Genetically engineered vaccine against both Enterovirus 71 and Coxsackie virus A16
An amino acid, antigen epitope technology, applied in the field of biomedicine, can solve the problem of CA16 without cross-protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1H
[0122] Expression and purification of embodiment 1 HBcPEP71 fusion protein
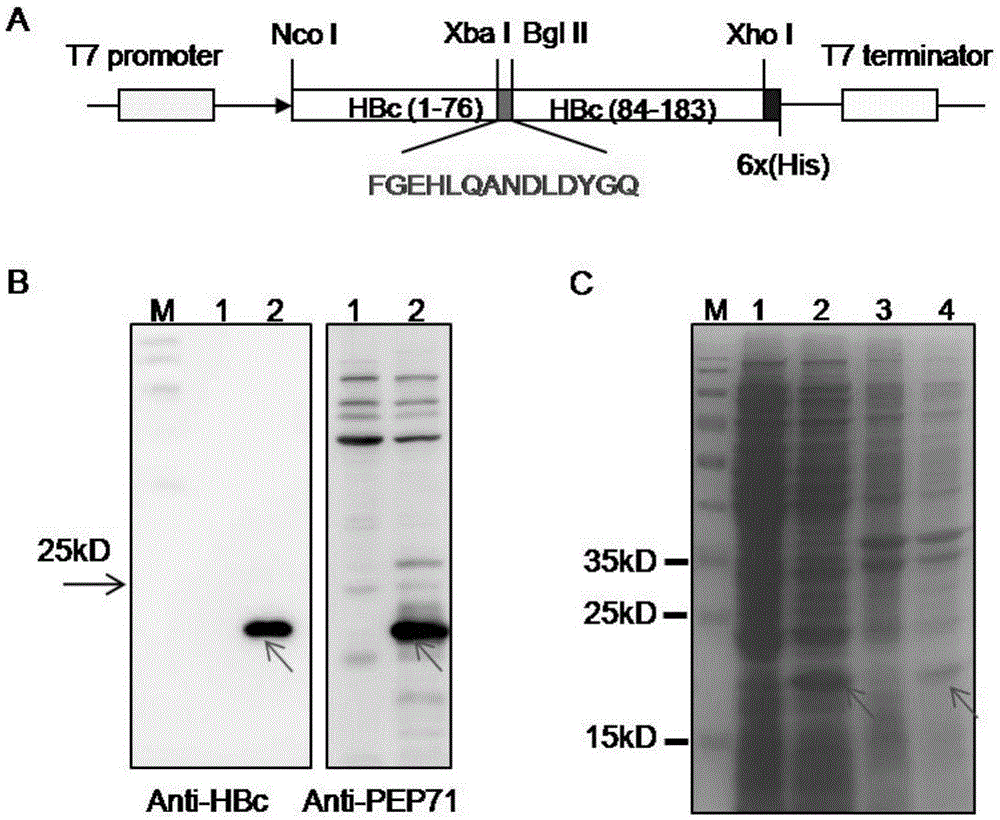
[0123] The 77th-83rd amino acid of HBc molecule is located at the top of its α-helical hairpin structure, which is the part that forms the spike on the surface of HBc virus-like particles, and is also the main immunodominant region (Major ImmunodominantRegion, MIR) of HBc protein. Such as figure 1 As shown in -A, amino acids 77-83 in the MIR region of the HBc molecule were replaced with the neutralizing epitope PEP71 (FGEHLQANDLDYGQ; SEQ ID NO.: 1) of CA16 to obtain a recombinant plasmid expressing the fusion protein HBcPEP71, and the sequence was confirmed to be correct by sequencing.
[0124] The amino acid sequence of Hbc is:
[0125] MDIDPYKEFGATVELLSFLPSDFFPSVRDLLDTASALYREALESPEHCSPHHTALRQAILCWGELMTLATWVGNNLEDPASRDLVVNYVNTNVGLKIRQLLWFHISCLTFGRETVLEYLVSFGVWIRTPPAYRPPNAPILSTLPETTVVRRRDRGRSPRRTPSPPRRRRSPSPRRRRSQSRESQC (SEQ ID NO.: 2)
[0126] The amino acid sequence of HBcPEP71 is:
[0127] MDIDP...
Embodiment 2
[0132] Example 2 Assembly of HBcPEP71 fusion protein into chimeric virus-like particles
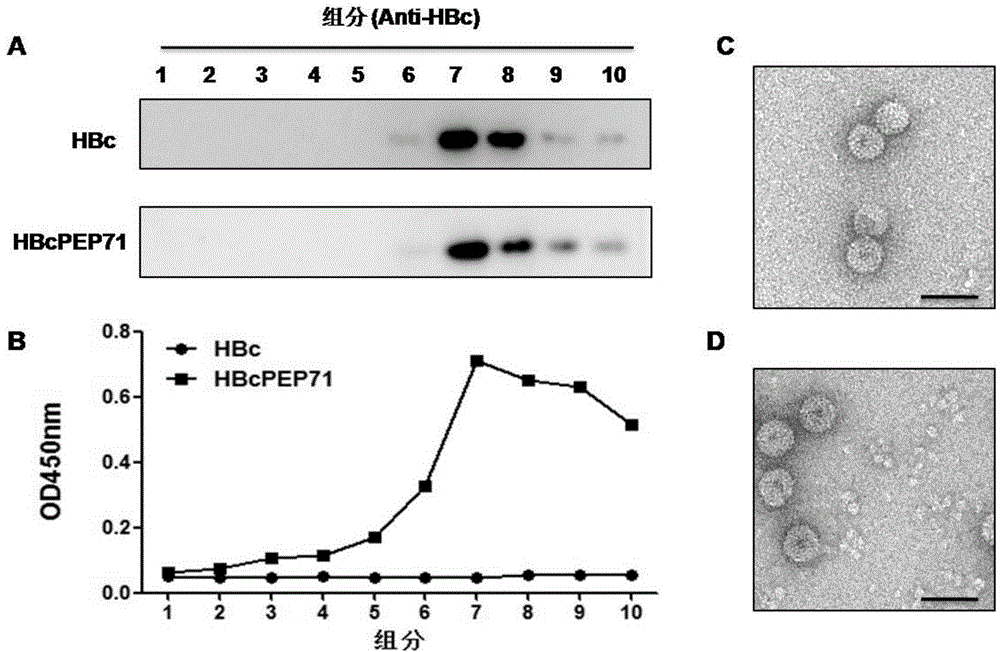
[0133] In order to identify the assembly of HBcPEP71, the purified protein was layered on the top layer of a 10-50% sucrose density gradient for ultracentrifugation, and then 10 fractions were taken from top to bottom for Western Blot detection with HBc-specific monoclonal antibody. Such as image 3 As shown in -A, consistent with unmodified HBc, the HBcPEP71 fusion protein signal has specific signals in the last few components, and the signal of the seventh component is the strongest, indicating that HBcPEP71 exists in the form of particles. In order to detect the display of epitope PEP71 on the surface of HBcPEP71 particles, each component was detected by ELISA with PEP71-specific serum, and it was found that HBcPEP71 could be well recognized by PEP71-specific serum, while HBc could not ( image 3 -B), and consistent with the results of WesternBlot, the signal of the 7th component is t...
Embodiment 3
[0135] Example 3 HBcPEP71 immunized mouse serum can neutralize CA16
[0136] Three groups (6 / group) of 6-8 week-old female ICR mice were used to detect the immunogenicity of chimeric VLPs. The mice in each group were injected intraperitoneally with aluminum adjuvant-adsorbed PBS, HBc or HBcPEP71, in which HBc and PBS The group is the control. The neutralizing activity of the mixed sera of mice in each group two weeks after the fourth immunization was measured against CA16 / SZ05, and it was found that the immune sera of the PBS and HBc groups had no neutralizing activity when diluted 1:8, while the neutralizing activity of the immune sera of the HBcPEP71 group was and a titer of 16 (Table 1). The neutralizing activity of a single mouse serum to CA16 / SZ05 was further determined, and the results showed that the serum of 2 of the 6 HBcPEP71-immunized mice was not neutralized at 1 / 8 dilution, and the neutralizing titer of the other four mice was the lowest 8, up to 64 ( Figure 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com