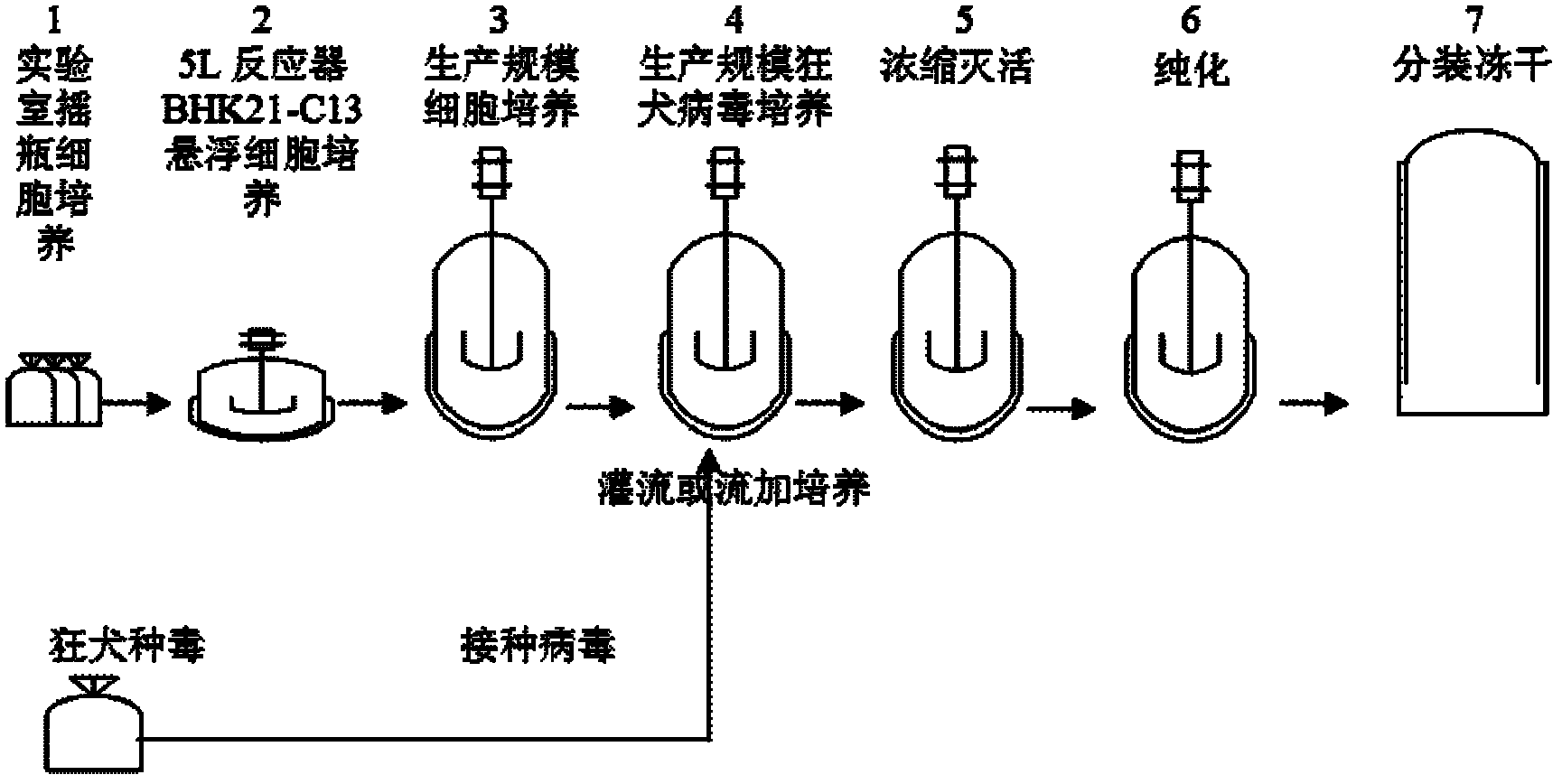
Process for preparing veterinary rabies inactivated and freeze-dried vaccine through suspension culture cell
A suspension culture cell and suspension culture technology, which is applied in the field of suspension culture cells to manufacture veterinary rabies inactivated freeze-dried vaccine technology, can solve the problems of unfavorable animal rabies prevention and control, high cost, and process conditions to be optimized, etc. Titer and antigen yield, easy scale-up of the process, and the effect of process automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 laboratory shake flask culture BHK21-C13 cell
[0019] 1. Seed cell recovery
[0020] Take out a frozen BHK21-C13 suspension cell line from liquid nitrogen, thaw quickly at 37°C, and centrifuge at 1000rpm for 5 minutes. Discard the supernatant, add appropriate amount of MEM nutrient solution (containing 10% newborn bovine serum) to resuspend, transfer to 75cm 2 Fill the cell culture flask with MEM nutrient solution to 30ml, and let it stand at 37°C for adherent culture.
[0021] 2. Cell passage
[0022] Discard the nutrient solution after 48-72 hours of cell culture, add 10ml of digestive solution (0.02% EDTA-0.25% trypsin solution), digest for about 2 minutes, discard the digestive solution, let it stand for 3-5 minutes, and add MEM nutrition Liquid 90ml, divided into 3 new culture bottles, the volume of each bottle is about 30ml, 37 ℃ static culture.
[0023] 3. Cell shake flask culture
[0024] After 48-72 hours of cell adherent culture, discard the...
Embodiment 2
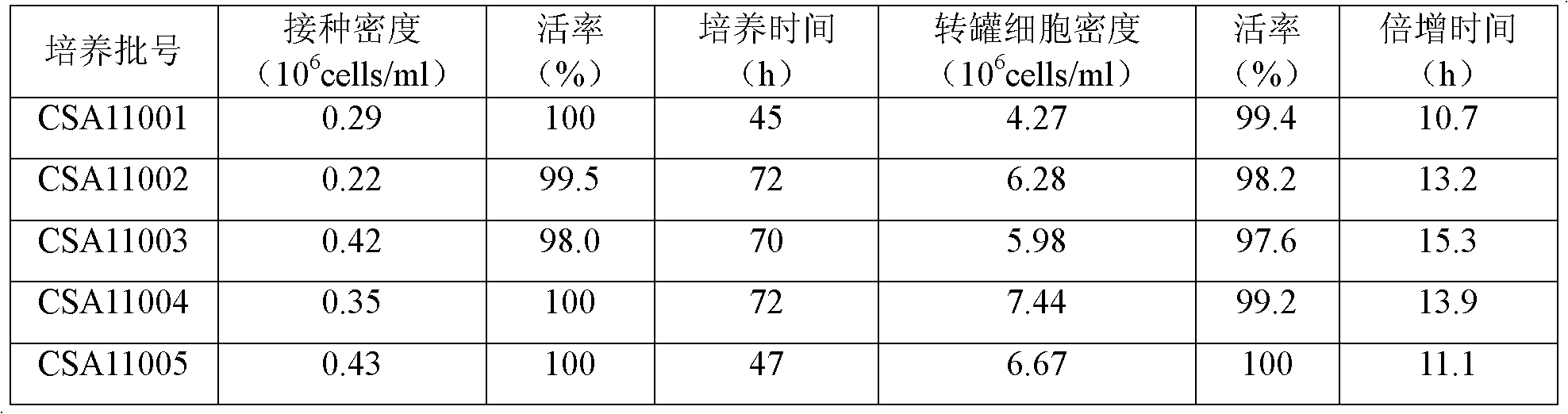
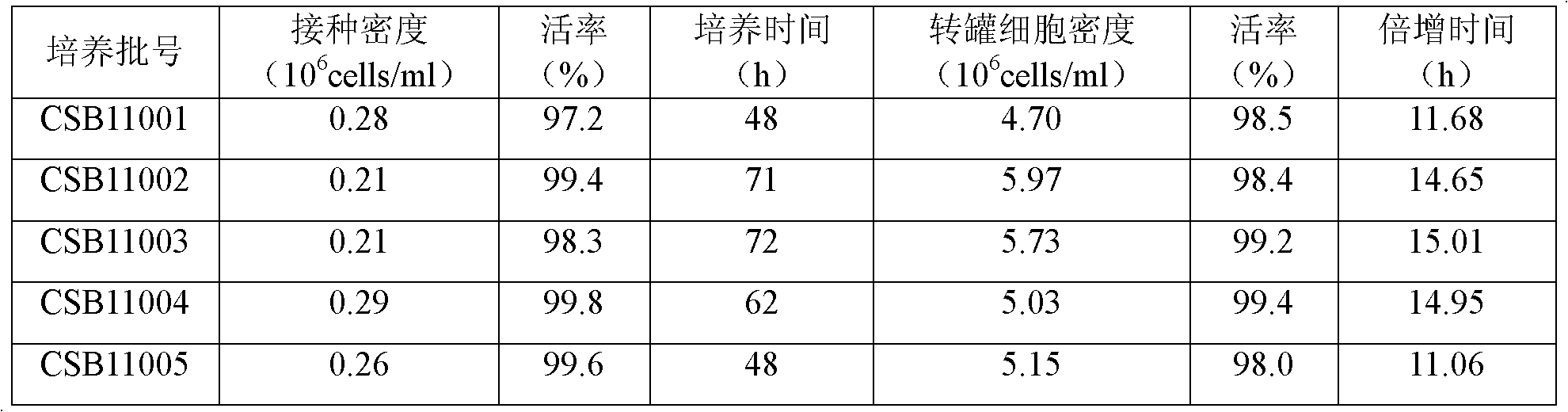
[0025] Example two 5L and production scale reactor BHK21-C13 cell suspension culture
[0026] 1. BHK21-C13 cell suspension culture in 5L reactor
[0027] Cultured shake flask cells (cell density ≥ 2.0×10 6 Cells / ml, activity rate greater than 90%) 800-1000ml mixed in a special inoculation bottle, sampling and counting, sterility test. Transfer the cells in the inoculation bottle to a 5L reactor, add about 4000ml of suspension nutrient solution, and dilute to 0.2-0.5×10 6 Cells / ml, set cell culture temperature: 36.5±0.5°C, pH: 7.20±0.1, DO: 20%-60%, speed varies with cell density, culture volume, stirring method, generally at 60-110rpm . Sampling and counting after 48 hours of cell culture, when the cell density ≥ 2.0×10 6 Transfer the cell liquid to a 50L reactor for culture at the cell / ml, otherwise continue to culture to the desired cell density.
[0028] 2. BHK21-C13 cell suspension culture in 50L reactor
[0029] Add about 45L of suspension nutrient solution to dilut...
Embodiment 3
[0038] Embodiment three production scale rabies virus cultivation
[0039] 1. Cultivation of adherent rabies
[0040] Take well-growing BHK21-C13 adherent cells (175cm 2 Kerry bottle), add about 10ml of digestive juice, digest for about 2 minutes, discard the digestive juice, let it stand for 3-5 minutes, add about 720ml of MEM maintenance solution (containing 1% newborn bovine serum), and insert rabies adherent species Poison 1% (v / v), divided into 8 pieces of 175cm 2 Cultivate statically at 34±1°C in a Kirschner flask; after culturing for 48-72 hours, add about 5-10ml of 7.5% sodium bicarbonate to adjust the pH to 7.4±0.5. Continue to cultivate until 96-120h to harvest the virus liquid, store it in a freezer at -20°C, and take a sample to detect LD 50 , virus content ≥ 10 per 0.03ml 5.5 .
[0041] 2. Suspension Seed Virus Culture
[0042] Get well-grown suspension BHK21-C13 cells in a 5L reactor (the operation method is the same as in Example 2), discard the suspension...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com