Kit for performing direct immunofluorescent detection on enterovirus 71 and coxsackievirus A16 and application thereof
A technology for immunofluorescence detection and coxsackie virus, which is applied in the field of pathogen detection, can solve the problems of easy pollution, long time-consuming, low sensitivity, etc., and achieve the effect of high-efficiency detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of kit
[0044] 1 Cell collection and processing
[0045] 1.1 Cell collection: The kit of the present invention is used to detect EV71 and CA16 virus antigens in the sample cells, and EV71 and CA16 virus infection involves a wide range, therefore, theoretically, any cell sample that can be prepared into a single cell suspension is applicable In the kit of the present invention. Judging from the path of patients infected with EV71 and CA16 viruses and the experience of collecting samples, the collection of nasopharyngeal swabs is easy, non-invasive and fast, and the collection of patients after rinsing is basically free of impurities and does not need to be washed repeatedly. Therefore, the collection of cell samples is the first choice For nasopharyngeal swabs, the embodiments all take nasopharyngeal swabs as an example.
[0046] 1.2 Preparation of single-cell suspension: Put the test sample into a sampling tube containing 3ml PBS or viru...
Embodiment 2
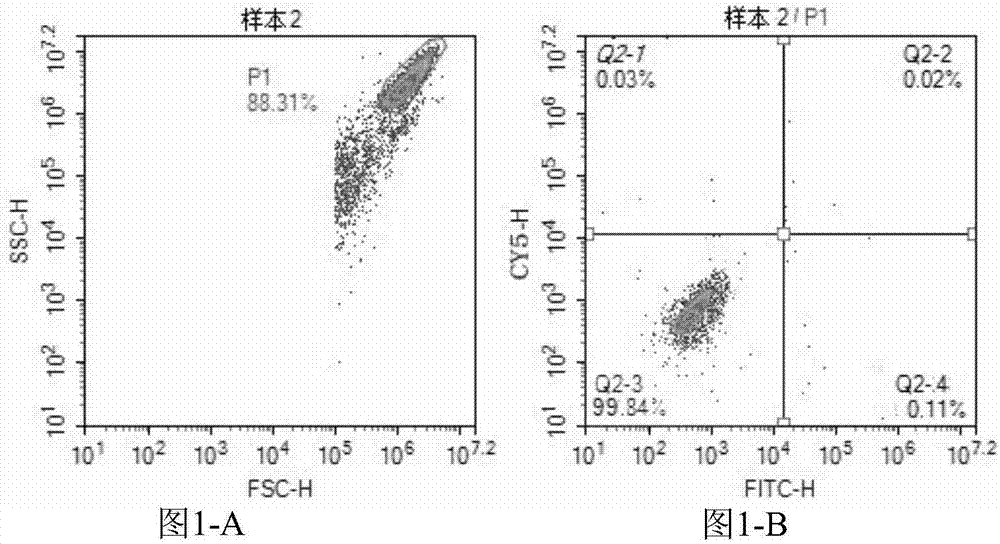
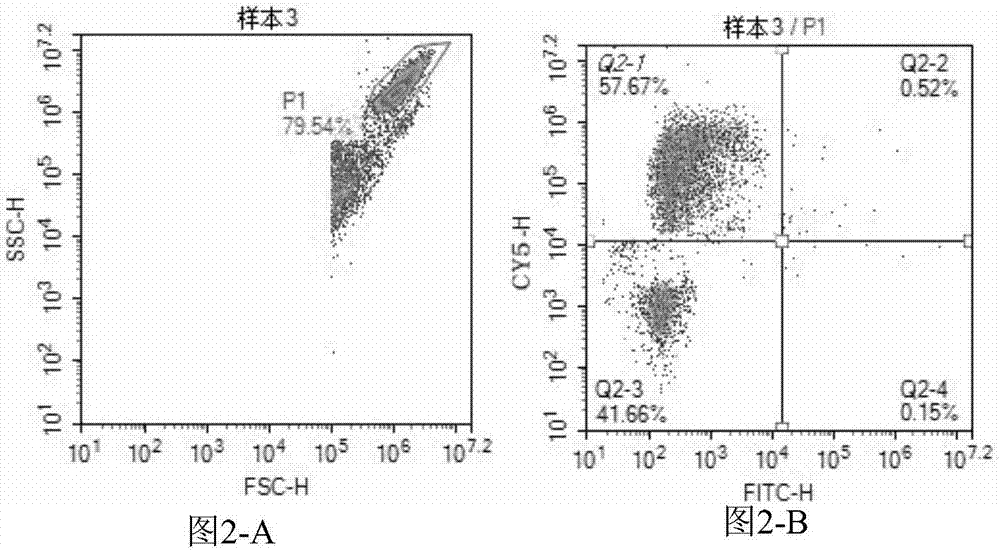
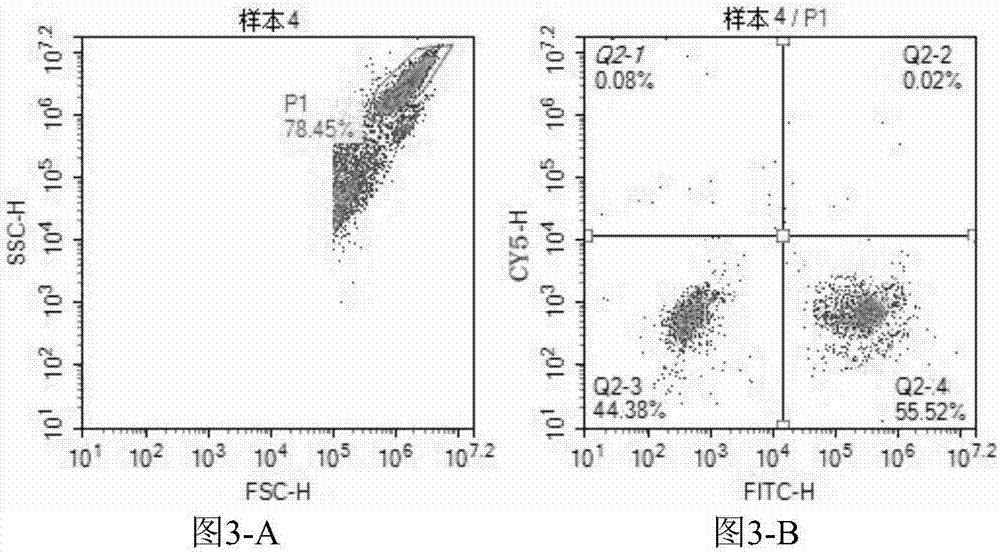
[0057] Embodiment 2: Enterovirus 71 type and Coxsackie virus A16 type detection kit (flow cytometry)
[0058] This embodiment provides a kit for directly detecting Enterovirus 71 and Coxsackievirus A16 by using a flow cytometer. The kit can be used to assist clinical diagnosis of hand, foot and mouth disease.
[0059] 1. The components of the kit:
[0060]
[0061] Note: For the convenience of use, the antibody, preservative, antibody stabilizer and 1% Triton-X100 in the kit were all prepared as staining solution according to the operation method in Example 1, and packed in a brown bottle; is the mass volume ratio (g / 100ml);
[0062] 2. Preparation of reagents in the kit
[0063] 2.1 Composition of PBS and PBS-T
[0064]
[0065]
[0066] with dH 2 Dilute the volume of O to 1L to be the PBS solution, and add 0.05% tween-20 to the PBST solution on this basis;
[0067] 2.2 Cell fixation solution: 4% paraformaldehyde: 4g paraformaldehyde was dissolved in 100ml PBS ...
Embodiment 3
[0084] Embodiment 3: Enterovirus 71 type and Coxsackie virus A16 type detection kit (direct immunofluorescence method)
[0085] This embodiment provides a kit for directly detecting Enterovirus 71 and Coxsackievirus A16 using a fluorescence microscope. The kit can be used to assist clinical diagnosis of hand, foot and mouth disease.
[0086] 1 The kit consists of:
[0087]
[0088]
[0089] Quality Control Slides
[0090] Note: For the convenience of use, the antibodies, preservatives, antibody stabilizers, Evans Blue and 1% Triton-X100 in the kit were all prepared as fluorescent identification reagents according to the operation method in Example 1, and packed in a brown bottle Medium; Percentages are mass volume ratio (g / 100ml);
[0091] 2 Preparation of reagents in the kit
[0092] 2.1 PBS
[0093]
[0094] with dH 2 O dilute to 1L of PBS solution.
[0095] 2.2 Cell fixation solution: Prepare by dissolving 4g of paraformaldehyde in 100ml of PBS solution.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com