Building and evaluation of an animal model infected with a coxsackievirus A10 domesticated strain TA151R-1
A coxsackie virus, a technology for infecting animals, applied in the biological field, can solve the problems of less reported A10 virus strains, affect the efficiency of virus strains, and unstable passage, so as to achieve increased expression levels, high virus titers, and viral loads Increased effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Isolation, Screening and Domestication of Coxsackievirus A10 Type Strain
[0043] The inventors carried out a lot of isolation and screening work in the early stage. In 2015, the Coxsackie virus A10 strain TA151R was isolated from the stool sample of a 4-year-old child in Shandong Province, and the infection was established using this strain. Animal model, prepared the whole virus inactivated vaccine, and studied the immune protection effect of the vaccine ("Protective Efficacies of Formaldehyde-Inactivated Whole-Virus Vaccine and Antivirals in a Murine Model of Coxsackievirus A10 Infection", Zhenjie Zhang et al., "Journal of Virology", Volume 91, 2017, e00333-17). In the follow-up research process, we found that TA151R would have the problems of decreased titer and unstable passage during the passage process, which seriously restricted the application of this strain; in order to solve this problem, we carried out domestication screening for TA151R.
[0044] ...
Embodiment 2
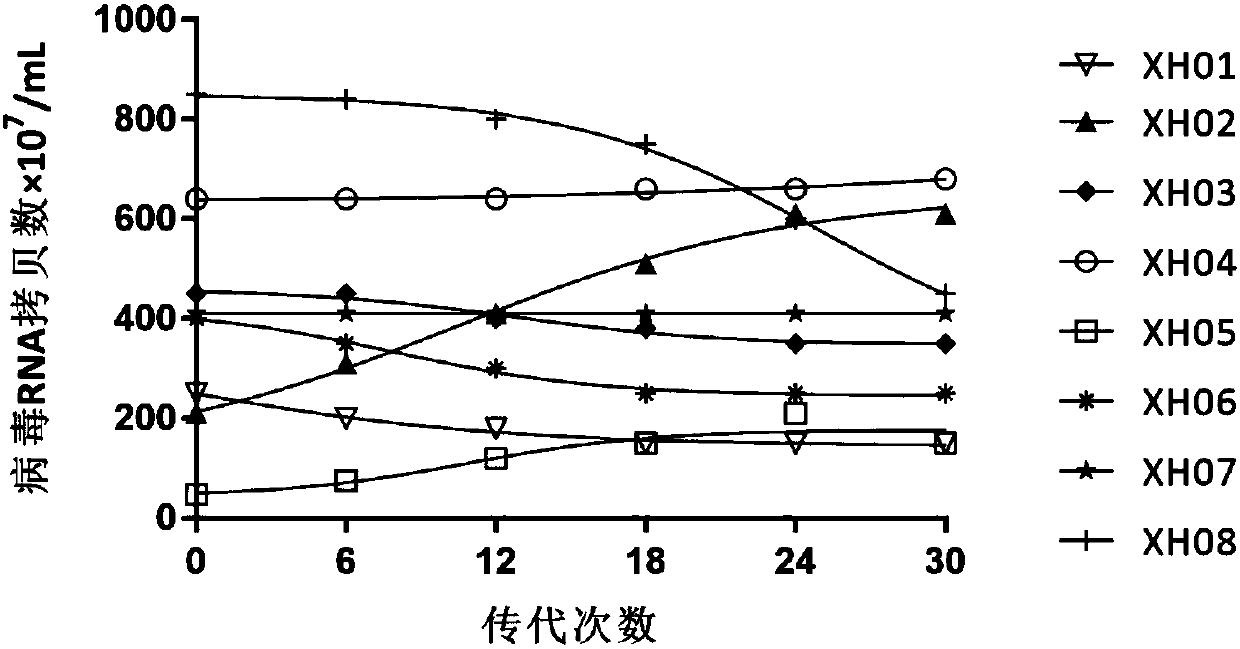
[0047] Example 2 Passage stability of high titer domesticated strain XH01-08
[0048] RD cells were used to continuously subculture the high-titer domesticated strain XH01-08 in Example 1. When the cells showed a cytopathic effect area of more than 80%, they were repeatedly frozen and thawed 3 times, and the first-generation virus liquid was collected and assayed. Virus titer: The harvested first-generation virus was continuously passaged 30 times in the same way, and the virus was collected and titered at each generation, and the virus titer of different generations was compared. like figure 1 As shown, XH01, XH02, XH05, XH06, and XH08 showed different titer reduction or titer instability during the passage process, while XH03, XH04, and XH07 were more stable and could produce high titer of virus.
Embodiment 3
[0049] Example 3 Antiserum Test of Domesticated Strain XH03 / XH04 / XH07
[0050] 0.4% formaldehyde-inactivated TA151R, XH03, XH04, and XH07 antigens were mixed with complete Freund's adjuvant and incomplete Freund's adjuvant in a volume ratio of 1:1, and after ultrasonic emulsification, 6-week-old ICR mice (n= 6) Inject complete Freund's adjuvant and antigen emulsion 300ul intraperitoneally, and after an interval of 7 days, inject incomplete Freund's adjuvant and antigen emulsion 300ul intraperitoneally, and take peripheral blood from the orbital venous plexus of immunized mice 20 days later and centrifuge serum. After 2-fold serial dilution of the immune serum starting from 1:8, they were mixed with 100CCID50 (Cell culture infective dose 50%) of CVA10 respectively, placed at 37°C for 2 hours, and the mixture was added to a 96-well plate of monolayer RD cells (1 ×10 5 / hole), put CO 2 Cultivate in an incubator at 37°C for 48 hours, observe the CPE of the cells, and take the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com