Nucleic acid construct and method for preparing coxsackievirus A16 virus-like particles
A Coxsackie virus and nucleic acid construct technology, applied in the fields of virology, immunology, and molecular biology, can solve the problems of protein expression and misfolding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1: VP1, VP2, VP3, VP4 tandem soluble co-expression recombinant vector construction
[0124] The full length of the Coxsackie virus type A16 capsid protein (VP1, VP2, VP3 and VP4) gene, which was codon optimized by E. coli, was synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd. (SEQ ID NO: 5-8). And synthesize the primers shown in Table 1 above.
[0125] The full-length fragments of the Coxsackie virus A16 capsid protein (VP1, VP2, VP3, VP4) gene synthesized previously were used as the PCR reaction template. The primer sequences and names used are shown in Table 1 above.
[0126] Coxsackie virus A16-VP1F was used as the forward primer, Coxsackie virus A16-VP1R was used as the reverse primer, and VP1 was used as a PCR template to amplify the Coxsackie virus A16 capsid protein VP1 gene; Saatchi virus A16-VP2F was used as forward primer, Coxsackie virus A16-VP2R was used as reverse primer, and VP2 was used as PCR template to amplify the Coxsackie virus A16 capsid p...
Embodiment 2
[0140] Example 2: Tandem co-expression of Coxsackie virus A16 capsid protein (VP1, VP2, VP3, VP4)
[0141] Transform the pET-Q-CoxA16-VP3124 plasmid prepared in Example 1 into 40 μl of competent E. coli BL21 (DE3) prepared by the calcium chloride method, and spread it on kanamycin-resistant solid LB medium, Incubate at 37°C for 10-12 hours until a single colony is clearly identifiable. Pick a single colony into a test tube containing 4ml of kanamycin-resistant liquid LB medium, culture it with shaking at 230 rpm at 37°C for 12 hours, and take 1 mL of bacterial solution from it and freeze-dry it at -80°C for storage.
[0142] Take out the Escherichia coli strain with recombinant plasmid pET-Q-CoxA16-VP3124 from -80℃, insert 50ml LB liquid medium resistant to kanamycin, 230rpm, 37℃, cultivate for about 12 hours, then transfer Connect to 1L LB liquid medium, express a lot at 37℃, wait for OD 600 After the value reached 0.6, 0.1 mM IPTG was added to induce protein expression, overnig...
Embodiment 3
[0155] Example 3: Affinity color of Coxsackie virus A16 capsid protein (VP1, VP2, VP3, VP4) with SUMO tag Spectrum purification preparation
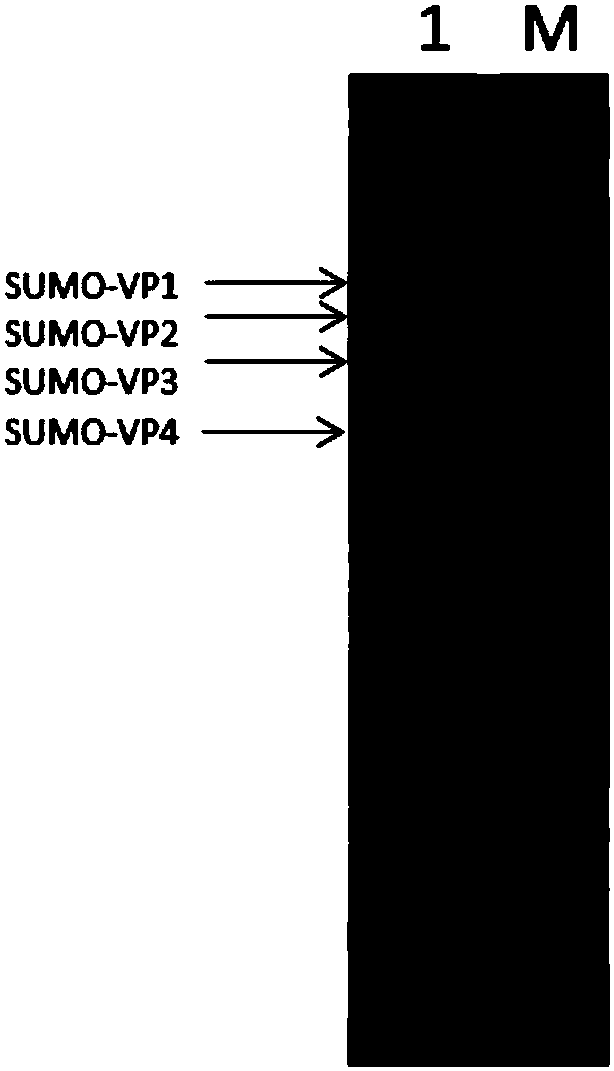
[0156] The bacterial cells prepared in Example 2 were resuspended in a ratio of 1 g bacterial cells to 10 mL lysate (20mM Tris buffer pH 7.2, 300mM NaCl), and the bacterial cells were disrupted 4 times with a homogenizer at a pressure of 700 bar. JA-14 rotor 13500rpm (28000g), centrifugation for 40 minutes, the supernatant was collected, and the supernatant was detected by 15% SDS-polyacrylamide gel electrophoresis. At this time, the SUMO-labeled Coxsackie virus A16 capsid protein ( The purity of SUMO-VP1, SUMO-VP2, SUMO-VP3, SUMO-VP4) is about 10%.
[0157] After the supernatant was filtered with a 0.45 μm pore filter membrane, the XK 26 / 60 column (GE Healthcare Life Sciences) packed with His beads (GE Healthcare Life Sciences) was used for chromatographic purification.
[0158] Instrument system: AKTA purified preparative liquid chromatogr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com