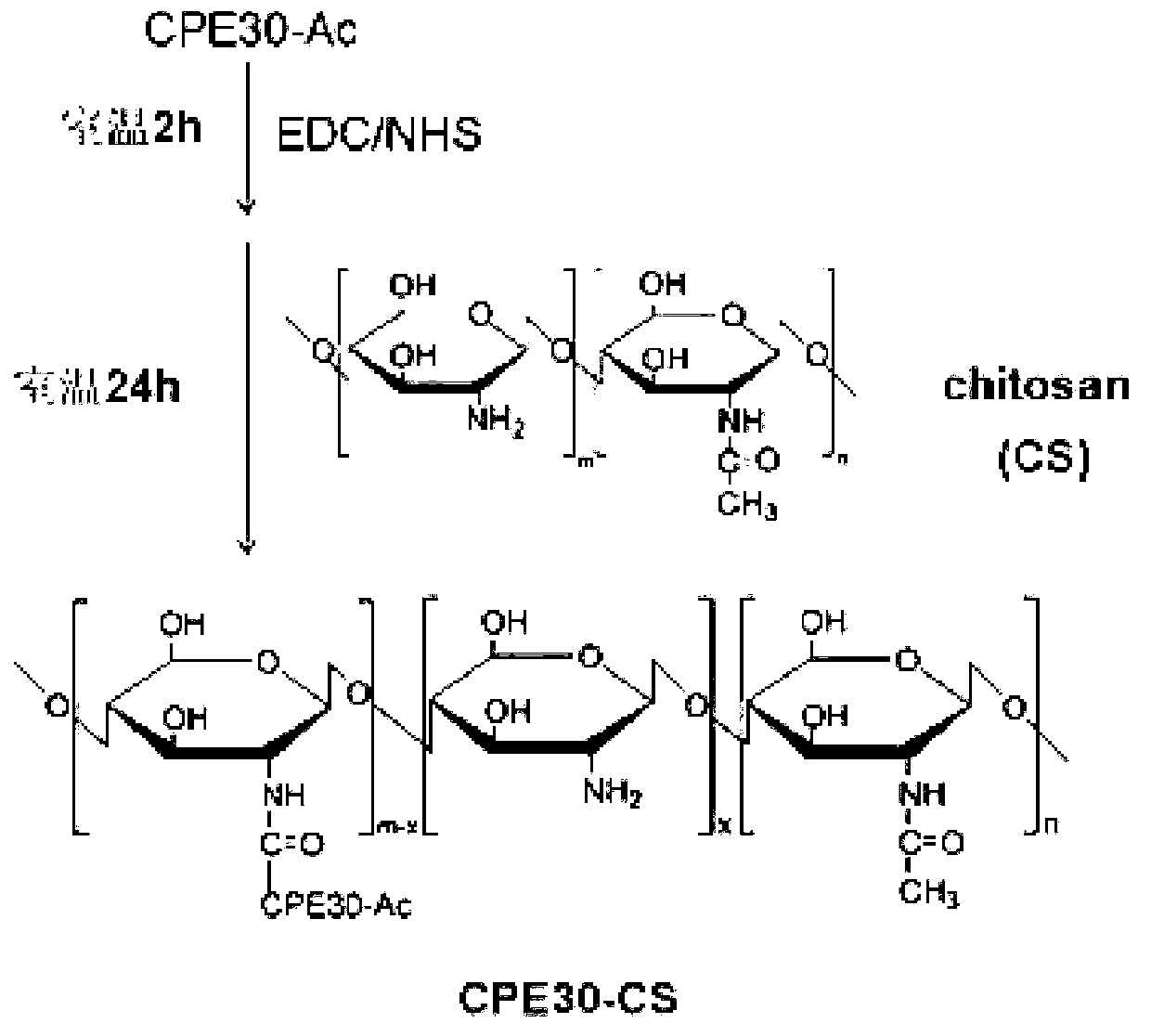
The invention discloses a mucosa M-cell targeted viral myocarditis gene vaccine. The vaccine is prepared by compounding a mucosa delivery system capable of targeting mucosa M-cells and B3-type coxsackie virus antigen encoding plasmids through cross-linking copolymerization. The invention further discloses a preparation method of the gene vaccine. The invention further discloses a preparation method of the mucosa delivery system capable of targeting the mucosa M-cells, and the mucosa delivery system is acquired after stable amide-ester bonds are formed by carboxyl groups and amino groups in deacetylated chitosan, wherein the carboxyl groups in peptide fragment CPE30 are targeted by M-cells which are activated by using EDC (Dichloroethane) and NHS (N-Hydroxysuccinimide). By nasally dropping the gene vaccine onto immunized mice, the gene vaccine is proved to be capable of effectively inducing the response between specific antigen serum and mucoantibody, obviously enhancing the local specific T-cell killing ability of the whole body and gastrointestinal mucosa and significantly improving the ability of mice for resisting B3-type coxsackie virus, thereby being an excellent prophylactic vaccine for viral myocarditis.
Owner:SUZHOU UNIV
Traditional Chinese medicine composition for treating viral respiratory tract infection and preparation method thereof
The invention discloses a traditional Chinese medicine composition for treating viral respiratory tract infection and a preparation method thereof. The composition comprises the following raw medicinal materials in parts by weight: 3-20 parts of honey-fried ephedra, 3-20 parts of flos farfarae, 5-25 parts of schizonepeta spike, 5-20 parts of radix asteris, 5-15 parts of apricot kernel, 5-20 parts of roasted perilla seed, 3-15 parts of rhizoma anemarrhenae, 3-15 parts of bulbus fritillariae thunbergii, 5-25 parts of fructus arctii, and 2-15 parts of liquorice. The raw medicinal materials are matched with auxiliary materials and a flavoring agent to be directly or indirectly made into pharmaceutically acceptable capsules, oral liquid, granules, syrup, tablets, effervescent particles, dropping pills, aerosol, spraying agent, gargle, mouthwash and a variety of dosage forms via conventional processes. The traditional Chinese medicine composition can be clinically used for treating or preventing the respiratory tract viral infectious diseases caused by influenza A virus, influenza B virus, rhino virus, coronavirus, respiratory syncytial virus, hand, foot and mouth virus, adenovirus, parainfluenza virus, echovirus, coxsackievirus and the like.
Owner:SHENZHEN TRADITIONAL CHINESE MEDICINE HOSPITAL
Building and evaluation of an animal model infected with a coxsackievirus A10 domesticated strain TA151R-1
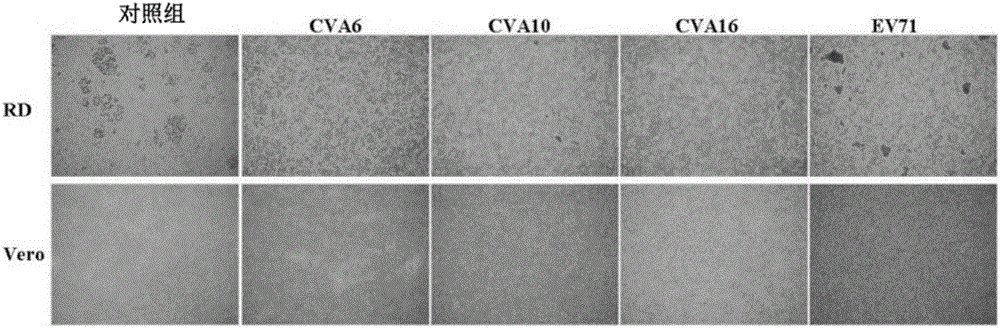
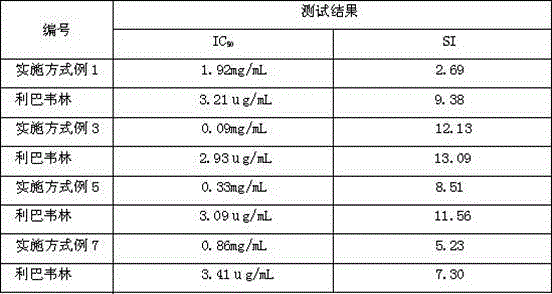
InactiveCN107744530AImprove replication efficiencyImprove efficiencyCompounds screening/testingViral/bacteriophage medical ingredientsCoxsackievirusPolyvalent Vaccine
A coxsackievirus A10 domesticated strain TA151R-1 high in tilter and stable in passage is disclosed. The virus strain can infect RD cells, HEK293 cells, Vero cells, MRC-5 cells, Hep-2 cells, WI-38 cells, and other cell lines, and can be used for preparing a univalent vaccine or a polyvalent vaccine. The prepared vaccine can protect a body from being harmed by the coxsackievirus, and can completelyprotect the body from attack by heterogenous viruses. An infected animal model can be built efficiently.
Owner:SHANDONG FIRST MEDICAL UNIV & SHANDONG ACADEMY OF MEDICAL SCI
Recombinant expression plasmids used for packaging coxsackievirus B5 (CV-B5) pseudovirus, pseudovirus, kit and method
PendingCN106884017ADetection securityQuick checkViruses/bacteriophagesFermentationCoxsackievirusStructural protein
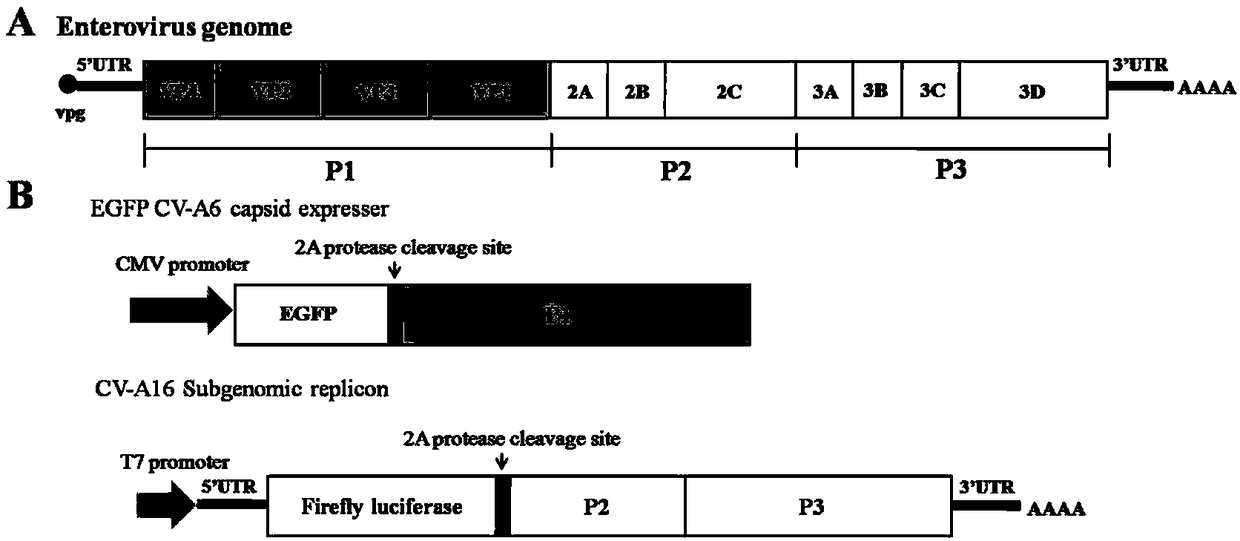
The invention relates to recombinant expression plasmids used for packaging a coxsackievirus B5 (CV-B5) pseudovirus, the pseudovirus, a kit and a method. The recombinant expression plasmids used for packaging the coxsackievirus B5 pseudovirus are respectively named as the pEGFP-CV-B5 (417) plasmid and the pCVB3-replicon, the CV-B5 structural protein expressed by the pEGFP-CV-B5 (417) plasmid can be used for packaging CV-B3 subgenome RNA transcribed by the pCVB3-replicon in the cell, thus the CV-B5 pseudovirus is generated, the pseudovirus can be used for detecting the neutralizing antibody, and since the pseudovirus with single-cycle infection is adopted, the safety problem caused when the live virus is used is avoided. After a plurality of experiments, the result shows that the invention provides the method for detecting the CV-B5 neutralizing antibody which is safe, sensitive, rapid, specific, simple and convenient, and is low in cost. Based on the abovementioned features, the method is particularly suitable for the experiment for rapidly detecting the neutralizing antibody in large scale, and thus the method has the significant application value in developing viral vaccines and detecting the level of the CV-B5 specific neutralizing antibody of individual and group patients.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Nucleic acid composition for detecting four enteroviruses simultaneously and kit and detection method
InactiveCN109504805AMicrobiological testing/measurementMicroorganism based processesCoxsackievirusMicrobiology
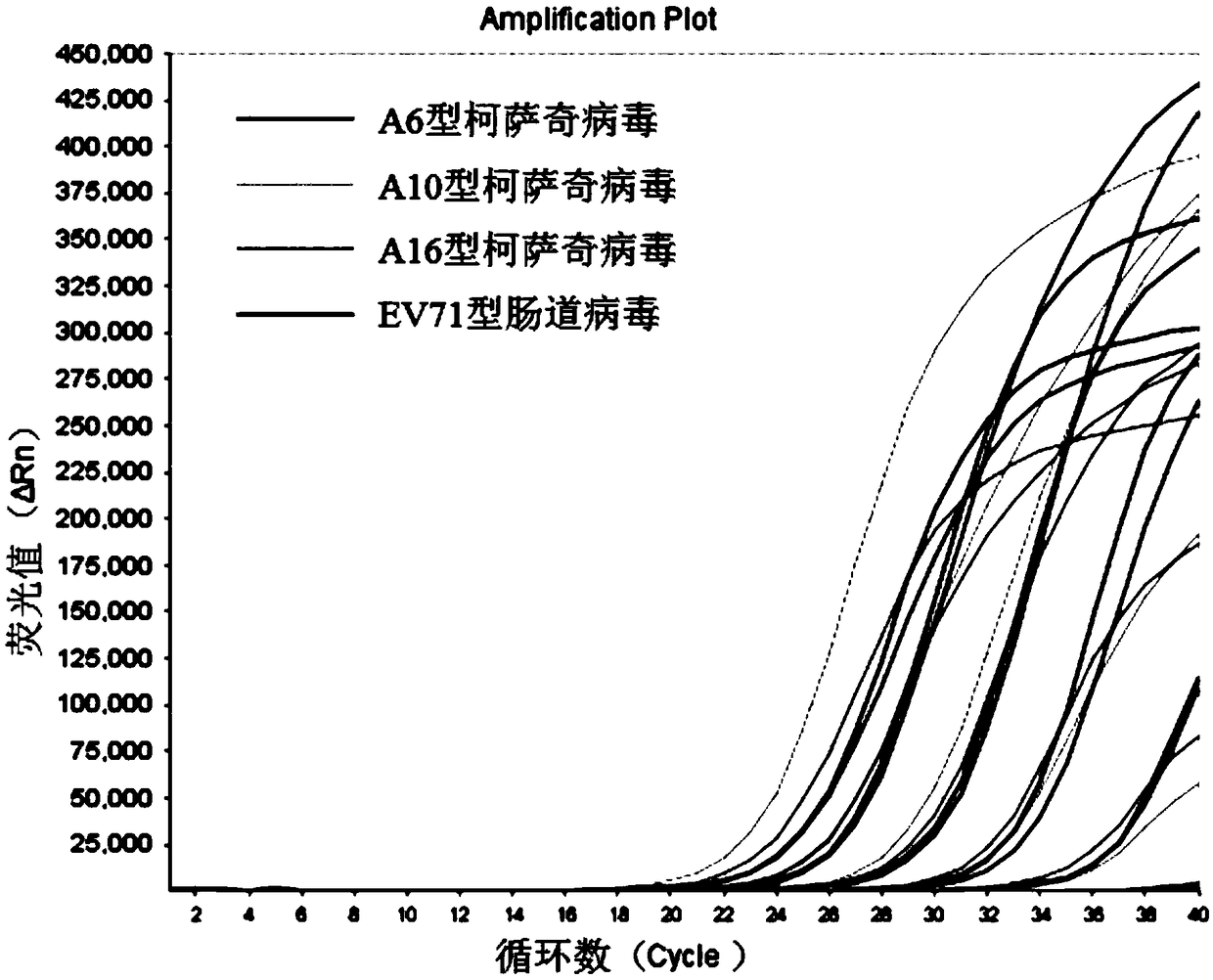
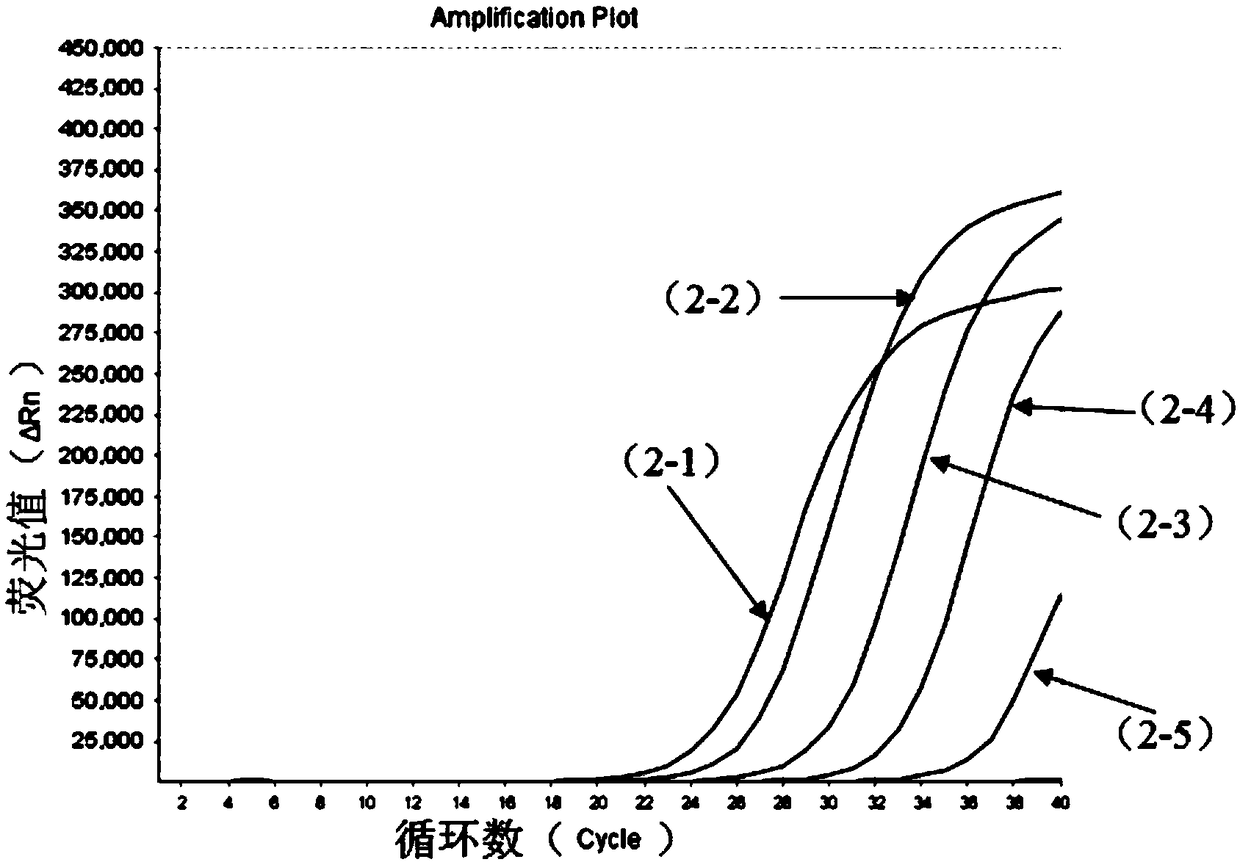
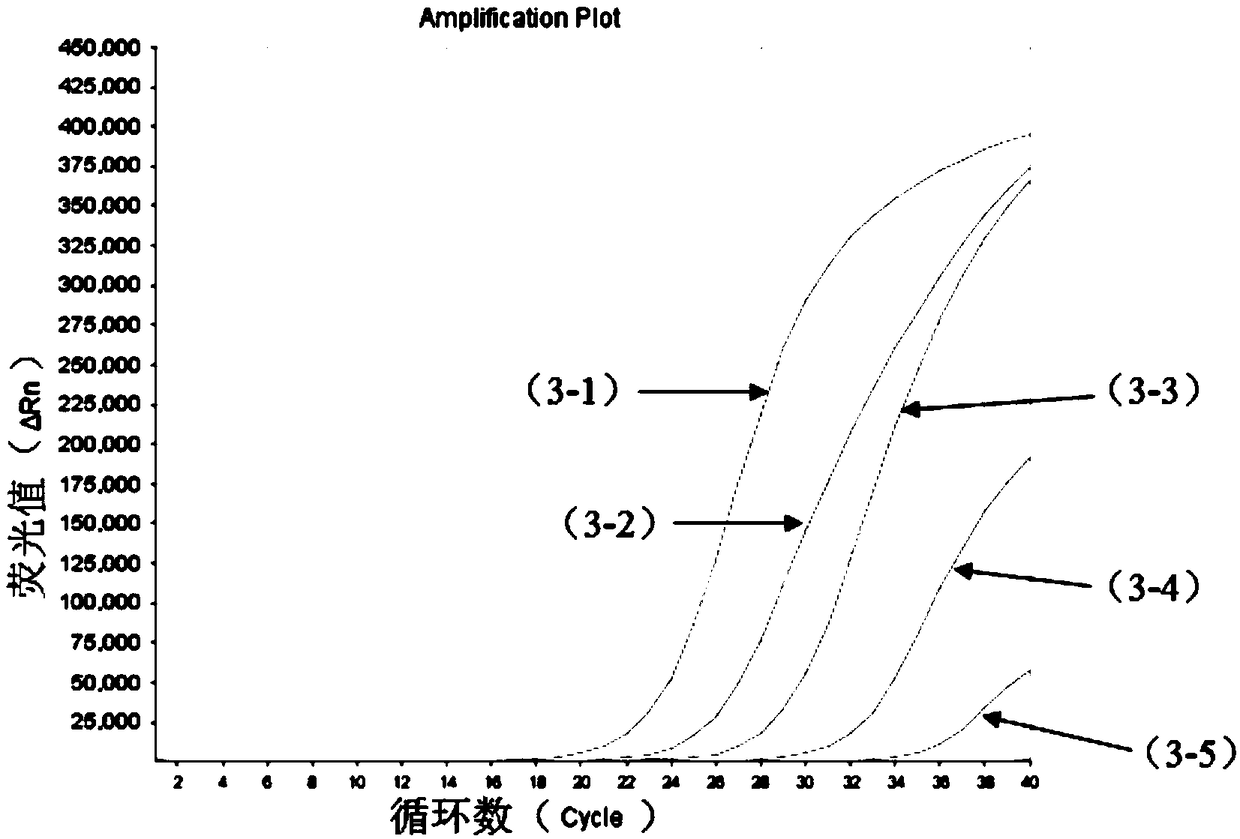
The invention relates to a nucleic acid composition for detecting four enteroviruses simultaneously and a kit and a detection method. The nucleic acid composition comprises type A6 coxsackievirus amplification primer pairs as shown in SEQ ID No.1 and SEQ ID No.2, type A10 coxsackievirus amplification primer pairs as shown in SEQ ID No.3 and SEQ ID No.4, type A16 coxsackievirus amplification primerpairs as shown in SEQ ID No.5 and SEQ ID No.6 and type EV71 enterovirus amplification primer pairs as shown in SEQ ID No.7 and SEQ ID No.8. The nucleic acid composition can detect type A6, type A10 and type A16 coxsackieviruses and type EV71 enterovirus simultaneously.
Owner:深圳市艾伟迪生物科技有限公司
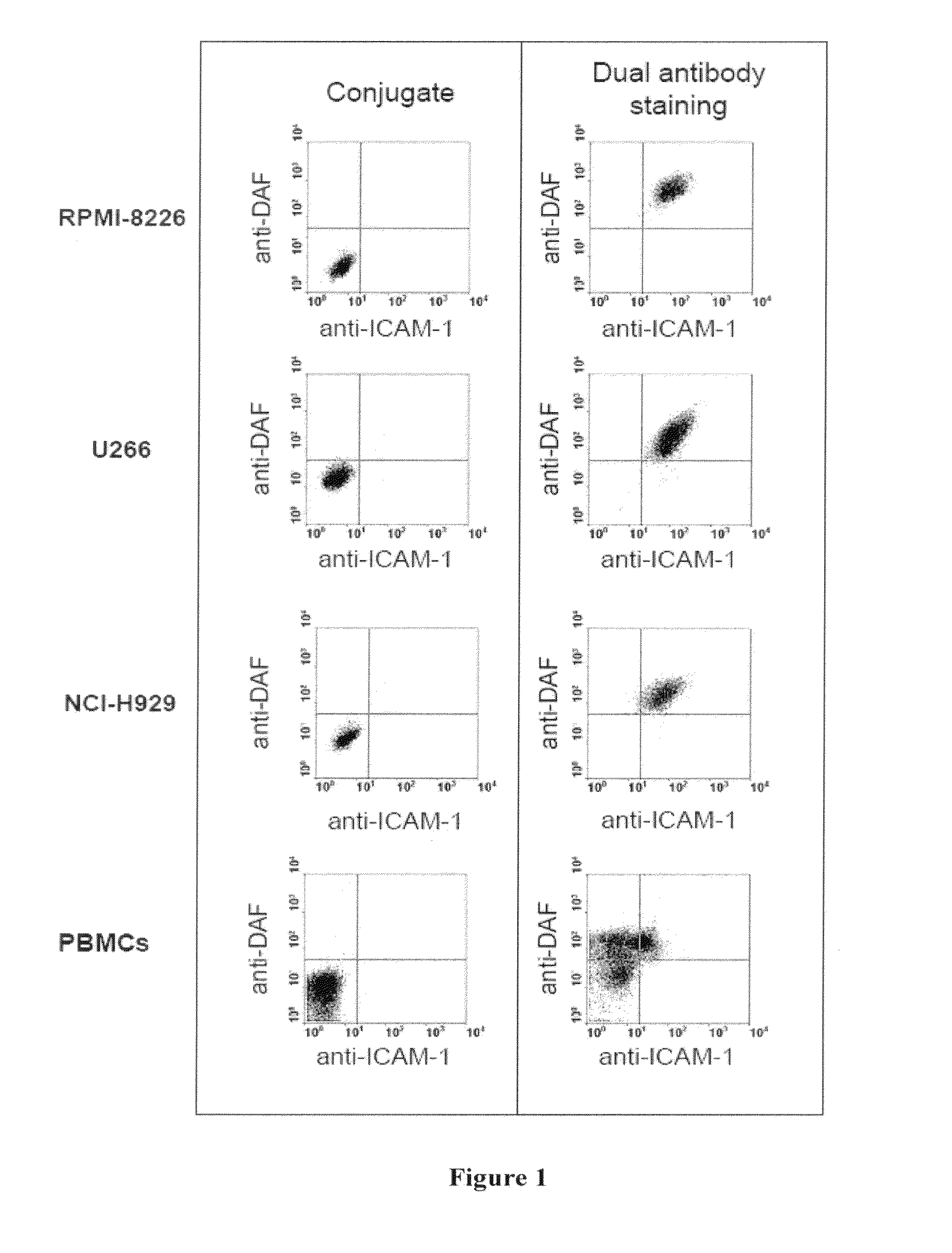
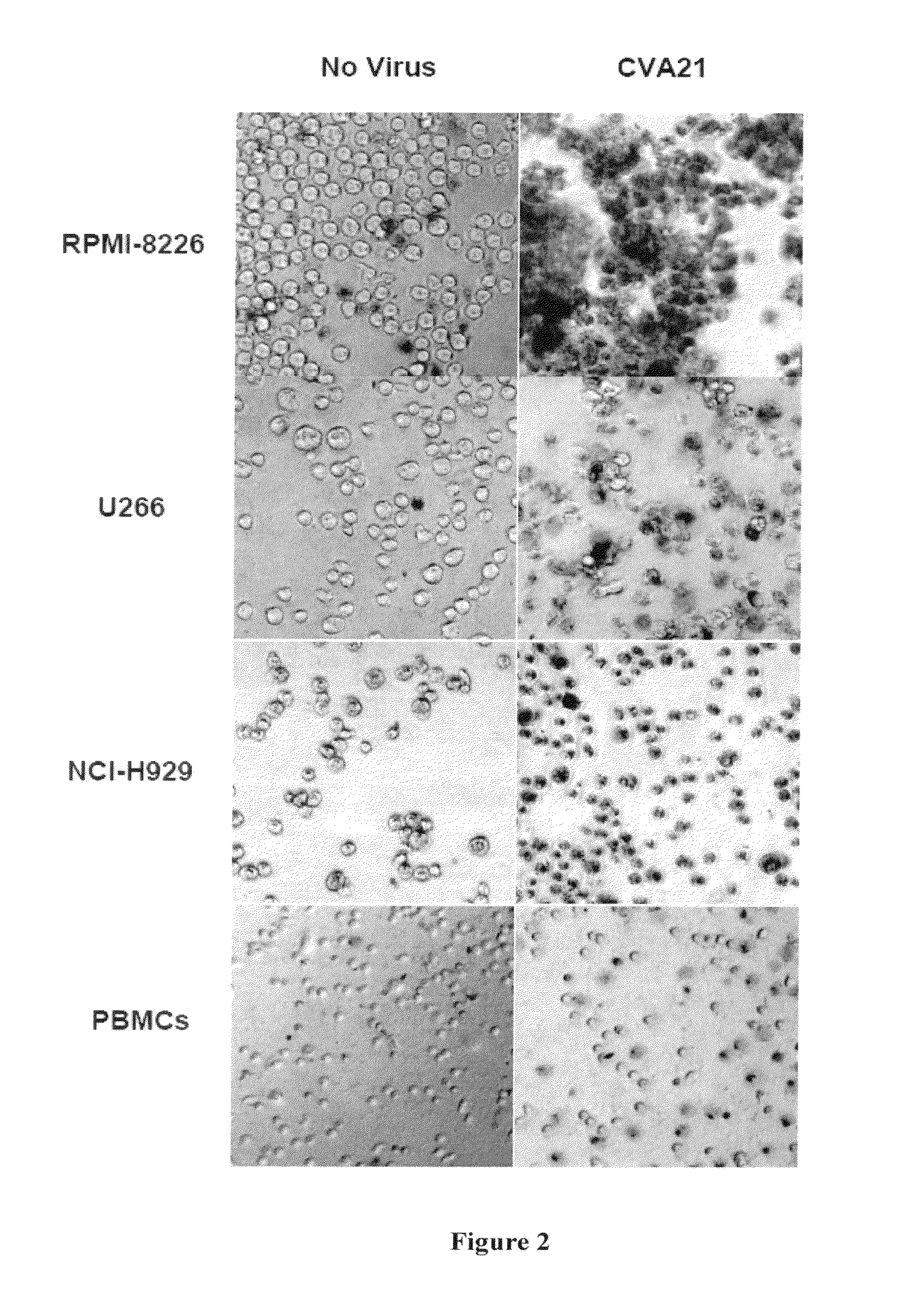
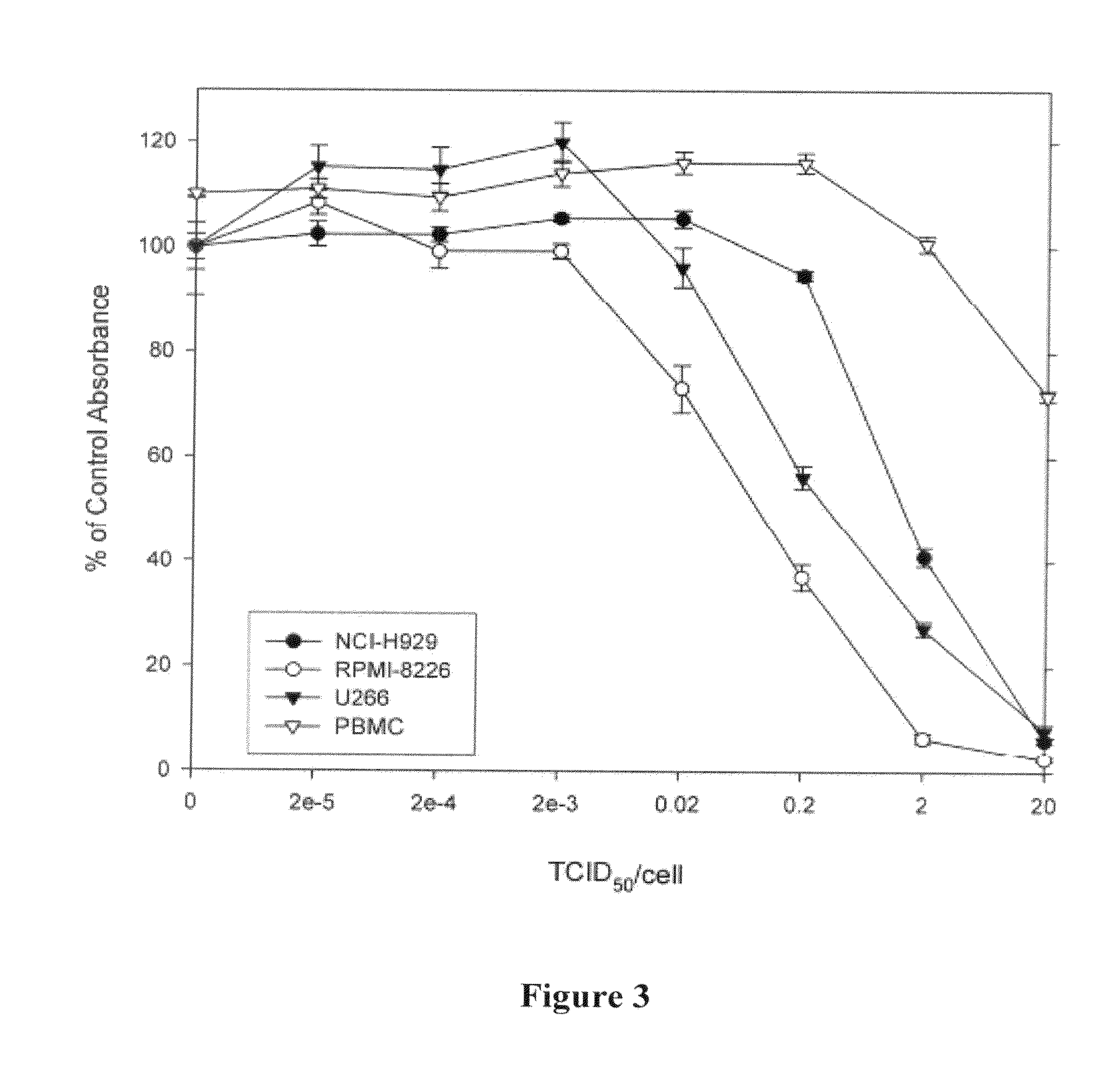
Methods and compositions for treatment of hematologic cancers
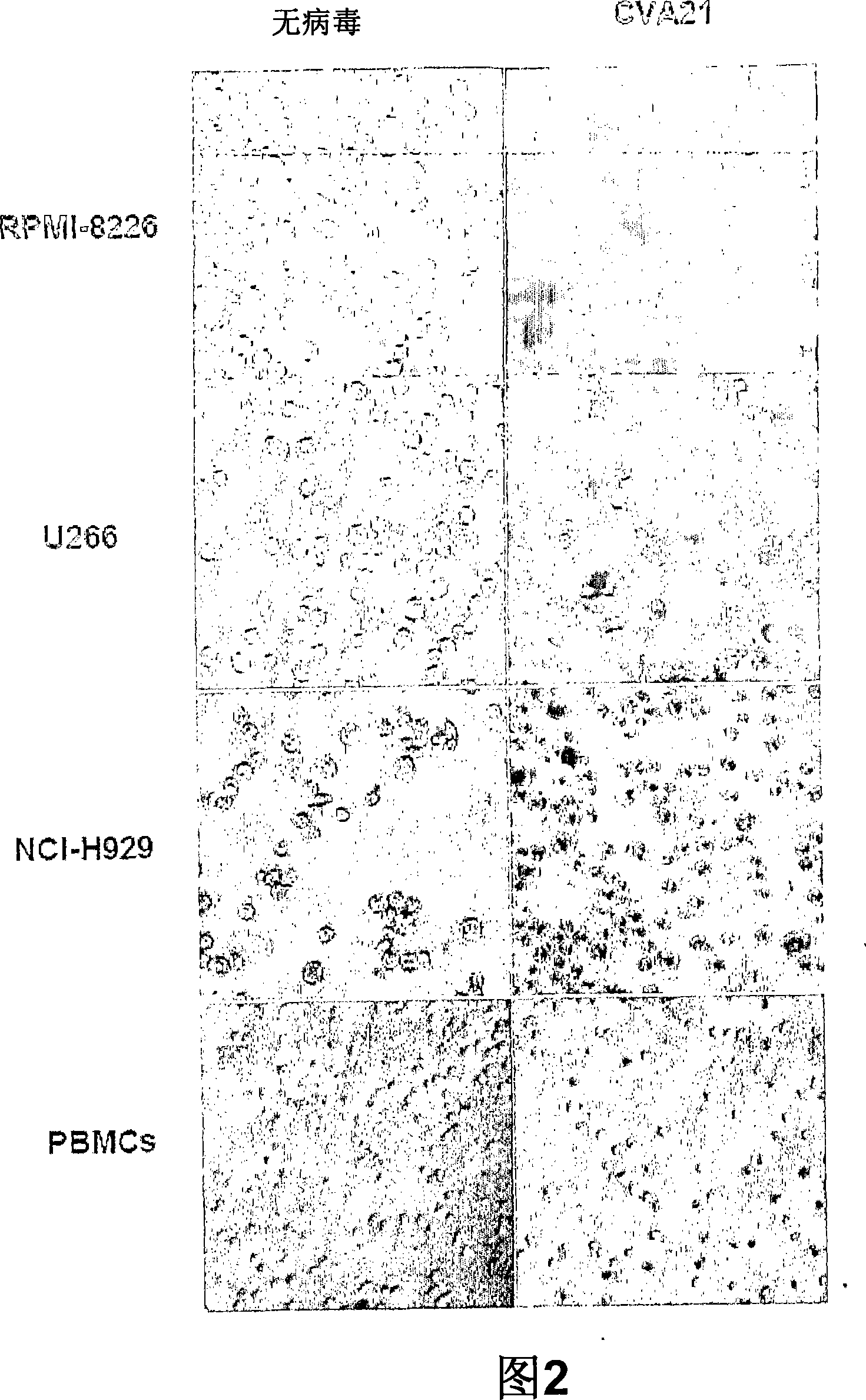
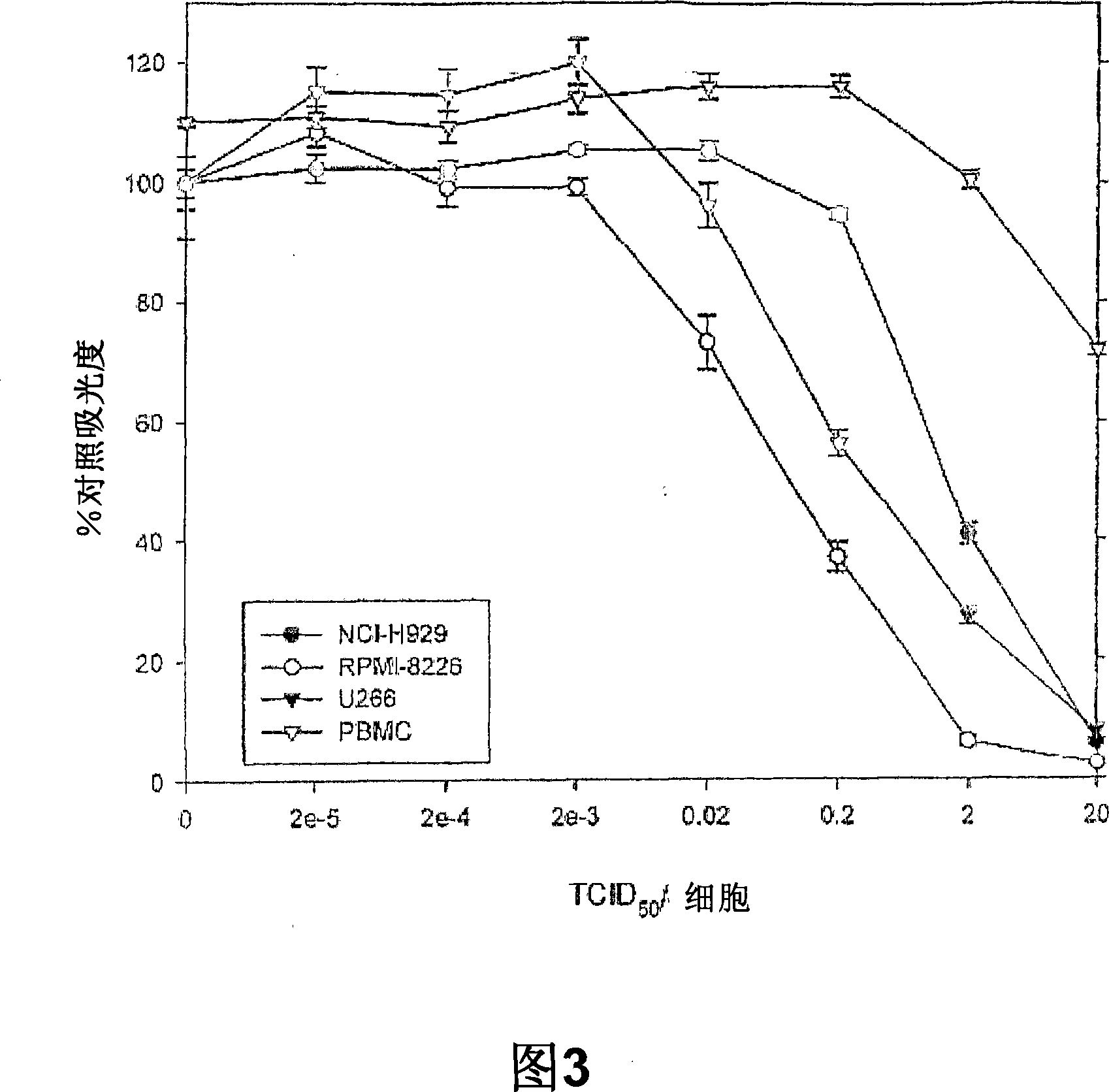
The present invention relates to oncolytic picornaviruses and methods and compositions for treating subjects having hematologic cancers. These include methods and compositions for treatment of myeloma, using disclosed Picornavirus such as Coxsackievirus, in methods of direct or indirect administration to subjects and ex vivo purging of malignant cells within auto grafts prior to transplantation.
Owner:MERCK & CO INC
Coxsackievirus B5 CV-B5 and application thereof in preparing infectious animal models and kits
PendingCN107236713AEffective infectionSsRNA viruses positive-senseViral antigen ingredientsCoxsackieviruses BNeural cell
The invention relates to Coxsackievirus B5 CV-B5 CVB5 / JS417 capable of being stably copied and abdicated. The preservation number of the virus is CGMCC (China general microbiological culture collection center) No. 13851. The invention further relates to an application of Coxsackievirus B5 CV-B5 the CVB5 / JS417 in preparing a Coxsackievirus B5 CV-B5 infected mouse. The experimental results show that obvious eosinophilic necrosis and vacuolar degeneration necrosis in spots of the brain, spinal cord, cardiac muscle and skeletal muscle of hind limbs of the Coxsackievirus B5 CV-B5 infected mouse can be seen, and meanwhile, immunohistochemistry shows that positive or strong positive reaction appears in brain and spinal neural cells and cardiac muscle and skeletal muscle cells of the hind limbs, which verifies that the CVB5 / JS417 can cause obvious lesions and necrosis to the brain, spinal nerves, cardiac muscle and skeletal muscle of the hind limbs of the infected mouse.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Fusion protein and fusion protein expression vector thereof
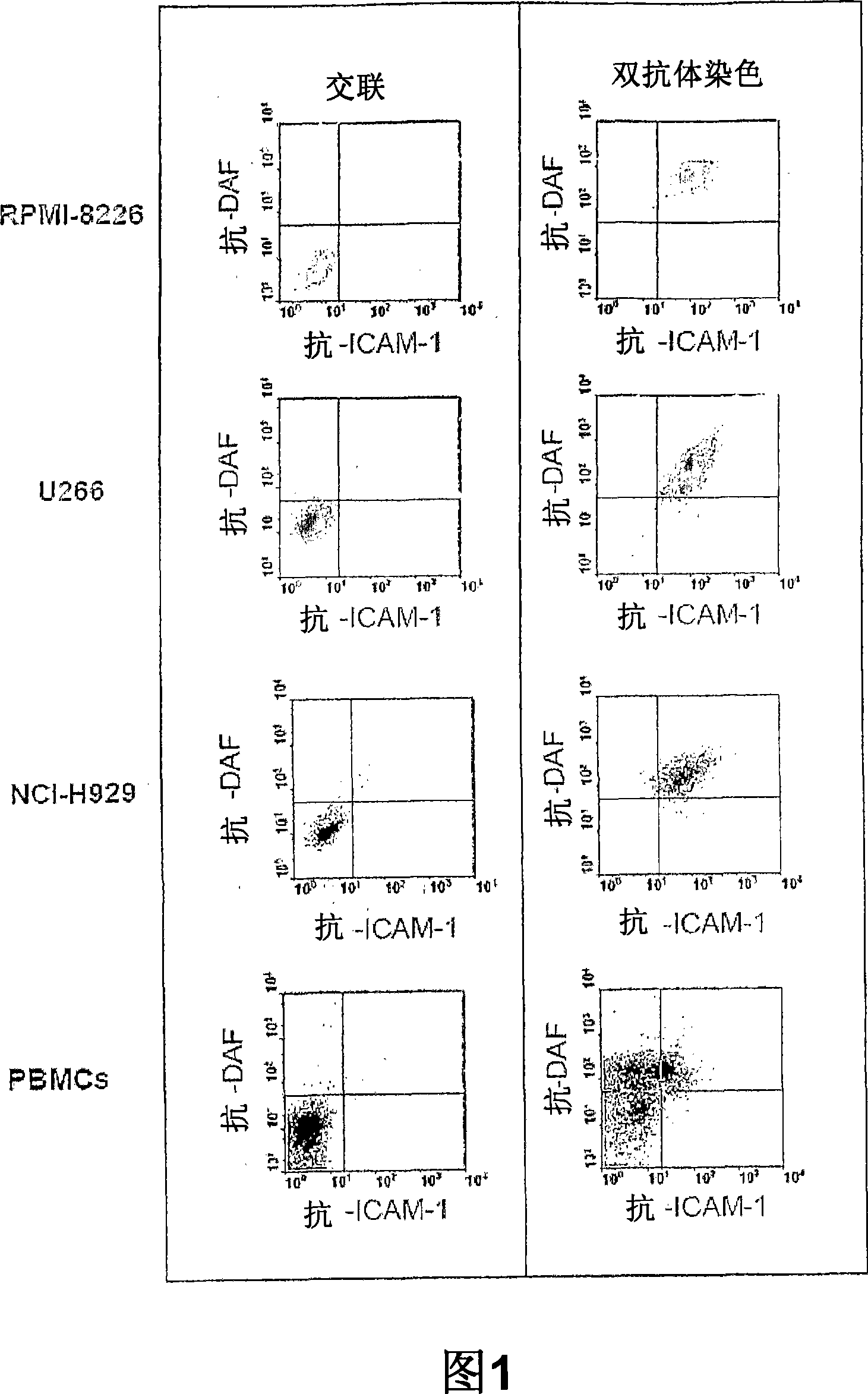
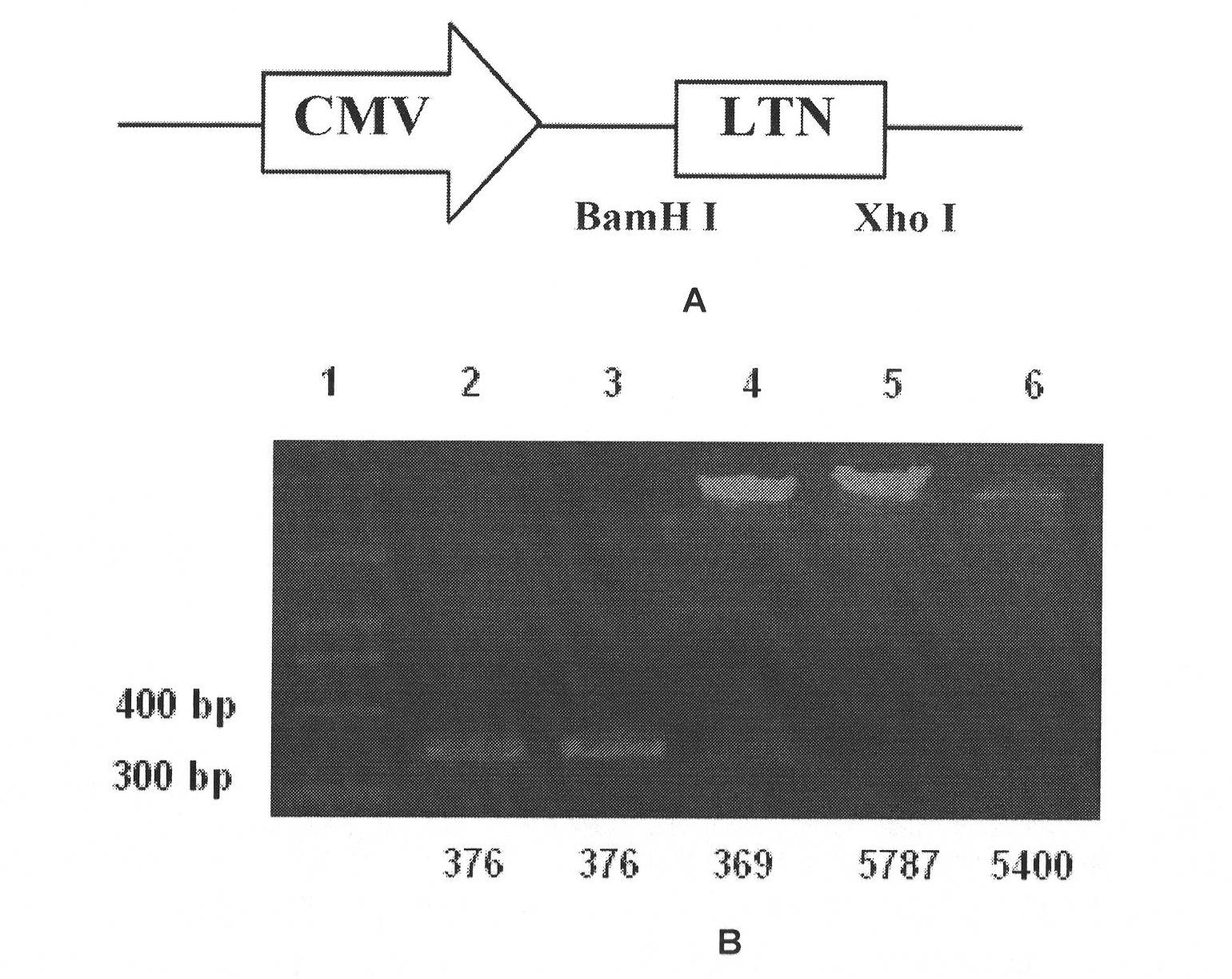
ActiveCN102816240AImprove bindingAccelerated phagocytosisFungiPeptide/protein ingredientsFusion Protein ExpressionCoxsackie meningitis
The invention provides a fusion protein and a fusion protein expression vector thereof. The fusion protein comprises human-derived Coxsackie virus-adenovirus receptor extracellular region and Fc fragment of human IgG1. The gene sequence of the fusion protein is subjected to codon optimization. The fusion protein expression vector comprises an optimized gene sequence of the fusion protein, and a pPIC3.5K plasmid gene sequence. The fusion protein can be combined with high affinity with Coxsackie virus / adenovirus, and can prevent the Coxsackie virus / adenovirus from infecting body cells especially cells expressing CAR, such as myocardial cells. The fusion protein provided by the invention can be used in treatments of Coxsackie virus and / or adenovirus infectious diseases.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Method for detecting neutralizing antibody in coxsackievirus A6 (CV-A6), and recombinant virus applied in method
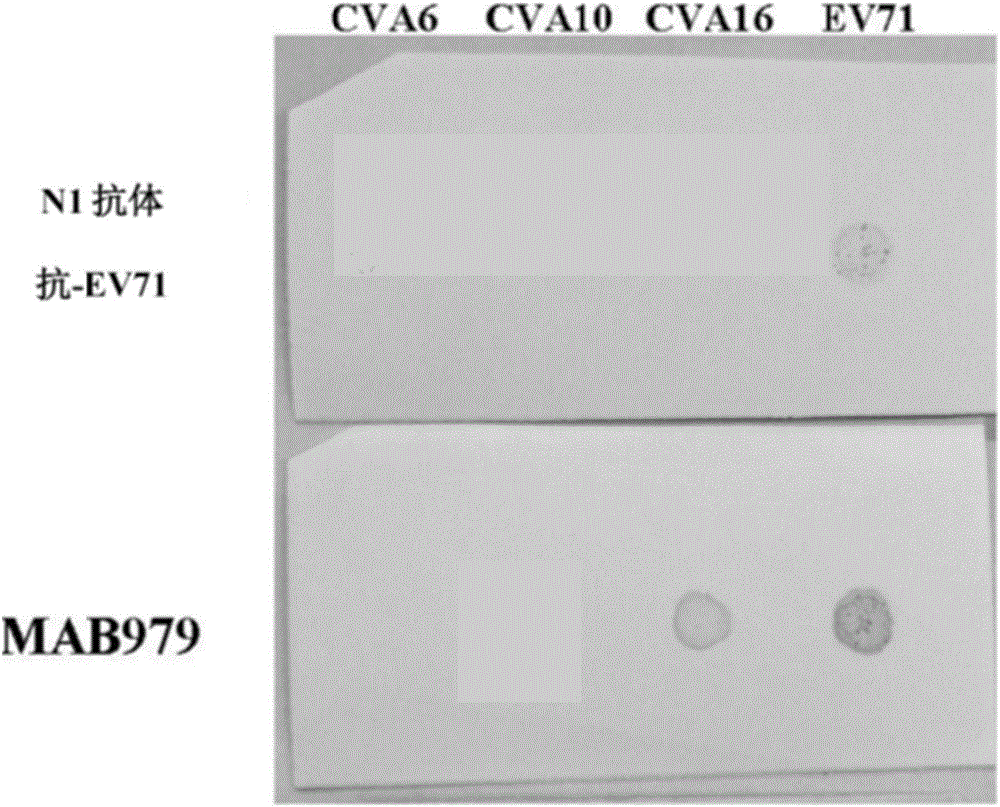
ActiveCN108060172ALower requirementGood linear relationshipSsRNA viruses positive-senseVector-based foreign material introductionViral VaccineNeutralizing antibody
The invention discloses a method for detecting a neutralizing antibody in coxsackievirus A6 (CV-A6), and a recombinant virus applied in the method. According to the method, the neutralizing antibody is detected by using a pseudovirus system packaged by two types of recombinant plasmids; by adopting a single-cycle infected pseudovirus, the safety problem caused by use of live viruses is avoided. The results of a plurality of tests prove that the pseudovirus detection system is a CV-A6 neutralizing antibody detection method which is safe, sensitive, rapid, specific, simple and convenient, and low in cost. Based on the characteristics, the pseudovirus detection system is very suitable for the tests for rapid and large-scale detection of the neutralizing antibody, and has an import applicationvalue for development of a CV-A6 viral vaccine and detection of CV-A6 specific neutralizing antibody level of patients and populations.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Primer and kit for detecting coxsackievirus A6 type RT-LAMP (Reverse Transcription Loop-mediated Isothermal Amplification) nucleic acid
InactiveCN102816870ALow costStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceHand-foot-and-mouth diseaseSocial benefits
The invention relates to the application field of biological detection technologies, and in particular relates to a primer pair and a kit for coxsackievirus A6 type RT-LAMP (Reverse Transcription Loop-mediated Isothermal Amplification) nucleic acid detection. The primer pair comprises two outer primers F3 and B3, two inner primers FIP and BIP and two loop primers LF and LB; and the kit comprises the primer pair. The CA6RT-LAMP has the characteristics of strong specificity, high sensitivity, simplicity in operation methods, quick detection, easiness in result judgment, low cost and the like, can better satisfy the requirement of on-site quick detection, is easy to popularize and apply in grassroots units, can be used for early rapid diagnosis of epidemic outbreaks, such as hand-foot-and-mouth diseases and the like and clinical cases in disease prevention and control organizations, hospitals, kindergarten units, and has broad market prospect and enormous economic and social benefits.
Owner:何雅青 +2
Mucosal adjuvant and its preparation method and use
InactiveCN102688488ASafe and non-toxicReduce releaseAntibacterial agentsGenetic material ingredientsNasal cavityVaccination
The invention provides a mucosal adjuvant. The mucosal adjuvant is prepared by copolymerization crosslinking compounding of oligo-chitosan and lymphotactin encoding plasmids. The invention also discloses a preparation method and a use of the mucosal adjuvant. When the mucosal adjuvant and a gene vaccine composed of chitosan or oligo-chitosan are synchronously dropped into a nasal cavity for immunization, coxsackievirus specific serum antibody and systemic (spleen and lymph glands) Th1-type immune responses are induced and especially, intestinal mucosa partially-reinforced specific-secretion-type sIgA and IFNgama+Th1 responses are induced, and thus a gene vaccine used with the mucosal adjuvant is obviously superior to an exposed gene vaccine and effectively prevents coxsackievirus-caused myocarditis. The mucosal adjuvant can be used as a novel mucosal vaccination adjuvant.
Owner:FUDAN UNIV
Preparation method of coxsackievirus antigen and rapid detection kit prepared by utilizing antigen and used for detecting coxsackievirus antibody
InactiveCN106046124AHigh antigen yieldImproving immunogenicitySsRNA viruses positive-senseVirus peptidesEscherichia coliInclusion bodies
The invention relates to an artificial genetic engineering-expressed coxsackievirus antigen, a method for preparing the antigen, a method for rapidly detecting a coxsackievirus antibody and a rapid detection kit used for detecting the coxsackievirus antibody. The method for preparing the antigen comprises the following steps: artificially synthesizing optimized coxsackievirus VP1 protein antigen gene sequences, constructing a prokaryotic expression vector and an escherichia coli-expressed coxsackievirus VP1 protein antigen, and renaturing an inclusion body by adopting a dialysis method, a gradient dilution method and a gel chromatography to obtain a recombinant coxsackievirus VP1 protein antigen with a three-dimensional structure and immunocompetence. The method for rapidly detecting the coxsackievirus antibody comprises the step of applying the coxsackievirus VP1 protein antigen. The rapid detection kit used for detecting the coxsackievirus antibody comprises the coxsackievirus VP1 protein antigen which can be directly used for whole blood detection. The kit comprises a rheumatoid factor processing pad which can remove rheumatoid factors in a sample and directly detect IgM in the sample. The coxsackievirus antigen provided by the invention has high specificity. The invention provides the method for preparing the antigen, the method for rapidly detecting the coxsackievirus antibody and the kit for rapidly detecting the coxsackievirus antibody.
Owner:LANZHOU YAHUA BIOTECH
Genetically engineered vaccine against both Enterovirus 71 and Coxsackie virus A16
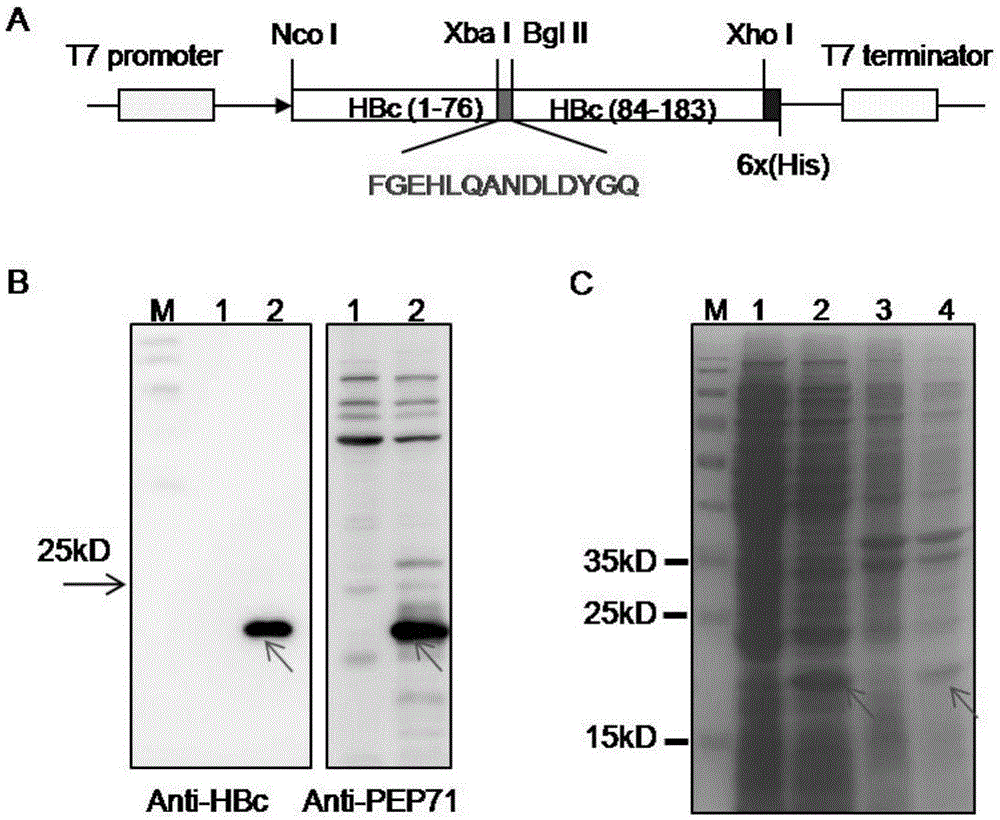
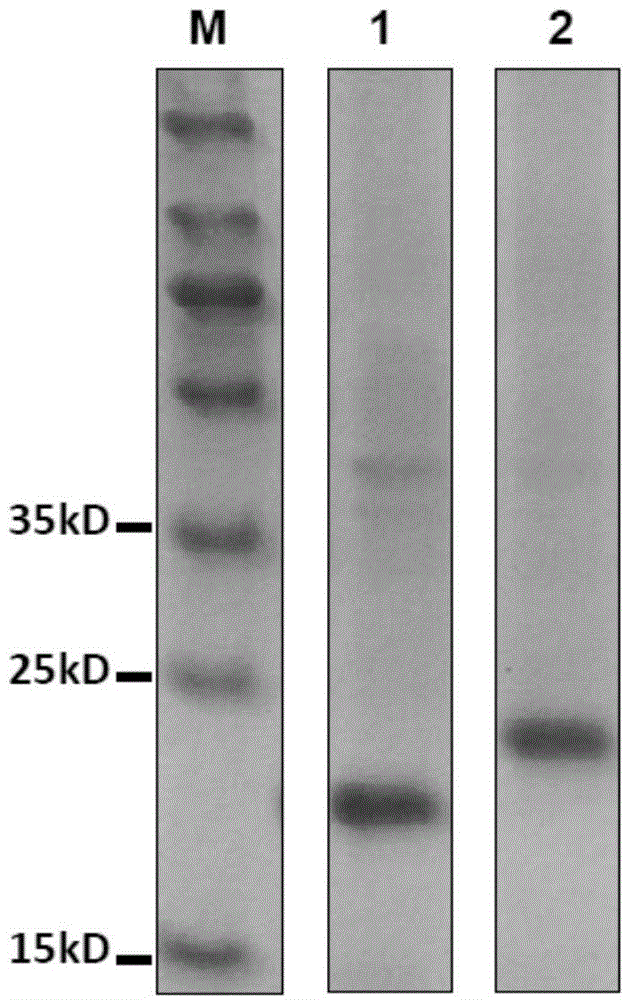
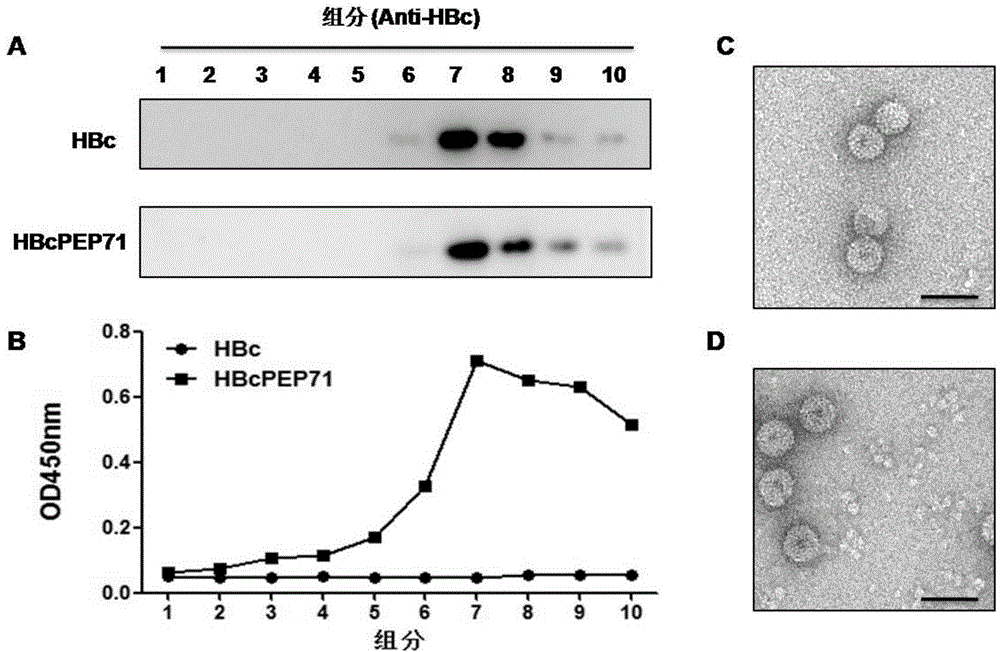
The invention provides an epitope peptide. The epitope peptide is originated from the neutralizing epitope PEP71 of the Coxsackie virus and has a length of 5 to 15 amino acids; and recombinant protein formed by fusion between the epitope peptide and carrier protein can induce immunoreaction of a mammal to the epitope peptid. Experiments prove that the fusion protein including the epitope peptide can be used as a genetically engineered vaccine against both EV 71 and CA16.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Coxsackievirus for treating tumor
ActiveCN109568350AEnhance killing activityHigh activitySsRNA viruses positive-senseVirus peptidesCoxsackieviruses BWilms' tumor
The invention relates to the field of viruses and the field of tumor treatment, in particular to to coxsackievirus B3 (CVB3) and a modification form thereof, a genomic sequence or a cDNA sequence containing the CVB3 or the modification form of the CVB3, or a nucleic acid molecule with a complementary sequence of the genomic sequence or the cDNA sequence, application to treat a tumor of a subject (for example, a human), and application to prepare a pharmaceutical composition for treating the tumor of the subject (such as the human). The invention further relates to a method for treating the tumor, and the method comprises the step that the CVB3 or the modification form of the CVB3, or the genomic sequence or the cDNA sequence containing the CVB3 or the modification form of the CVB3, or thenucleic acid molecular of the complementary sequence of the genomic sequence or the cDNA sequence are applied to the subject needing tumor treating.
Owner:XIAMEN UNIV +1
Methods and compositions for treatment of hematologic cancers
The present invention relates to oncolytic Picornaviruses and methods and compositions for treating subjects having hematologic cancers. These include methods and compositions for treatment of myeloma, using disclosed Picornavirus such as Coxsackievirus, in methods of direct or indirect administration to subjects and ex vivo purging of malignant cells within auto grafts prior to transplantation.
Owner:MERCK SHARP & DOHME LLC
Detection method of coxsackievirus A10 in mixed infection of hand-foot-mouth disease
InactiveCN107815510ALow costShorten the timeMicrobiological testing/measurementHand-foot-and-mouth diseaseAgricultural science
The invention discloses a detection method of coxsackievirus A10 (CV-A10) in mixed infection of hand-foot-mouth disease. The method is as follows: extracting viral RNA, using enterovirus universal primer 224 and 222 for RT-PCR amplification, then using primers AN89 and AN88 for nested PCR amplification, if the virus is non-CV-A10 serotype after comparison, designing specific CV-A10 upstream primerCVA10TF and downstream primer CVA10TR according to the CV-A10 sequence, using the RT-PCR product as a template for nested PCR amplification, and taking the PCR product for 1% agarose gel electrophoresis to identify. The method has the advantages of simple operation, rapid detection, high accuracy, low cost, and the like, and is particularly suitable for large-scale molecular epidemiological investigation.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com